Modelling the Dynamics of P. aeruginosa in the Formation of Biofilms †
Abstract
1. Introduction
2. Methods
2.1. Experimental Design
2.2. Model for Bacteria Accumulation and Mobilization
2.3. Determined the Bacteria Accumulation and Mobilization Velocity
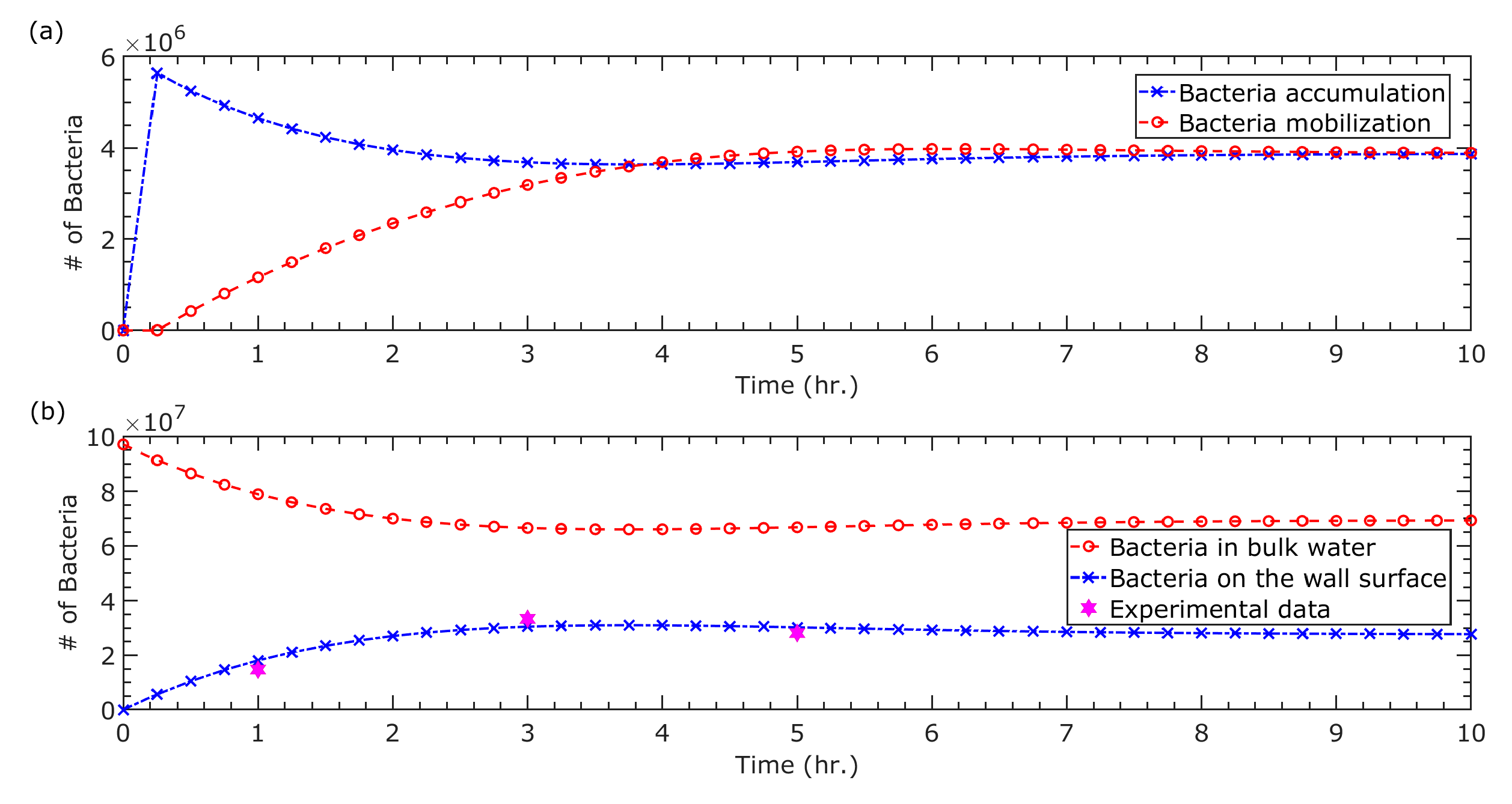
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Potable Water Quality and Bacteria in Water Distribution Systems. Available online: https://www.wcs-group.co.uk/wcs-blog/bacteria-in-water-distribution-systems (accessed on 27 March 2024).
- Erdei-Tombor, P.; Kiskó, G.; Taczman-Brückner, A. Biofilm Formation in Water Distribution Systems. Processes 2024, 12, 280. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tonner, P.D.; Darnell, C.L.; Engelhardt, B.E.; Schmid, A.K. Detecting differential growth of microbial populations with Gaussian process regression. Genome Res. 2017, 27, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Childs, P. Rotating Cylinders, Annuli, and Spheres. In Rotating Flow, 1st ed.; Elsevier Science & Technology Books: Shanghai, China, 2011; pp. 177–247. [Google Scholar]
- Janjaroen, D.; Ling, F.; Monroy, G.; Derlon, N.; Mogenroth, E.; Boppart, S.A.; Liu, W.T.; Nguyen, T.H. Roles of ionic strength and biofilm roughness on adhesion kinetics of Escherichia coli onto groundwater biofilm grown on PVC surfaces. Water Res. 2013, 47, 2531–2542. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Value |
|---|---|
| Water density | |
| Dynamic Viscosity | |
| Temperature |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, D.S.; Quinn, D.; Tsagkari, E.; Fish, K.; Pick, F.; Boxall, J.; Smith, C.; You, S.; Sloan, W. Modelling the Dynamics of P. aeruginosa in the Formation of Biofilms. Eng. Proc. 2024, 69, 141. https://doi.org/10.3390/engproc2024069141
Bhandari DS, Quinn D, Tsagkari E, Fish K, Pick F, Boxall J, Smith C, You S, Sloan W. Modelling the Dynamics of P. aeruginosa in the Formation of Biofilms. Engineering Proceedings. 2024; 69(1):141. https://doi.org/10.3390/engproc2024069141
Chicago/Turabian StyleBhandari, Dinesh Singh, Dominic Quinn, Erifyli Tsagkari, Katherine Fish, Frances Pick, Joby Boxall, Cindy Smith, Siming You, and William Sloan. 2024. "Modelling the Dynamics of P. aeruginosa in the Formation of Biofilms" Engineering Proceedings 69, no. 1: 141. https://doi.org/10.3390/engproc2024069141
APA StyleBhandari, D. S., Quinn, D., Tsagkari, E., Fish, K., Pick, F., Boxall, J., Smith, C., You, S., & Sloan, W. (2024). Modelling the Dynamics of P. aeruginosa in the Formation of Biofilms. Engineering Proceedings, 69(1), 141. https://doi.org/10.3390/engproc2024069141







