The Effect of the Hardener on the Characteristics of the Polyester-Based Coating †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method of Obtaining Samples
2.3. Methods for the Analysis of the Samples
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.M.; Akhtarul Islam, M. Application of epoxy resins in building materials: Progress and prospects. Polym. Bull. 2022, 79, 1949–1975. [Google Scholar] [CrossRef]
- Shundo, A.; Yamamoto, S.; Tanaka, K. Network formation and physical properties of epoxy resins for future practical applications. Jacs Au 2022, 2, 1522–1542. [Google Scholar] [CrossRef]
- Mi, X.; Liang, N.; Xu, H.; Wu, J.; Jiang, Y.; Nie, B.; Zhang, D. Toughness and its mechanisms in epoxy resins. Prog. Mater. Sci. 2022, 130, 100977. [Google Scholar] [CrossRef]
- Hsissou, R.; Dagdag, O.; Berradi, M.; El Bouchti, M.; Assouag, M.; El Bachiri, A.; Elharfi, A. Investigation of structure and rheological behavior of a new epoxy polymer pentaglycidyl ether pentabisphenol A of phosphorus and of its composite with natural phosphate. SN Appl. Sci. 2019, 1, 869. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Pulikkalparambil, H.; Rangappa, S.M.; Siengchin, S. (Eds.) Epoxy Composites: Fabrication, Characterization and Applications; KGaA. Epoxy Composites448; Wiley-VCH Verlag GmbH & Co: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Zanghellini, B.; Knaack, P.; Schörpf, S.; Semlitsch, K.H.; Lichtenegger, H.C.; Praher, B.; Rennhofer, H. Solvent-free ultrasonic dispersion of nanofillers in epoxy matrix. Polymers 2021, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Dallaev, R.; Pisarenko, T.; Papež, N.; Sadovský, P.; Holcman, V. A Brief Overview on Epoxies in Electronics: Properties, Applications, and Modifications. Polymers 2023, 15, 3964. [Google Scholar] [CrossRef]
- Rudawska, A.; Frigione, M. Effect of Diluents on Mechanical Characteristics of Epoxy Compounds. Polymers 2022, 14, 2277. [Google Scholar] [CrossRef]
- Heck, C.T.; Volkmann, G.; Woodward, H.N. Polyester or epoxy: Assessing embedding product efficacy in paleohistological methods. PeerJ 2020, 8, e10495. [Google Scholar] [CrossRef] [PubMed]
- Baron, C.; Donadio, F.; Scherdel, M.; Taha, I. Bio-based epoxy and unsaturated polyester resins: Research and market overview. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2024, 09544062241245552. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Aliphatic Polyesters: Synthesis, Properties and Applications BT—Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–40. ISBN 978-3-540-45734-3. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab: Golden, CO, USA, 2004. [Google Scholar]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Poly(glycerol-co-diacids) Polyesters: From Glycerol Biorefinery to Sustainable Engineering Applications, A Review. ACS Sustain. Chem. Eng. 2018, 6, 5681–5693. [Google Scholar] [CrossRef]
- Puri, S.; Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; Garnett, M.C. Drug incorporation and release of water soluble drugs from novel functionalised poly(glycerol adipate) nanoparticles. J. Control. Release 2008, 125, 59–67. [Google Scholar] [CrossRef]
- Godinho, B.; Gama, N.; Ferreira, A. Different methods of synthesizing poly(glycerol sebacate) (PGS): A review. Front. Bioeng. Biotechnol. 2022, 10, 1033827. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.; Cahill, J.; Jolanki, R.; Nixon, R. Polyester resins. In Kanerva’s Occupational Dermatology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 809–819. [Google Scholar]
- Ramakrishnan, T.; Mohan Gift, M.D.; Chitradevi, S.; Jegan, R.; Hency Jose, P.S.; Nagaraja, H.N.; Sharma, R.; Selvakumar, P.; Hailegiorgis, S.M. Study of numerous resins used in polymer matrix composite materials. Adv. Mater. Sci. Eng. 2022, 1, 1088926. [Google Scholar]
- Nodehi, M. Epoxy, polyester and vinyl ester based polymer concrete: A review. Innov. Infrastruct. Solut. 2022, 7, 64. [Google Scholar] [CrossRef]
- Savotchenko, S.; Kovaleva, E. Mechanical properties of cured epoxy resin. Mod. Phys. Lett. B 2021, 35, 2150445. [Google Scholar] [CrossRef]
- Tkachuk, A.I.; Zagora, A.G.; Terekhov, I.V.; Mukhametov, R.R. Isophorone Diamine—A Curing Agent for Epoxy Resins: Production, Application, Prospects. A Review. Polym. Sci. Ser. D 2022, 15, 171–176. [Google Scholar] [CrossRef]
- Rakhmatullina, A.P.; Satbaeva, N.S.; Cherezova, E.N.; Izergina, A.S. New Complex Epoxy Ester Resin-Based Binder: Synthesis and Influence on the Curing Process of Epoxyamine Compositions. Russ. J. Appl. Chem. 2020, 93, 182–187. [Google Scholar] [CrossRef]
- Aziz, T.; Haq, F.; Farid, A.; Cheng, L.; Chuah, L.F.; Bokhari, A.; Mubashir, M.; Tang, D.Y.Y.; Show, P.L. The epoxy resin system: Function and role of curing agents. Carbon Lett. 2024, 34, 477–494. [Google Scholar] [CrossRef]
- Li, J.; Aung, H.H.; Du, B. Curing regime-modulating insulation performance of anhydride-cured epoxy resin: A review. Molecules 2023, 28, 547. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, S.; Tang, Z.; Liu, Y.; Xu, X.; Li, Q.; Zhang, K.; Wang, B.; Wang, S.; Zhu, J. Amino acids as latent curing agents and their application in fully bio-based epoxy resins. Green Chem. 2021, 23, 6566–6575. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, S.; Guo, A.; Li, J.; Liu, D. The curing kinetics analysis of four epoxy resins using a diamine terminated polyether as curing agent. Thermochim. Acta 2021, 702, 178987. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Q.; Lu, M.; Meng, H.; Qu, Z.; Xu, C.A.; Jiao, E. Synthesis of a novel lignin-based epoxy resin curing agent and study of cure kinetics, thermal, and mechanical properties. J. Appl. Polym. Sci. 2021, 138, 50523. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, N.; Faisal, S.; Ali, A.; Khan, F.U.; You, S.; Bokhari, A.; et al. Role of silica-based porous cellulose nanocrystals in improving water absorption and mechanical properties. Environ. Res 2023, 222, 115253. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.L.; Soares, B.G.; Duchet-Rumeau, J.; Livi, S. Dual functions of ILs in the core-shell particle reinforced epoxy networks: Curing agent vs dispersion aids. Compos. Sci. Technol. 2017, 140, 30–38. [Google Scholar] [CrossRef]
- El-Aouni, N.; Hsissou, R.; El Azzaoui, J.; El Bouchti, M.; Elharfi, A. Synthesis rheological and thermal studies of epoxy polymer and its composite. Chem. Data Collect. 2020, 30, 100584. [Google Scholar] [CrossRef]
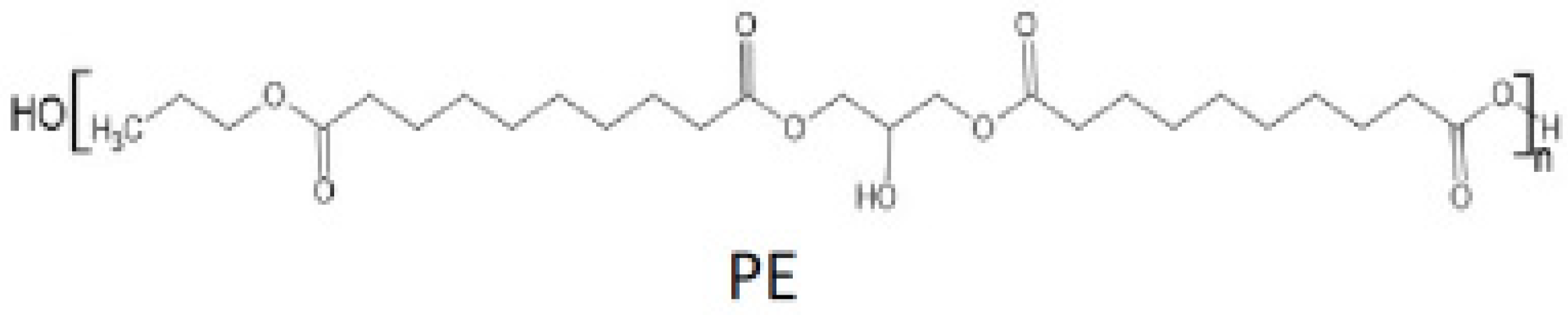
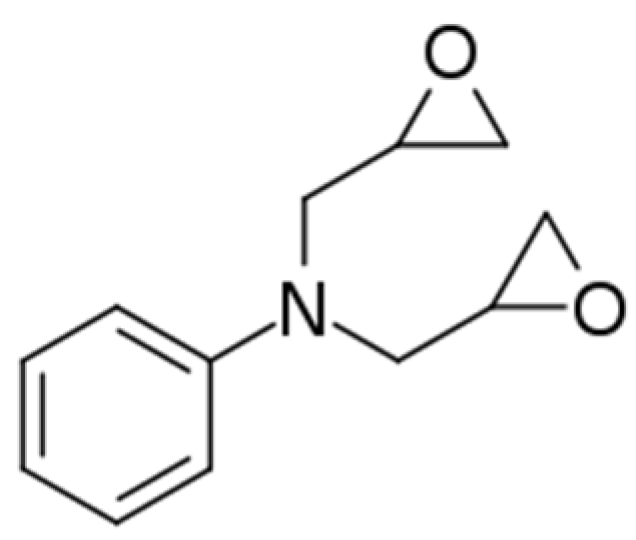
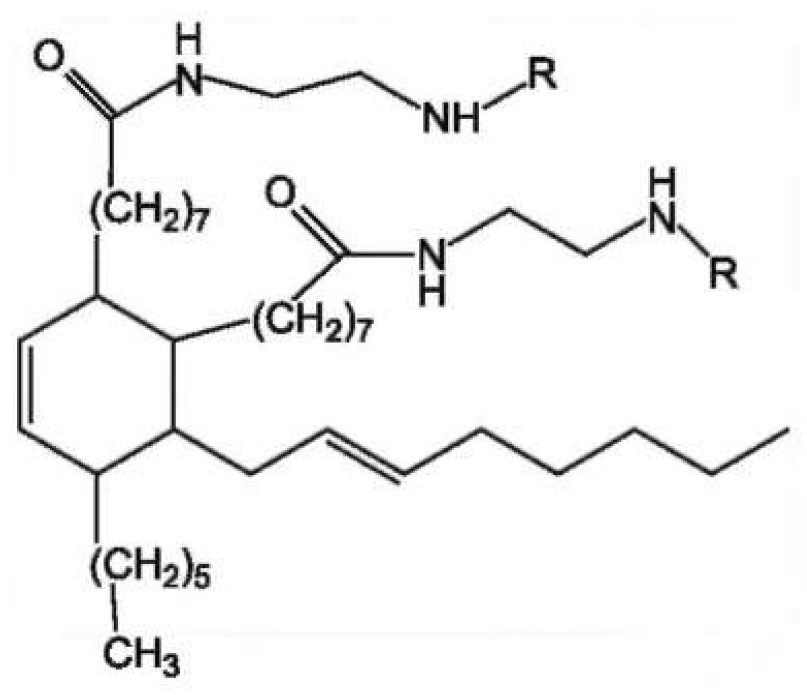

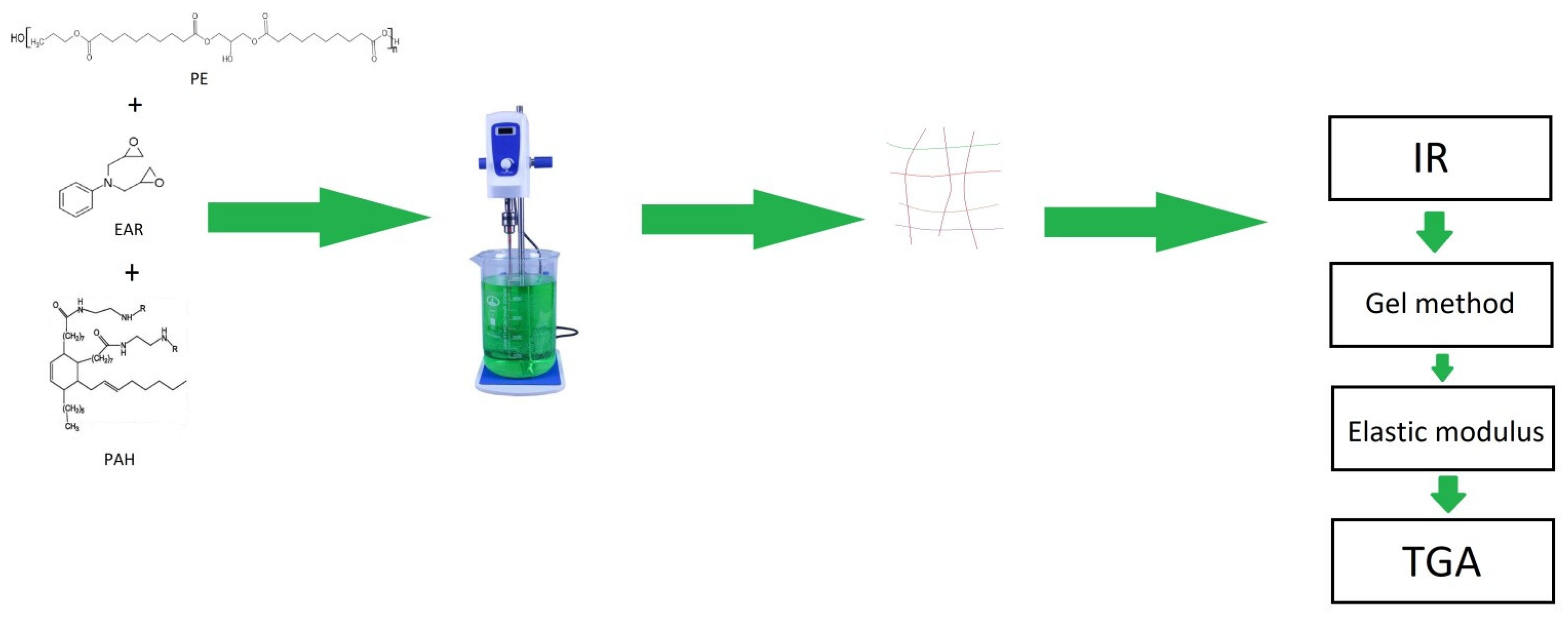
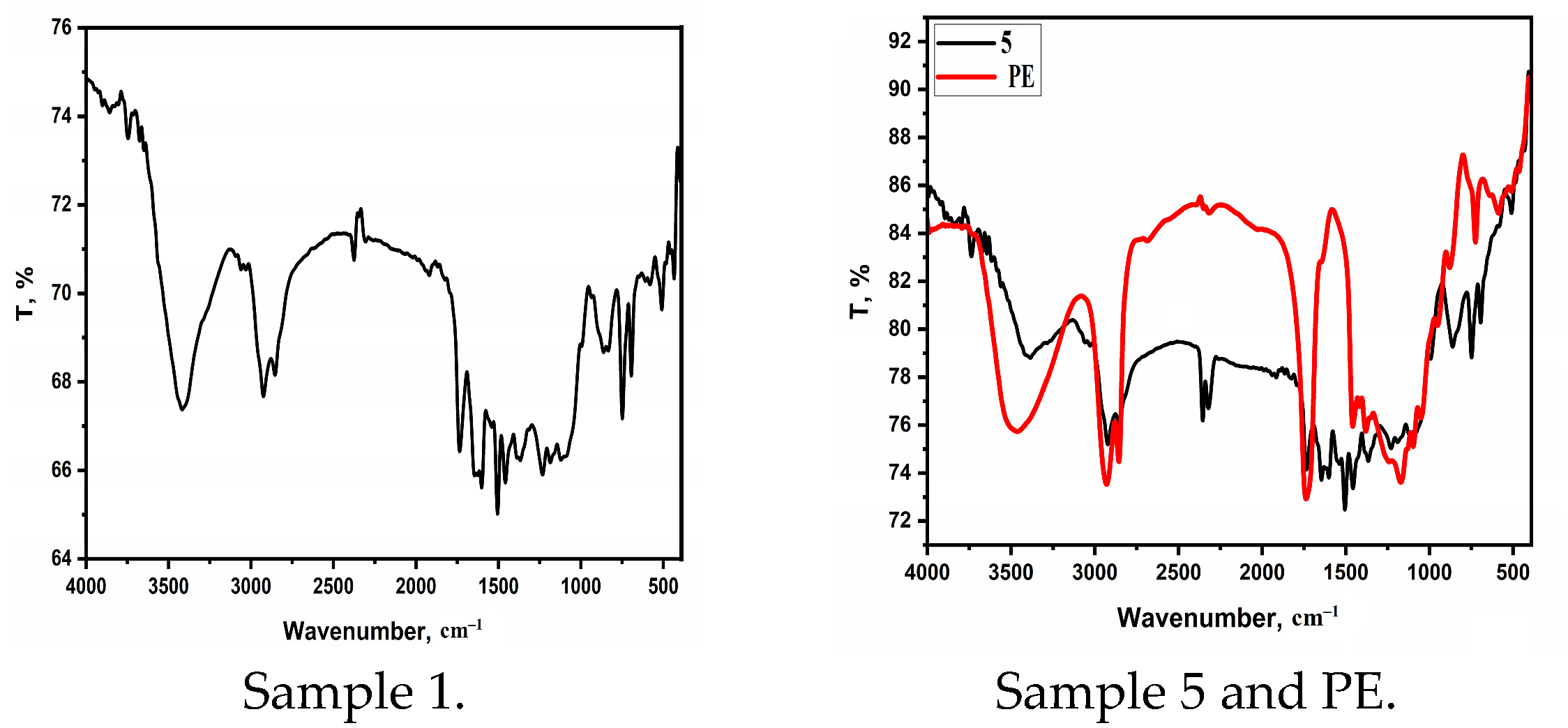
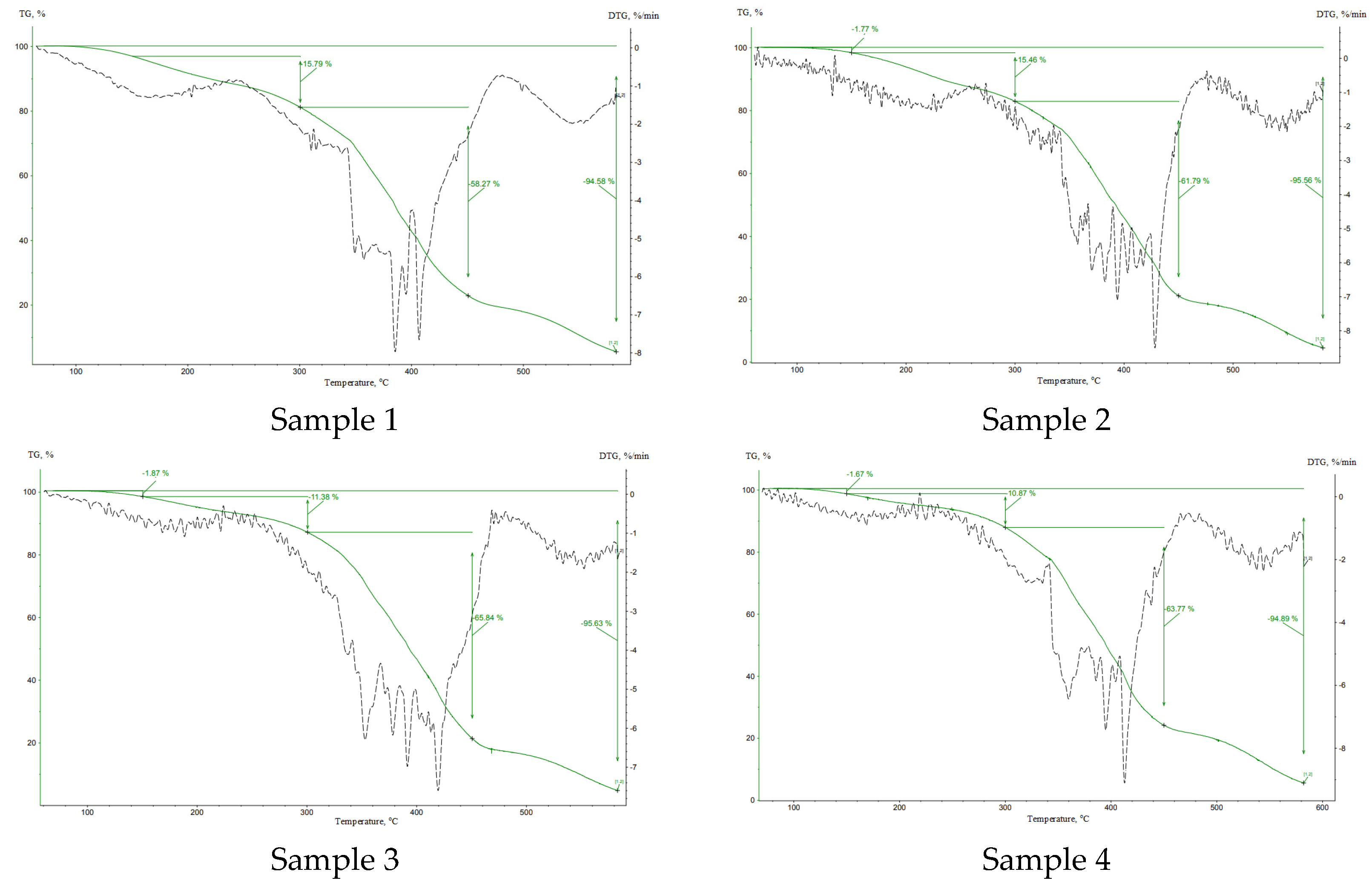
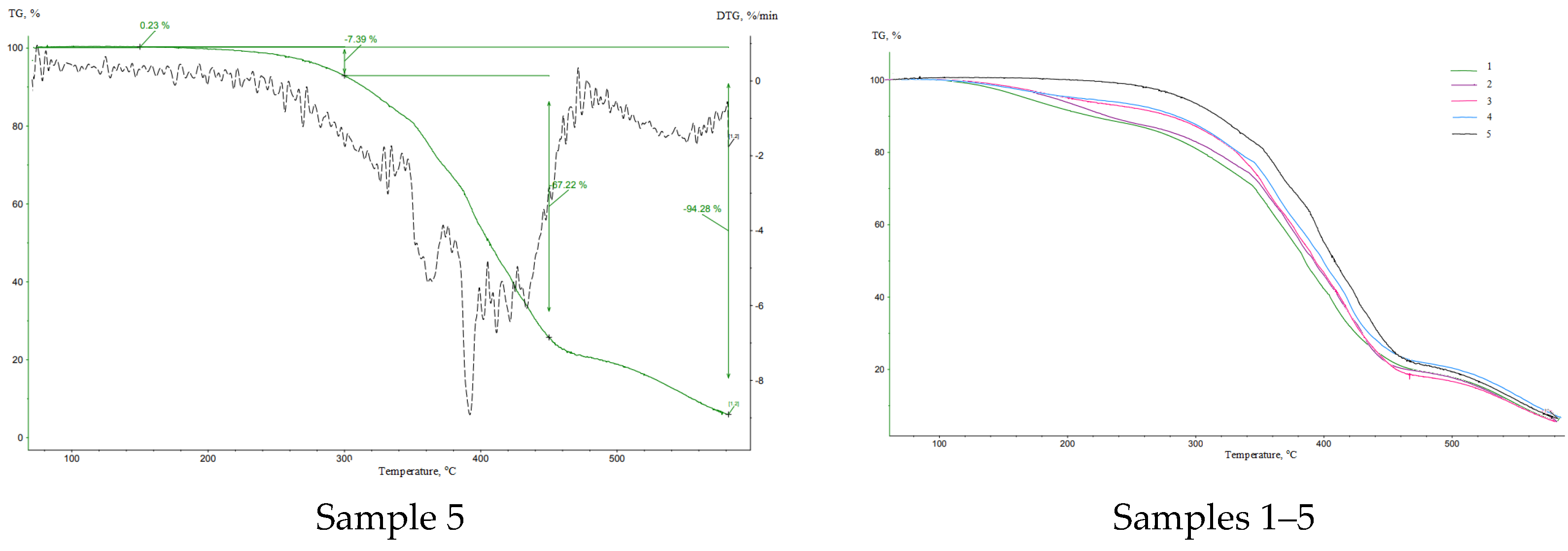
| Sample | Curing Modes |
|---|---|
| 1 | 22 °C/56 h |
| 2 | 22 °C/24 h, 60 °C/1 h, 80 °C/1 h |
| 3 | 22 °C/24 h, 80 °C/1 h, 100 °C/1 h, 120 °C/1 h |
| 4 | 60 °C/1 h, 80 °C/1 h, 120 °C/1 h |
| 5 | 22 °C/24 h, 80 °C/1 h, 120 °C/3 h |
| Sample | Percentage of Sol Fractions, % |
|---|---|
| 1 | 21.78 |
| 2 | 27.54 |
| 3 | 20.69 |
| 4 | 27.15 |
| 5 | 24.21 |
| Sample | Modulus, MPa | Tensile Strength, MPa | Elongation at Breaking, % | Elongation, mm | Maximum Strength, N |
|---|---|---|---|---|---|
| Standard | 37.57 | 2.54 | 72.05 | 44.83 | 54.79 |
| 1 | 2.56 | 0.43 | 34.99 | 21.77 | 6.08 |
| 2 | 2.67 | 0.59 | 42.27 | 22.65 | 6.66 |
| 3 | 2.75 | 0.72 | 48.76 | 23.53 | 9.67 |
| 4 | 3.62 | 0.89 | 54.28 | 26.35 | 14.89 |
| 5 | 4.36 | 1.12 | 59.10 | 30.05 | 24.12 |
| Sample | Δm (0–150 °C), % | Δm (150–300 °C), % | Δm (300–450 °C), % | Δm (450–600 °C), % | mf, g |
|---|---|---|---|---|---|
| 1 | 5.11 | 15.79 | 58.27 | 17.29 | 4.42 |
| 2 | 1.77 | 15.46 | 61.79 | 16.52 | 4.46 |
| 3 | 1.87 | 11.38 | 65.84 | 16.53 | 4.38 |
| 4 | 1.67 | 10.87 | 63.77 | 18.57 | 5.12 |
| 5 | 0.23 | 7.39 | 67.22 | 19.76 | 5.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikhareva, I.N. The Effect of the Hardener on the Characteristics of the Polyester-Based Coating. Eng. Proc. 2024, 67, 16. https://doi.org/10.3390/engproc2024067016
Vikhareva IN. The Effect of the Hardener on the Characteristics of the Polyester-Based Coating. Engineering Proceedings. 2024; 67(1):16. https://doi.org/10.3390/engproc2024067016
Chicago/Turabian StyleVikhareva, Irina N. 2024. "The Effect of the Hardener on the Characteristics of the Polyester-Based Coating" Engineering Proceedings 67, no. 1: 16. https://doi.org/10.3390/engproc2024067016
APA StyleVikhareva, I. N. (2024). The Effect of the Hardener on the Characteristics of the Polyester-Based Coating. Engineering Proceedings, 67(1), 16. https://doi.org/10.3390/engproc2024067016






