Abstract
Biofilm formation in drinking water distribution systems (DWDSs) poses challenges to water quality and system integrity. Traditional measurement methods often involve intrusive techniques, disrupting the biofilm ecosystem, while non-intrusive methods offer promising alternatives. This paper explores the feasibility of using non-intrusive temperature sensing to monitor biofilm growth in PVC pipes. Through experiments using the SLIMER 2.0 setup, the biofilm accumulation’s impact on the heat transfer properties is investigated. Preliminary results show successful biofilm growth under controlled conditions, with temperature measurements revealing alterations in heat resistance, hence providing a basis for biofilm monitoring. This study contributes to advancing biofilm monitoring techniques, offering insights for improved water quality management in DWDSs.
1. Introduction
Drinking water distribution systems (DWDSs) ensure the delivery of safe water through rigorous treatment processes. However, despite these measures, microorganisms that survive treatment or enter through leaks can form biofilms on the inner surfaces of pipes [1]. A biofilm, a heterogeneous and highly active microbiological ecosystem of its own, is composed of bacteria enveloped in extracellular polymeric substances (EPSs) and presents significant challenges such as accelerating pipe deterioration and providing a habitat for pathogens [2,3,4]. Consequently, extensive research has been conducted to measure biofilm in DWDSs.
Traditional intrusive measurement techniques involve removing the biofilm for analysis, which can disrupt its delicate balance [5,6]. In contrast, non-intrusive methods focus on indirect indicators of microbial activity. Optical sensors, for instance, detect biofilms through light-related phenomena, while electrochemical impedance sensors measure the added electrical resistance of bacteria [6]. However, non-intrusive sensors may encounter performance limitations under specific conditions, impacting their reliability [7].
A promising non-intrusive approach is temperature biofilm sensing, which quantifies biofilm accumulation by monitoring changes in heat transfer properties [8]. This method has demonstrated that even a minimal accumulation of biofilm, as little as 10 μm, can alter the overall thermal resistance significantly [5]. Importantly, temperature-based sensing is less influenced by pipe network discontinuities and offers broader coverage compared to other techniques, potentially reducing uncertainties associated with sparse sensor measurements.
Our ongoing research endeavors to explore the feasibility of utilizing heat transfer properties to measure biofilm growth in drinking water pipes. By adopting this non-intrusive approach, we aim to provide a more comprehensive and reliable method for monitoring biofilm in DWDSs, contributing to improved water quality management and infrastructure maintenance practices.
2. Materials and Methods
Biofilm build-up within pipes poses an impediment to heat transfer, impacting the heat flux. Compared to clean pipes, pipes fouled with biofilm exhibit diminished heat transfer efficiency, indicating increased heat resistance. Notably, this assessment method focuses solely on biofilm quantity, as this is expressed by the heat resistance, lacking insight into the biofilm’s composition or other microbiological attributes due to its non-intrusive nature.
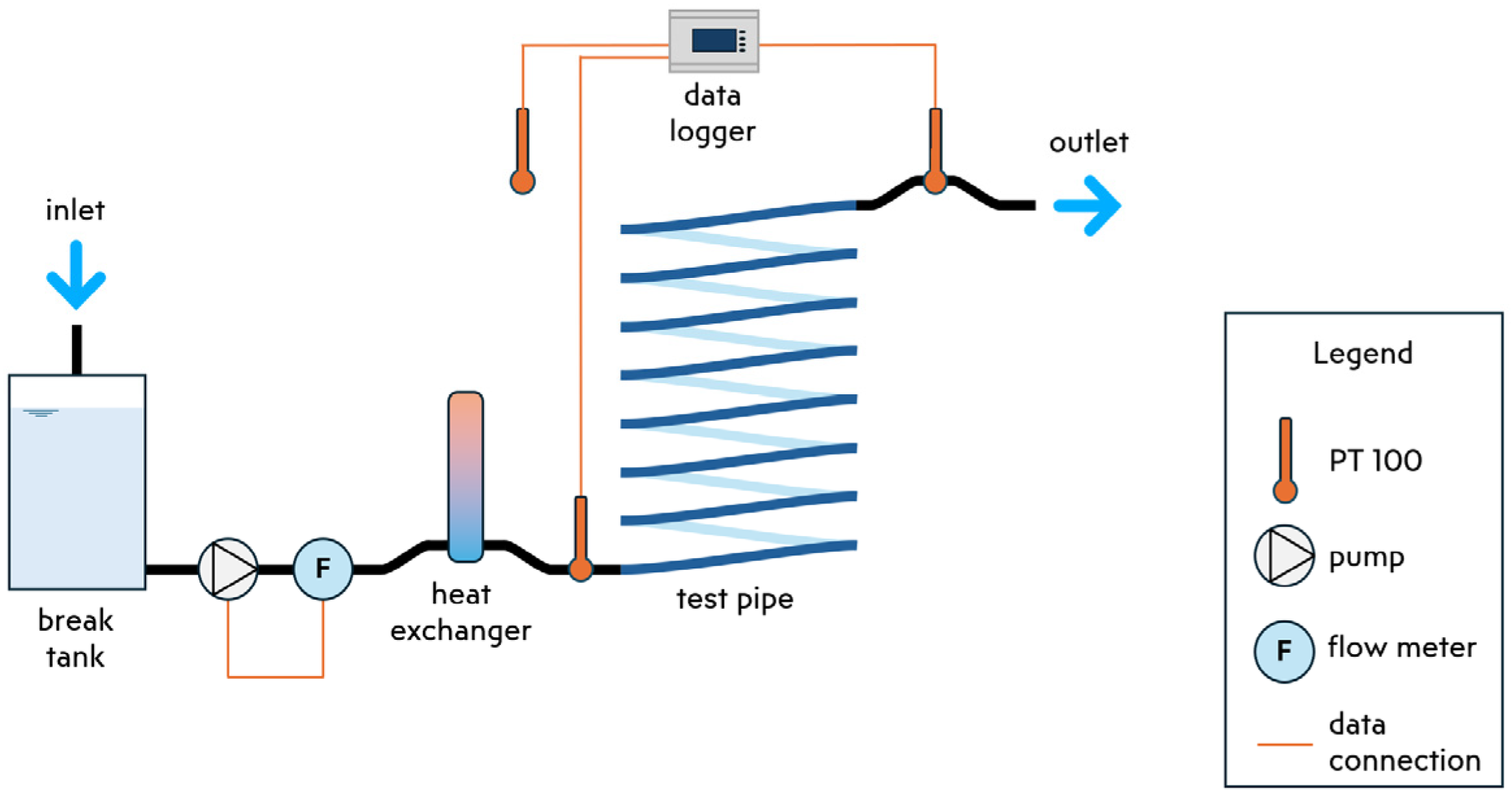
In our examination of biofouling effects on drinking water pipes, we utilize the SLIMER 2.0 experimental setup (see Figure 1), located at the KWR facilities in Nieuwegein, Netherlands. This experimental arrangement features a plasticized polyvinyl chloride (PVCp) transparent pipe, a material often found in garden hoses and showers. Tap water flows through the pipe without pre-treatment, offering insights into typical local water quality. Flow regulation is achieved through a break tank, a magnetic motor pump, and an ultrasonic flow meter, all housed within a temperature-controlled environment.
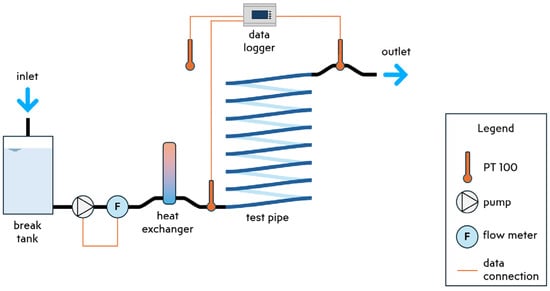
Figure 1.
SLIMER 2.0 experimental setup.
Temperature is continuously monitored using three PT100 class A temperature sensors (Labfacility Ltd., Bognor Regis, UK): one at the inlet, another at the outlet, and a third positioned externally to the pipe to gauge ambient temperature. Additionally, an ultrasonic flow meter installed at the inlet facilitates continuous flow rate measurement and pump speed control. Biofilm detection employs both an electronic microscope and adenosine triphosphate (ATP) measurements. The microscope enables us to observe biofouling at eleven different locations. A plano-convex lens flashlight ensures uniform lighting when using the microscope. Finally, we take post-experiment destructive ATP measurements to assess the biological activity of the grown biofilm [9].
Understanding the impact of biofilm build-up on pipe heat transfer involves considering the temporal aspect of biofilm growth. Initially absent, the biofilm gradually develops, leading to increased heat resistance and impacting the heat transfer. Equations linking heat flux through the pipe wall and in the water flow help quantify this effect, with total resistance decomposed into the internal, biofilm, wall, and external components. Calculating the external resistance at the experiment’s start enables tracking of biofilm-induced heat resistance changes over time while maintaining consistent experimental conditions.
3. Results and Discussion
In our current experimental iteration of SLIMER 2.0, we are utilizing a 50 m long REHAU RAUFILAM-E transparent PVCp pipe, boasting an internal diameter of 13.2 mm and a pipe wall thickness of 3.3 mm. This particular pipe material is rich in nutrients available to bacteria and ensures accelerated biofilm growth [10].
The default flow rate for our experiment is set at 400 L/h, resulting in a Reynolds number of 10,700, indicative of turbulent flow conditions given the aforementioned pipe characteristics. However, at specific time intervals, we deliberately vary the flow rate to 50, 100, 200, and 300 L/h for short periods of time to assess how the flow rate influences the heat transfer. This temporary adjustment allows for Reynolds numbers of 1340, 2680, 5360, and 8040, respectively. These instances of reduced flow rate, compared to the default, are brief, lasting only a few hours collectively, to evaluate heat transfer without disrupting the dynamics of biofilm growth. This approach is feasible because lower flows result in reduced pipe wall shear stress, which the developed biofilm is already accustomed to.
To control the inlet water temperature, we employ a heat exchanger plate. Initially, the inlet temperature is set to 12.5 °C for the first 30 days of the experiment, following which it is raised to 16.5 °C. This higher temperature accelerates biofilm growth within a shorter timeframe while remaining within the temperature range typically encountered in DWDSs. The ambient temperature is kept within the [20 °C, 28 °C] range, so that, for the flow rates and residence time tested, we have a sufficiently high temperature difference between the inlet and outlet.
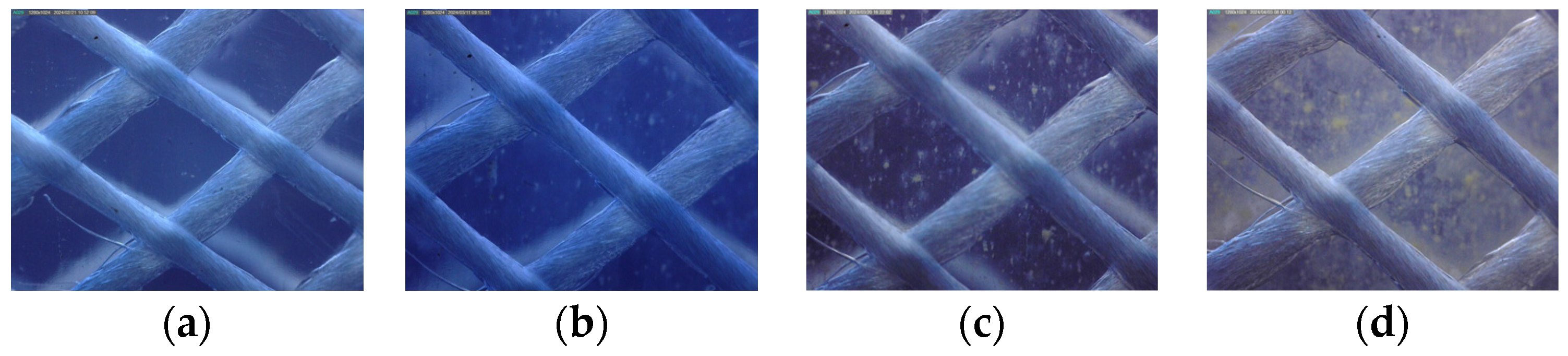
The preliminary findings suggest that the SLIMER 2.0 setup effectively cultivates biofilms using standard tap water, while consistently recording flow rates and temperatures. The examination of selected microscopic images (see Figure 2) revealed heterogeneous biofilm growth, following a non-linear temporal trend. In addition, biofilm growth seemed to be asynchronous at different cross-sections of the pipe, with parts of the pipe closer to the outlet exhibiting shorter maturation times.

Figure 2.
Microscope images of the biofilm grown in the PVCp pipe taken on different dates of the experiment: (a) biofilm on day 3; (b) biofilm on day 21; (c) biofilm on day 30; (d) biofilm on day 44.
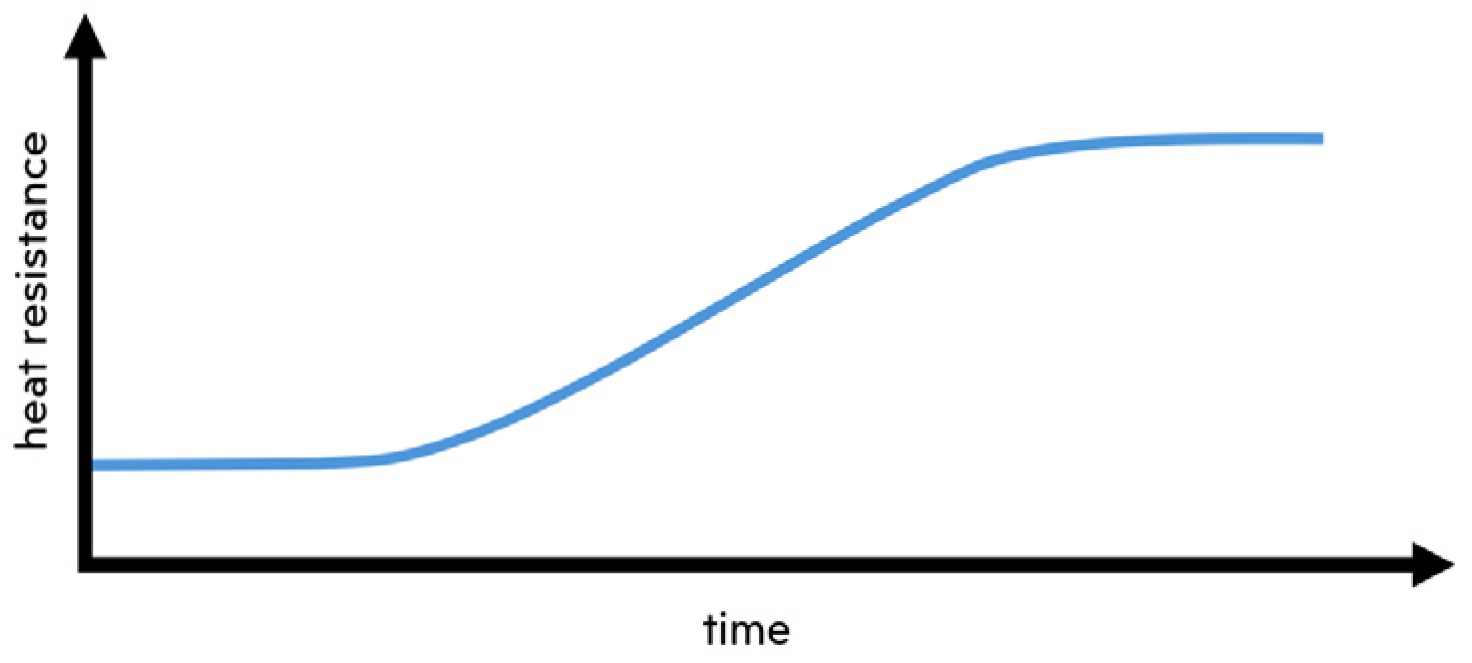
The temperature measurements, crucial for calculating the change biofouling induces in heat resistance, and a comprehensive array of images captured along the entire length of the pipe, will be presented at the upcoming conference. The results should provide evidence that heat resistance increases concurrently with biofouling, mirroring the trend illustrated in conceptual Figure 3.
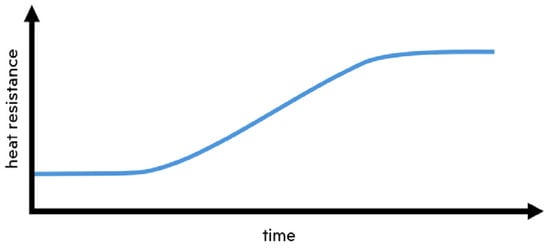
Figure 3.
Conceptual change in heat resistance induced by biofouling.
4. Conclusions
In this study, we investigate the potential of measuring biofilms in drinking water pipes by monitoring their impact on heat transfer properties. Our experiments using the SLIMER 2.0 setup demonstrate the successful growth of biofilms in a controlled environment, mimicking real-world conditions. Through non-intrusive temperature measurements, we observed an alteration in heat resistance associated with biofouling, indicating its potential as a quantifiable proxy for biofilm accumulation. The inhomogeneous growth pattern of biofilm observed across the pipe length underscores the complexity of biofilm dynamics. While preliminary results are promising, further analysis and detailed examination, including ATP measurements and comprehensive temperature data, will be presented to provide deeper insights into biofilm development and its implications for DWDSs. This research contributes to advancing our understanding of biofilm monitoring techniques and lays the groundwork for future studies aimed at enhancing water quality management in DWDSs.
Author Contributions
Conceptualization, K.G., M.B., Z.K. and D.S.; methodology, K.G.; formal analysis, K.G.; investigation, K.G.; resources, K.G.; data curation, K.G.; writing—original draft preparation, K.G.; writing—review and editing, K.G., M.B., Z.K. and D.S.; visualization, K.G.; supervision, M.B., Z.K. and D.S.; project administration, D.S.; funding acquisition, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Smart Water Futures” project, which has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program, grant agreement No. 951424.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank Paul van der Wielen (KWR and Wageningen University) for his support and advice on microbiological aspects of the research and Robbie van Pelt (KWR) for his technical advice and assistance in realizing and operating the SLIMER 2.0 setup.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pereira, A.; Melo, L.F. Online biofilm monitoring is missing in technical systems: How to build stronger case-studies? npj Clean Water 2023, 6, 36. [Google Scholar] [CrossRef]
- Liu, S.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ. Sci. Technol. 2016, 50, 8954–8976. [Google Scholar] [CrossRef] [PubMed]
- Neu, L.; Proctor, C.R.; Walser, J.C.; Hammes, F. Small-scale heterogeneity in drinking water biofilms. Front. Microbiol. 2019, 10, 490474. [Google Scholar] [CrossRef] [PubMed]
- Blokker, E.J.M.; van der Wielen, P.W.J.J. Modelling Steady-State Biofilm in a Drinking Water Distribution System. In Proceedings of the 1st International WDSA/CCWI 2018 Joint Conference, Kingston, ON, Canada, 23–25 July 2018. [Google Scholar]
- Janknecht, P.; Melo, L.F. Online biofilm monitoring. Rev. Environ. Sci. Biotechnol. 2003, 2, 269–283. [Google Scholar] [CrossRef]
- Saccomano, S.C.; Jewell, M.P.; Cash, K.J. A review of chemosensors and biosensors for monitoring biofilm dynamics. Sens. Actuators Rep. 2021, 3, 100043. [Google Scholar] [CrossRef]
- Strathmann, M.; Mittenzwey, K.H.; Sinn, G.; Papadakis, W.; Flemming, H.C. Simultaneous monitoring of biofilm growth, microbial activity, and inorganic deposits on surfaces with an in situ, online, real-time, non-destructive, optical sensor. Biofouling 2013, 29, 573–583. [Google Scholar] [CrossRef]
- Boukazia, Y.; Delaplace, G.; Cade, M.; Bellouard, F.; Bégué, M.; Semmar, N.; Fillaudeau, L. Metrological performances of fouling sensors based on steady thermal excitation applied to bioprocess. Food Bioprod. Process. 2020, 119, 226–237. [Google Scholar] [CrossRef]
- Delahaye, E.; Welte, B.; Levi, Y.; Leblon, G.; Montiel, A. An ATP-based method for monitoring the microbiological drinking water quality in a distribution network. Water Res. 2003, 37, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Learbuch, K.L.G.; Smidt, H.; Van Der Wielen, P.W.J.J. Influence of pipe materials on the microbial community in unchlorinated drinking water and biofilm. Water Res. 2021, 194, 116922. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).