Abstract
Methylotrophic yeast Ogataea polymorpha BKM Y-2559 was immobilized in organosilicon sol–gel matrices using precursors isobutyltriethoxysilane (iBTES) and tetraethoxysilane (TEOS) to create an effective biocatalyst. The analytical and metrological performance of the biosensor permitted the determination of the optimum ratio of iBTES and TEOS, which was found to be 20/80 vol.%. The results of the scanning electron microscopy method demonstrated the formation of organosilicon material around microorganisms, as well as the ease with which metabolic products of yeast cells and substrates could diffuse through the obtained pores. A laboratory model of the biofilter was developed, exhibiting an oxidative capacity that varied from 0.14 to 1.25 gO2/(m3 × cycle) in accordance with the initial level of water pollution and the degree of purification of moderately polluted water. The latter was found to be 20%, which aligns with the norm for drip biofilters operating in cyclic mode.
1. Introduction
The initial biosensor systems were developed on the basis of the biochemical interactions between living microbial cells and enzymes [1,2,3,4,5]. These systems convert the analytical signal generated by the interaction of the biomaterial with the substance into a form that is readily understandable for the researcher. The strength of the analytical signal is contingent upon the quantity of the analyzed substance, thereby enabling the determination of the content of the component within the sample. The catalytic activity of biological objects is contingent upon the methodology employed for the fixation of the biomaterial on the surface of the transducer, in addition to various environmental factors, including temperature, pH, the presence of heavy metal ions, and radiation. The biomaterial used in biosensors can be employed in drop-type biofilters. This indicates that the resulting biocatalyst can be utilized for both the determination of contaminant levels and the utilization of such contaminants.
The development of biofilters based on microorganisms presents a number of challenges that can impact the efficiency and effectiveness of the biofiltration process. In many cases, biofilters are dominated by a single microbial species, which can result in competition with introduced or bio-added microorganisms. This can restrict the efficacy of particular microbial strains that are employed to facilitate the degradation of contaminants [6]. The dynamic nature of microbial communities means that the desired functional microorganisms may not establish themselves or persist in the biofilter media, resulting in unstable performance over time. Conversely, the secure attachment of the biomaterial can be employed as a solution to this issue. Harmful environmental factors can affect microbial viability, and maintaining a balance is essential to ensure the continued activity of the requisite microbial populations and prevent clogging of the filter media. Fluctuations in temperature and pH can significantly affect microbial activity and in turn, biofilter performance. Deviation from optimal pH ranges can result in reduced degradation rates or even microbial extinction. Therefore, encapsulation in protective shells can extend the life of the biofilter and increase its efficiency.
Consequently, organizations engaged in the development of microbial-based biofilters must contend with a number of challenges, including those related to microbial community dynamics, environmental conditions, contaminant characteristics, operational requirements, and design considerations. Addressing these issues is critical to optimize biofilter performance and ensure the effective contaminant removal in practical applications. One potential solution to these problems may be the immobilization of microorganisms in organosilicon matrices. Among the methods of protecting the bioagent from unfavorable conditions, the incorporation of living cells into the silicon matrix stands out as a particularly effective approach. The cell is immobilized within a porous shell that allows for the effective exchange of substances between the cell and the surrounding environment while preventing the cell from being exposed to aggressive environmental factors.
A novel biofilter has been developed by researchers at Georgia Tech, comprising yeast encapsulated in hydrogel capsules [7]. The capsules facilitate the effective removal of contaminants from drinking water. The yeast cells within the capsules function as biosorbents, binding to lead ions as water flows through the filter. The capsules have been designed to be mechanically robust, thereby enabling their use in a variety of water treatment systems, including small household filters and larger municipal applications. It should be noted that this biofilter is only effective in the removal of metal ions from an aquatic environment. The concept of yeast biocapsules has been a subject of investigation, whereby yeast cells are immobilized within a matrix that allows for the controlled release of cells into the water [8]. These biocapsules can be designed to optimize the interaction between the yeast and the contaminants, thereby enhancing the efficiency of pollutant removal. The encapsulation technique not only protects the yeast cells but also facilitates their application in various biofilter designs. However, microbial contamination of wastewater does occur.
Our research team has extensive experience in the immobilization of live microbial cells into organosilicon sol–gel matrices [9,10,11]. The synthesis commences with the utilization of alkoxysilanes, with tetraethoxysilane (TEOS) serving as a particularly prevalent precursor. Nevertheless, it has been demonstrated that the sole use of tetraethoxysilane results in the formation of a sol–gel matrix with a compact, solid structure, which subsequently diminishes the catalytic activity of the obtained biocatalyst [12,13]. This is due to the fact that cells are poorly fixed on the surface. The incorporation of a hydrophobic additive has been demonstrated to enhance the efficiency with which cells can be captured during the sol–gel synthesis process. A substance with a hydrophobic bond does not undergo hydrolysis and is not involved in polycondensation or matrix formation. Examples of such substances include methyltriethoxysilane (MTES), dimethyldiethoxysilane (DMDES), and isobutyltriethoxysilane (iBTES). Isobutyltriethoxysilane is a chemical compound that contains a non-hydrolysable silicon atom bond to a branched isobutyl radical. It is postulated that the use of small quantities of the substance results in the formation of a less rigid but stronger shell around microbial cells. In addition to silane precursors, structure-forming agents can be employed to achieve this result. The incorporation of this type of substance into the polymer chain can result in the formation of larger-diameter pores (e.g., polyethylene glycol (PEG)) or by forming a conducive environment for cells and participating in the creation of film structures (e.g., polyvinyl alcohol (PVA)).
2. Materials and Methods
2.1. Strains and Growth Conditions
The methylotrophic yeast Ogataea polymorpha BKM Y-2559 was received from the National Collection of the Institute of Biochemistry and Physiology of Microorganisms (Pushchino, Russia). The cultivation of microorganisms was conducted in accordance with the methodology employed in the aforementioned study [14].
2.2. Encapsulation of Cells by Sol–Gel Method
The silane precursors used were tetraethoxysilane (TEOS, Sigma-Aldrich, St. Louis, MO, USA) and isobutyltriethoxysilane (iBTES, Sigma-Aldrich, USA). A mixture of silane precursors, with a hydrophobic iBTES additive content ranging from 0 to 100 vol.% with respect to the total amount of the silane precursor, was used. The subsequent encapsulation of microorganisms was conducted in a manner analogous to that employed in our previous study [9].
2.3. Biocatalyst Bed Preparation for Biofilter Column Glass
This study was conducted in a similar manner [15]. Glass beads with an average diameter of 3.3 ± 0.3 mm were employed as the biofilter bed barrier. Prior to utilization, the glass beads were immersed in 0.1 N of HCl for a period of 2 h. An organosilica matrix containing entrapped yeast was applied to 500 glass beads that had been previously activated. The modified beads were then transferred to a 2.5 cm diameter, 16.5 cm long chromatographic column, cooled for 24 h and washed with a buffer (pH = 6.8).
3. Results and Discussion
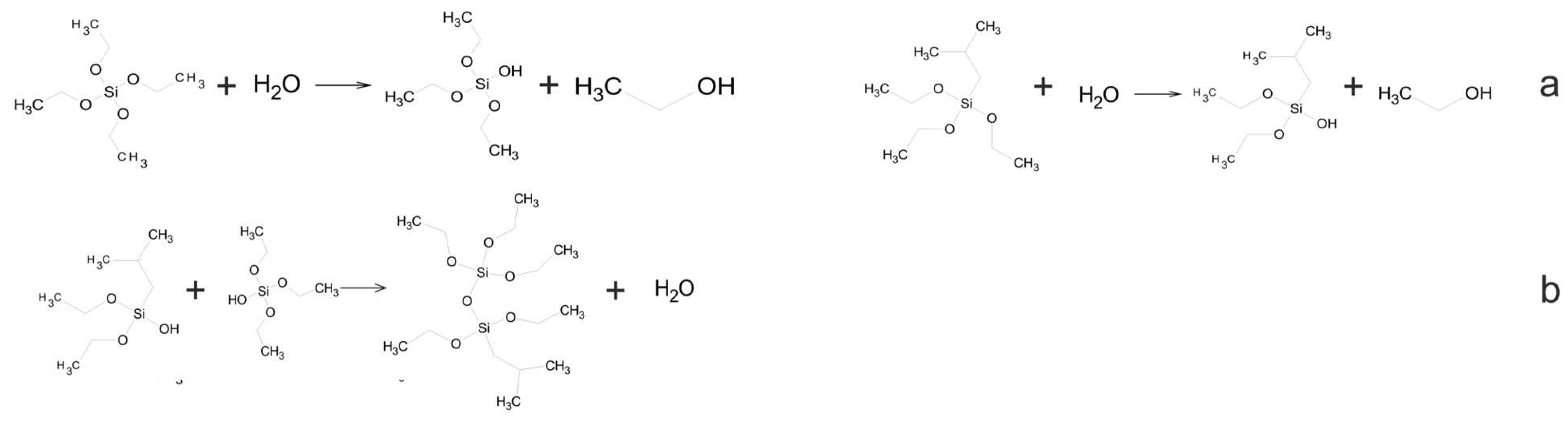
In the initial phase of this investigation, organosilicon materials were synthesized through the reaction of tetraethoxysilane and isobutyltriethoxysilane. Additionally, the hydrolysis and polycondensation of silicon precursors were also investigated (Figure 1).
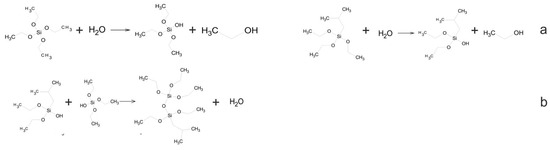
Figure 1.
Scheme of hydrolysis (a) and polycondensation (b) of tetraethoxysilane and isobutyltriethoxysilane.
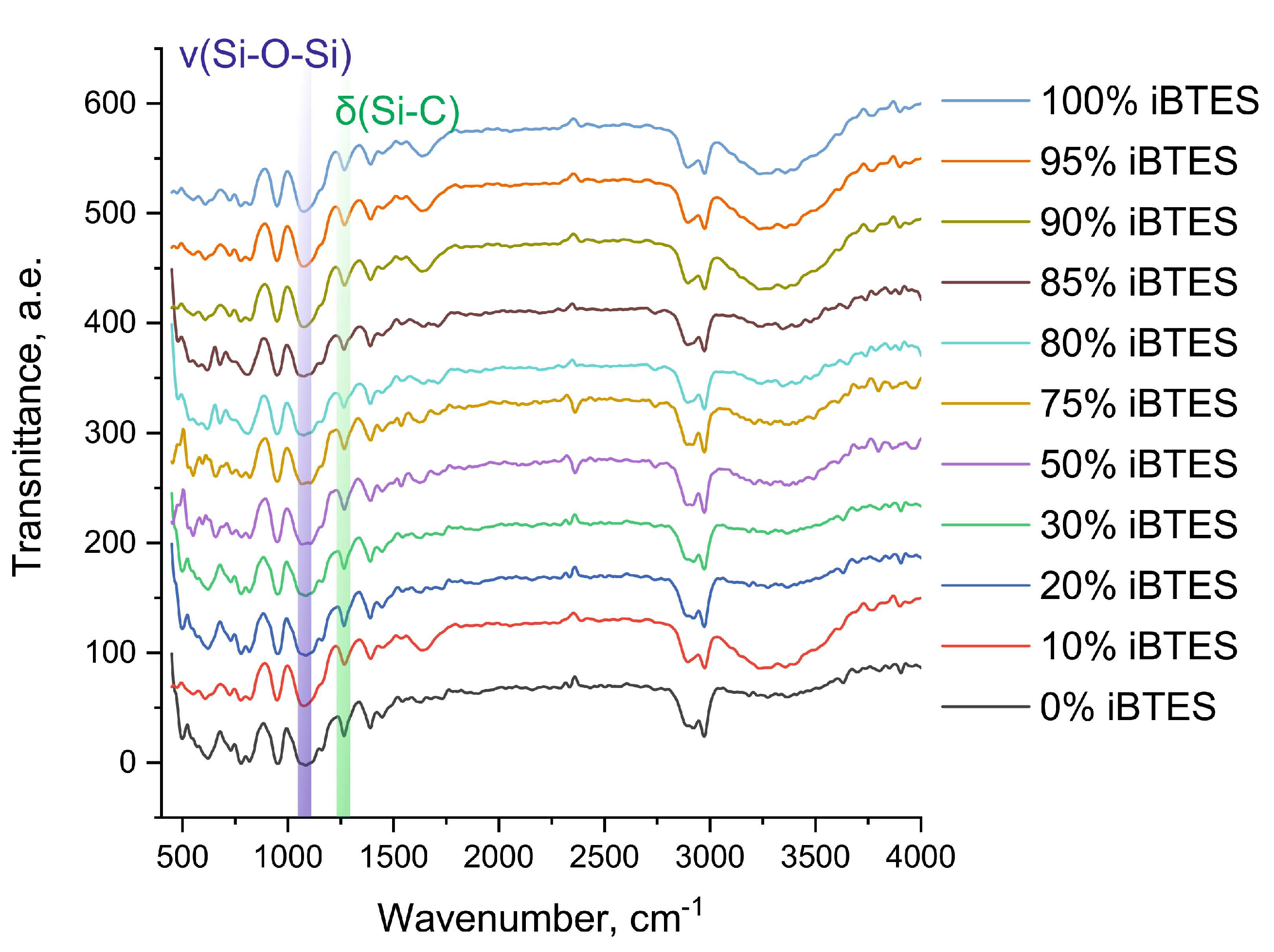
The subsequent stage of this investigation involved the analysis of the resulting material’s structural composition. The distinctive characteristics of the hybrid materials can be attributed to the degree of polymerization of the silicates. In the context of basic catalysis, where primary polymerization occurs in parallel with hydrolysis, the degree of polymerization is contingent upon the degree of hydrolysis. The number of formed Si–O–Si bonds, which can be determined by infrared spectroscopy, can estimate the degree of polymerization. The obtained spectra exhibited a high degree of similarity for all ratios of silane precursors. Figure 2 presents a typical view of the dependence of absorption intensities on the wave number.
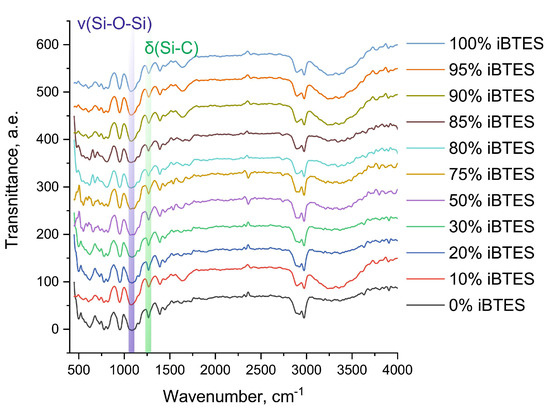
Figure 2.
IR spectrum of organosilicate sol–gel matrix of a sample consisting of iBTES, TEOS, and PVA.
In this study, IR spectra were obtained for the 11 materials under investigation. The IR spectra contain absorption bands in the range 1110–1000 cm−1, which correspond to Si–O–Si vibrations. Additionally, the spectra exhibit an intense absorption band at 1270 cm−1, which corresponds to the deformation vibrations of Si–C, which do not undergo hydrolysis. The results obtained are in complete agreement with the data described in the literature [16].
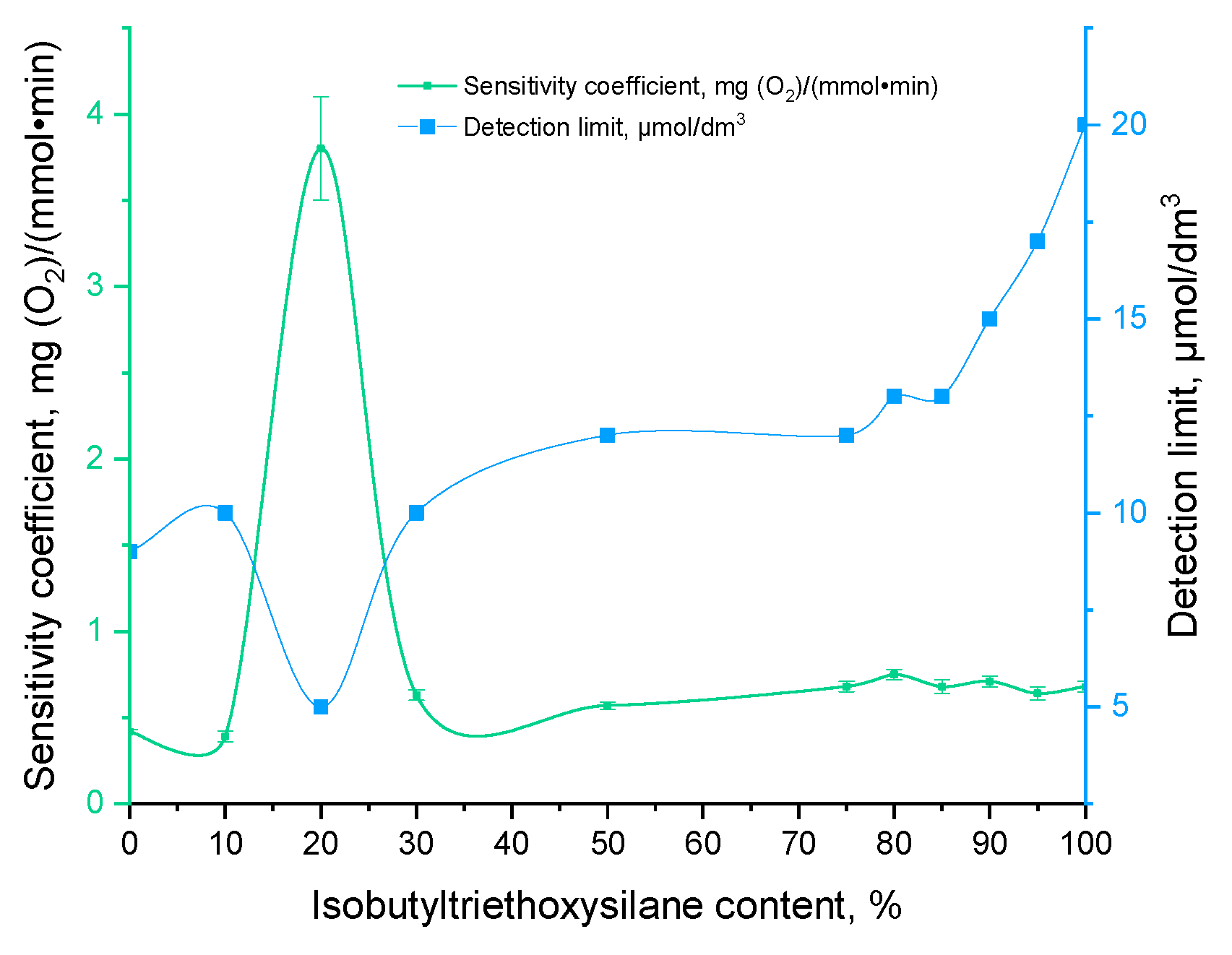
A biosensor approach was employed to characterize the functionality of living biomaterials. For this purpose, immobilized yeast cells were fixed on the surface of an oxygen electrode. The fundamental principle underlying the functioning of a biosensor is based on the increase in the respiratory activity of microorganisms in the presence of substrates, which subsequently leads to a decrease in the oxygen content in the near-electrode space. The change in oxygen content was recorded with an oxygen electrode. The biosensor response was quantified as the rate of change of oxygen content upon addition of the determined substance (mg(O2) × L−1 × min−1). In accordance with the recommendations of the International Union of Pure and Applied Chemistry (IUPAC) Division of Physical and Analytical Chemistry [17], the key characteristics of biosensors are as follows: sensitivity, an operating or linear concentration range, a detection limit, and a lower limit of detectable concentrations. The duration of biosensor operations without a replacement of the bio-recognition element (long-term stability) allows for the assessment of the stability of the biocatalyst based on immobilized microorganisms in an organosilicon matrix (Figure 3).
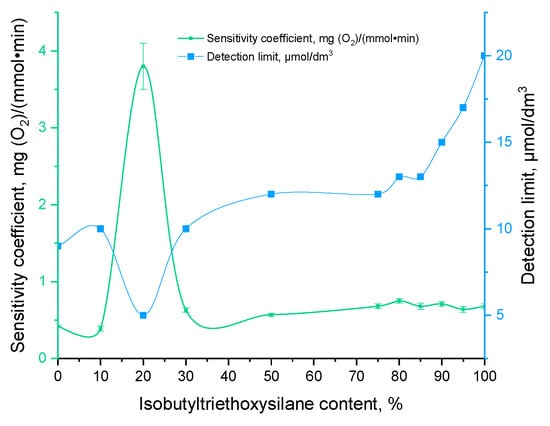
Figure 3.
Sensitivity parameters of sol–gel matrices investigated in this work.
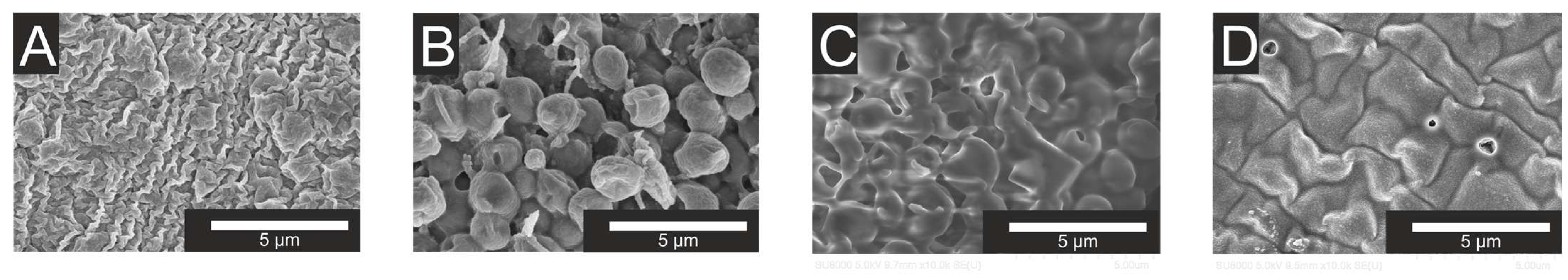
The biosensor based on biomaterial with a content of 20% vol. iBTES exhibited the highest sensitivity. It is postulated that this phenomenon is due to the sol–gel matrix, which contains a hydrophobic additive in a 20% vol. concentration, exhibiting a reduced degree of substrate diffusion. As the concentration of the hydrophobic additive iBTES exceeds 50%, a more porous matrix is formed, with larger pore diameters (Figure 4), which facilitates the straightforward removal of yeast cells. The data obtained are consistent with the findings of previous studies on the morphology of functional biomaterials.

Figure 4.
SEM images of the samples. (A)—10% iBTES; (B)—20% iBTES; (C)—90% iBTES; (D)—50% iBTES. Samples (A–C) contain yeast cells and sample (D) contains only iBTES without cells.
Consequently, the biomaterial comprising 20% iBTES exhibits the highest catalytic activity. The biosensor based on this sample is distinguished by the lowest lower limit of detectable concentrations and the maximum value of the sensitivity coefficient.
The characteristics of the biosensor based on methylotrophic yeast Og. polymorpha encapsulated in the organosilicon sol–gel using isobutyltriethoxysilane and tetraethoxysilane were compared with those of the previously studied bioreceptor element with a hydrophobic addition of methyltriethoxysilane (Table 1).

Table 1.
Comparison of characteristics of bioreceptor elements.
The data presented in this study indicate that the organosilicon sol–gel with a 20% isobutyltriethoxysilane content is the most effective method for immobilizing methylotrophic yeast Ogataea polymorpha VKM Y-2559. A comparison of the two samples reveals that the sample containing iBTES is more effective than the sample containing MTES. This may be attributed to the fact that the branched alkyl allows the formation of pores of a specific size, which facilitates the removal of reaction products and provides microorganisms with enhanced access to the substrate.
The biological treatment of industrial wastewater is a process that employs microorganisms to remove dissolved organic contaminants. This process is based on the ability of these microorganisms to utilize dissolved and colloidal organic contaminants as a food source during their life cycle. Such treatment can be implemented in natural environments, such as irrigation and filtration fields, as well as in specialized devices, including biofilters and aeration tanks. The biohybrid material obtained can be employed as biomaterial feedstock in the development of biofilters.
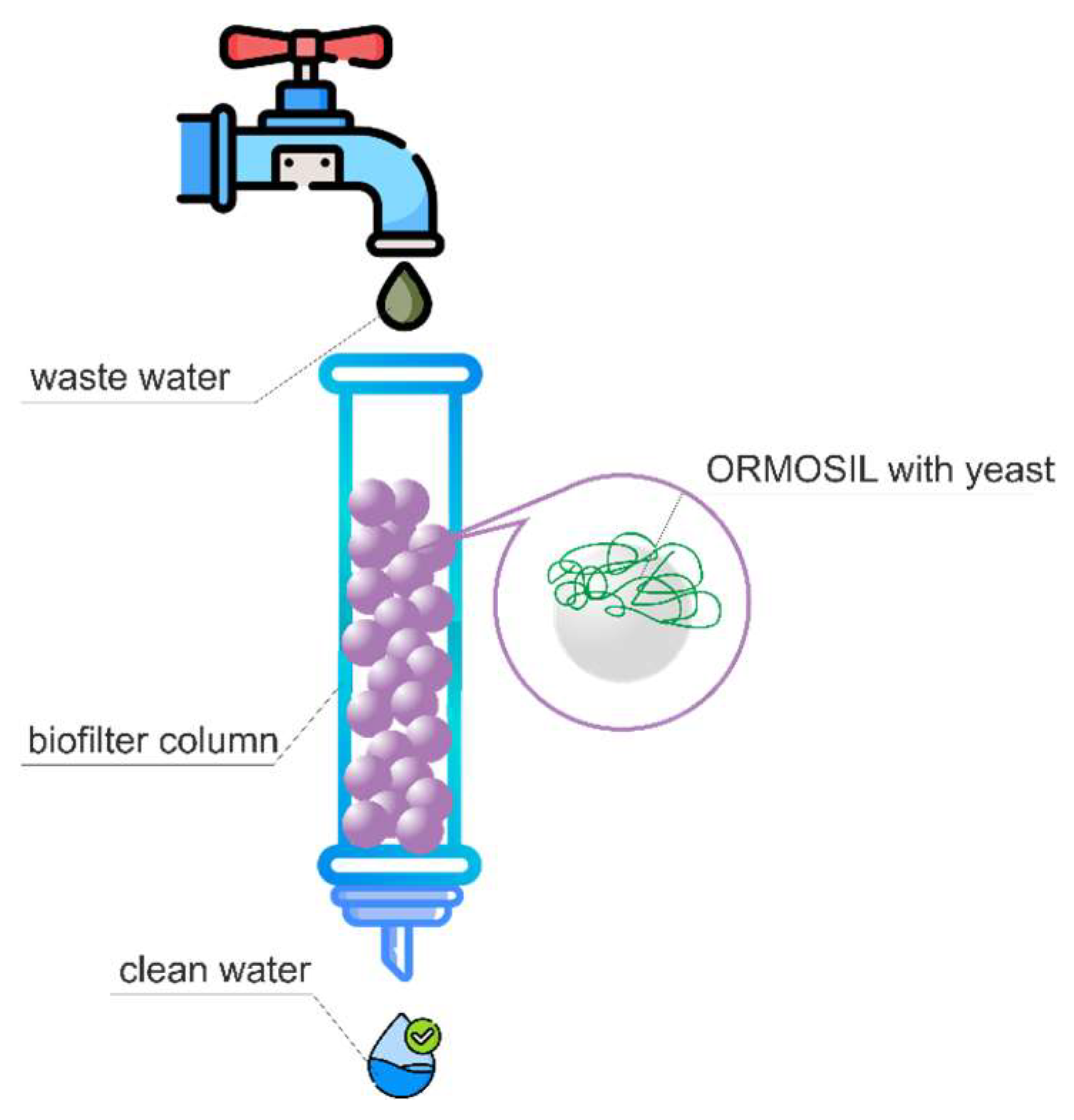
The laboratory biofilter (Figure 5) was constructed as follows: glass beads with immobilized whole microorganism cells in an organosilicate matrix were placed in a chromatographic column (length of 16.5 cm, diameter of 2.5 cm) and washed with a buffer solution (pH 6.8) until reaching the complete purification from alcohol, which was monitored by gas chromatography. The results are presented in Table 2.
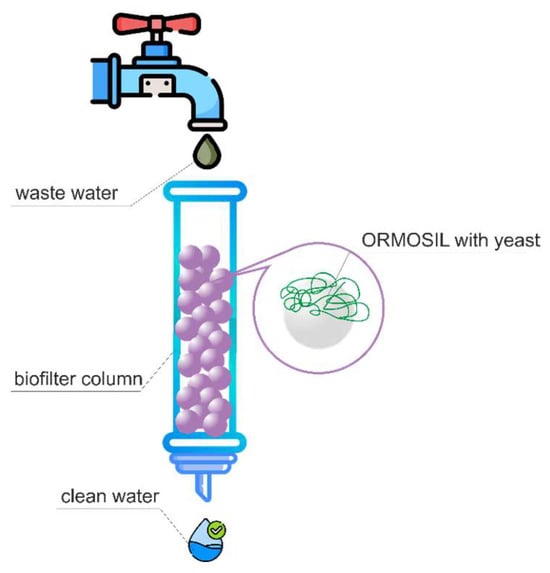
Figure 5.
Schematic diagram of a laboratory biofilter.

Table 2.
The efficiency of the biofilter and the degree of wastewater purification.
The capacity of the laboratory biofilter to utilize pollutants was examined under natural conditions at a model effluent flow rate of 0.5 mL/min (0.96 L/h × 1 loading). The capacity of the laboratory biofilter to purify wastewater was evaluated in accordance with the established protocol for BOD5 determination [18]. Consequently, glass beads (diameter of 3.3 mm) with a surface modified with encapsulated yeast cells were employed as a stationary phase in the laboratory biofilter. The biofilter efficiency was determined by measuring the oxidative capacity (OC, gO2/(m3 × cycle)).
- BODinp—BOD of incoming wastewater, mgO2/dm3;
- BODout—BOD of treated wastewater, mgO2/dm3;
- Q—the amount of wastewater, 5 × 10−5 (m3/cycle);
- Vbiofilter—feed volume, 3.1 × 10−5 m3.
It has been demonstrated that the oxidation capacity of a biofilter for a single operational cycle (70 min of operation time, 30 min of regeneration time) varies between 0.14 and 1.25 gO2/(m3 × cycle). This variation is contingent on the initial degree of water pollution and the degree of purification of moderately dirty water (20%), which is the norm for drip biofilters operating in cyclic mode. Following a washing of the column, the biofilter activity is restored, allowing for its repeated use. Nevertheless, for the full recovery of microbial activity, it is necessary to maintain the biofilter in a state of metabolic inactivity for a period of 12 h following three cycles of cleaning. It is recommended that the loading material be replaced according to the long-term stability of the developed heterogeneous biocatalyst. Silica gels are known to have sorption properties, but previous studies demonstrated that the sorption of pollutants does not exceed 5% [19]. It can be demonstrated that biohybrid materials based on encapsulated microorganisms in an organosilicate matrix of iBTES/TEOS in the ratio of 20/80 vol.% are effective biocatalysts for biofilters.
4. Conclusions
In this study, the methylotrophic yeast Ogataea polymorpha BKM Y-2559 was immobilized into organosilicon sol–gel matrices using the precursors iBTES and TEOS, thus creating an effective biocatalyst. The analytical and metrological performance of the biosensor was used to determine the optimal ratio of iBTES to TEOS, which was found to be 20/80 vol.%. The scanning electron microscopy (SEM) method demonstrated the formation of organosilicon material around the microorganisms, yeast cell metabolic products, and substrates, which were observed to easily diffuse through the pores in the obtained materials. It has been demonstrated that the replacement of MTES with iBTES in the sol–gel matrix results in enhanced sensitivity values. This is likely because branched alkyl allows the creation of pores of such a size that reaction products are more effectively removed, and microorganisms have better access to the substrate. A laboratory model of a biofilter was developed, with an oxidative capacity for one cycle of operation ranging from 0.14 to 1.25 gO2/(m3 × cycle), dependent on the initial value of water pollution and the degree of purification of moderately dirty water, which was set at 20% in accordance with the norm for drip biofilters operating in cyclic mode.
Author Contributions
Conceptualization, O.K., E.L. and P.R.; methodology, O.K., E.L., P.R. and V.S.; software, P.R.; validation, E.L.; investigation, O.K., E.L. and P.R.; resources, V.S.; data curation, O.K.; writing—original draft preparation, O.K., E.L., P.R. and V.S.; writing—review and editing, O.K. and V.S.; visualization, E.L., O.K., V.S. and P.R.; supervision, O.K.; project administration, O.K.; funding acquisition, O.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Government of the Tula region for science and technology in 2023 under contract DS/111/BASiB1/23/TO dated 27 September 2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article.
Acknowledgments
Electron microscopy characterization was performed in the Department of Structural Studies of Zelinsky Institute of Organic Chemistry, Moscow.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baca, H.K.; Carnes, E.C.; Ashley, C.E.; Lopez, D.M.; Douthit, C.; Karlin, S.; Brinker, C.J. Cell-directed-assembly: Directing the formation of nano/bio interfaces and architectures with living cells. Biochim. Et Biophys. Acta Gen. Subj. 2011, 1810, 259–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Sun, J.; Wang, J.; Bian, C.; Tong, J.; Li, Y.; Xia, S. A single-layer structured microbial sensor for fast detection of biochemical oxygen demand. Biochem. Eng. J. 2016, 112, 219–225. [Google Scholar] [CrossRef]
- Gonchar, M.; Maidan, M.; Korpan, Y.; Sibirny, V.; Kotylak, Z.; Sibirny, A. Metabolically engineered methylotrophic yeast cells and enzymes as sensor biorecognition elements. FEMS Yeast Res. 2002, 2, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Cornejo-Ponce, L.; Rajendran, R.; Manavalan, K.; Femilaa Rajan, V.; Awad, F. A review on biofiltration techniques: Recent advancements in the removal of volatile organic compounds and heavy metals in the treatment of polluted water. Bioengineered 2022, 13, 8432–8477. [Google Scholar] [CrossRef] [PubMed]
- Stathatou, P.M.; Athanasiou, C.E.; Tsezos, M.; Goss, J.W.; Blackburn, L.C.; Tourlomousis, F.; Mershin, A.; Sheldon, B.W.; Padture, N.P.; Darling, E.M.; et al. Lead removal at trace concentrations from water by inactive yeast cells. Commun. Earth Environ. 2022, 3, 132. [Google Scholar] [CrossRef]
- Lúquez-Caravaca, L.; Ogawa, M.; Rai, R.; Nitin, N.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Jiménez-Uceda, J.C.; Moreno-García, J. Yeast cell vacuum infusion into fungal pellets as a novel cell encapsulation methodology. Appl. Microbiol. Biotechnol. 2023, 107, 5715–5726. [Google Scholar] [CrossRef] [PubMed]
- Kamanina, O.A.; Lantsova, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Saverina, E.A.; Kulikovskaya, N.S.; Perepukhov, A.M.; Vereshchagin, A.N.; Ananikov, V.P. “3-in-1” Hybrid Biocatalysts: Association of Yeast Cells Immobilized in a Sol–Gel Matrix for Determining Sewage Pollution. ACS Appl. Mater. Interfaces 2023, 15, 47779–47789. [Google Scholar] [CrossRef] [PubMed]
- Lantsova, E.A.; Kamanina, O.A.; Rybochkin, P.V.; Saverina, E.A. Organosilicon Material in Combination with Structure-Controlling Agents as a Basis for Immobilization of the Enzyme Glucose Oxidase. Russ. J. Inorg. Chem. 2024. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Lantsova, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Plekhanova, Y.V.; Reshetilov, A.N. The Use of Diethoxydimethylsilane as the Basis of a Hybrid Organosilicon Material for the Production of Biosensitive Membranes for Sensory Devices. Membranes 2022, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Kamanina, O.A.; Fedoseeva, D.G.; Rogova, T.V.; Ponamoreva, O.N.; Blokhin, I.V.; Machulin, A.V.; Alferov, V.A. Synthesis of organosilicon sol-gel matrices and preparation of heterogeneous biocatalysts based on them. Russ. J. Appl. Chem. 2014, 87, 761–766. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Lavrova, D.G.; Arlyapov, V.A.; Alferov, V.A.; Ponamoreva, O.N. Silica sol-gel encapsulated methylotrophic yeast as filling of biofilters for the removal of methanol from industrial wastewater. Enzym. Microb. Technol. 2016, 92, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Ponamoreva, O.N.; Kamanina, O.A.; Alferov, V.A.; Machulin, A.V.; Rogova, T.V.; Arlyapov, V.A.; Alferov, S.V.; Suzina, N.E.; Ivanova, E.P. Yeast-based self-organized hybrid bio-silica sol–gels for the design of biosensors. Biosens. Bioelectron. 2015, 67, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Lavrova, D.G.; Kamanina, O.A.; Alferov, V.A.; Rybochkin, P.V.; Machulin, A.V.; Sidorov, A.I.; Ponamoreva, O.N. Impact of hydrophilic polymers in organosilica matrices on structure, stability, and biocatalytic activity of immobilized methylotrophic yeast used as biofilter bed. Enzym. Microb. Technol. 2021, 150, 109879. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A. Mesoporous materials and electrochemistry. Chem. Soc. Rev. 2013, 42, 4098–4140. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- ISO 5815–1:2003; Water Quality—Determination of Biochemical Oxygen Demand after N Days (BODn), Part 1: Dilution and Seeding Method with Allylthiourea Addition. International Organization for Standardization: Geneva, Switzerland, 2003.
- Farouk, S.M.; Brusewitz, G.H. Moisture sorption characteristics of dust contaminated silica gel. J. Agric. Eng. Res. 1980, 25, 209–216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).