Abstract
Fuel gas desulfurization is an important technological step toward achieving environmental neutrality in industrial production. The presence of sulfur compounds in fuel not only significantly increases the rate of corrosion processes, but also increases the amount of sulfur oxides in flue gases. Adsorption technologies are actively used to purify fuel. Materials that are mainly chosen as sorbents are determined by economic feasibility. These materials include bentonite clays. The paper presents an analysis of scientific works on the possible methods of using bentonite clays in industry. Due to its natural properties, this material has high adsorption properties. The authors present experimental laboratory studies to determine the adsorption efficiency of bentonite clay with a carbon substrate. Results on the adsorption capacity after regeneration of the composition are also presented. Based on the data obtained, the adsorption unit was calculated, and the process flow diagram was designed.
1. Introduction
In the modern world, environmental safety issues of industrial production are of significant importance. Technological solutions are being developed that make it possible to use by-product gaseous hydrocarbon products as fuel raw materials for power plants. But the use of gaseous hydrocarbon products is impossible without their preliminary purification from sulfur compounds. There are standards and requirements for the residual content of sulfur compounds in fuel, since power equipment is very sensitive and subject to intense corrosion and deposits on the internal surface. Today, there are various methods of desulfurization: adsorption, absorption, and membrane technologies. A convenient and effective technology is sorption purification using solid adsorbents. Adsorbents have selectivity toward sulfur compounds, and during regeneration of the spent composition, it is possible to obtain elemental sulfur. Today, there are many types of adsorbents that can be used to remove sulfur compounds with varying degrees of effectiveness. The price of these compositions differs greatly and depends on the quality of the adsorbent.
For industrial complexes, the choice of adsorbents is determined by economic feasibility, so enterprises strive to purchase cheap materials whose use does not entail a decrease in profits. Another important criterion for evaluating sorbents is their environmental safety. Therefore, the priority is to use natural materials as adsorbents that are low-cost and that fully comply with environmental safety criteria.
Among natural materials, bentonite clays are of greatest interest. Their composition is very diverse but mainly contains mixtures of various oxides, organic substances, soluble salts, and water. The main predominant chemical components of clays are SiO2 (silica), which sometimes reaches 80%, AI2O3 (alumina), and H2O. TiO2, Fe2O3, FeO, MnO, Na2O, MgO, CaO, and K2O are present in minor quantities. More accurate quantitative and qualitative composition is obtained as a result of analyzing a specific deposit.
The choice of bentonite clays as a sorbent is justified by their high dispersion and, as a consequence, their developed adsorption surface. Adsorption and chemical processes occur on the surface of the clay, which determines its ability to adsorb effectively. Studies have shown that, in addition to the developed surface, bentonite clays have a high cation-exchange capacity due to the content of various cations [1,2]. Considering the high ion-exchange ability of bentonite clays, their qualitative composition can be modified with the inclusion of ions that can increase the selectivity of adsorption for a certain component. This characteristic significantly expands the scope of bentonite clay applications. Most often, bentonite clays are used for the purification of both natural and waste waters. The use of clays for the purification of gaseous media is a new, promising direction for the industrial sector.
Today, more and more industrial enterprises use by-product gaseous hydrocarbon wastes as additives to the main fuel. The development of a gaseous fuel mixture purifying and preparation method is an urgent task. Therefore, this work is devoted to the problem of purifying fuel mixtures from sulfur compounds. The purpose of the work was to develop a technological installation for gaseous mixture purification with subsequent calculation of the main equipment. To achieve this goal, experimental studies were conducted to determine the efficiency of sulfur compounds adsorption by bentonite clay from synthesized and real gases. Based on the obtained results, a cleaning method was developed, an adsorption technology with a regeneration unit was presented, and the main characteristics of the adsorber were calculated.
2. Literature Review
There is information in literary sources and scientific works about the use of bentonite in the purification of gas mixtures and not only from sulfur compounds. E.I. Elkhalifa, M. Azmi Bustam, and A.M. Shariff T. Murugesan studied the properties of the magnesium form of bentonite clay modified with a solution of monoethanolamine. The adsorption properties of the resulting adsorbent were tested with respect to carbon dioxide. Previously, to obtain information on its structural characteristics, thermal stability, specific surface area, and porosity, the untreated bentonite was characterized using X-ray diffractometry (XRD), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and specific surface area determination using BET [3]. The experimental results showed that the treated bentonite has an adsorption capacity twice as high as that of the untreated sample. In addition, calculated values of the thermodynamic parameters showed that the adsorption process is physically spontaneous and exothermic in nature. Bentonite treated with a solution of monoethanolamine is able to withstand several cycles of adsorption–desorption without a noticeable decrease in its adsorption capacity. The modification increases the adsorption efficiency since not only physical adsorption occurs between contacted media but also chemisorption. Various compounds can act as modifiers [4].
Daniele Malferrari, Elena Castellini, Fabrizio Bernini, Aida Serrano Rubio, German Rafael Castro, and others have developed a hybrid material based on bentonite clay. This material is formed by intercalation of the μ-oxo Fe(III)–phenanthroline complex [(OH2)3(Phen)FeOFe(Phen)(OH2)3]4+ (Fe(III)Phen). The modified material is highly effective in adsorbing hydrogen sulfide from the gas phase. The adsorption reaction proceeds quite quickly, and the adsorbent traps even small concentrations of hydrogen sulfide. The purification mechanism is based on the occurrence of a redox reaction between iron (III) and one S2- ion, followed by binding of the second S2− ion to the metal center [5].
Danh Nguyen-Thanh, Karin Block, and Teresa J. Bandosz proposed a modification of bentonite clays in the sodium form. Sodium-rich montmorillonite was modified with iron to introduce active hydrogen sulfide adsorption sites. The modification affected interlayer cations, i.e., sodium cations were replaced by iron cations. This modification involved the inclusion of iron compounds in the interlayer space of clay with aluminum posts. During the modification process, the characteristics of the feedstock changed, and a noticeable decrease in microporosity occurred. This composition has been tested as an adsorbent of sulfur compounds. Despite the change in the physical characteristics of the modified material, the high adsorption capacity of sulfur compounds was retained. The results obtained suggest that on the surface of iron-modified clay, hydrogen sulfide reacts with Fe3+, forming sulfides, or is catalytically oxidized to SO2 on iron hydroxides. Subsequent oxidation may lead to the formation of sulfate [6].
Most often, modified forms of bentonite clays are used to purify gaseous mixtures. Scientists have proposed the co-combustion of fuel with a modified form of bentonite clay to reduce sulfur oxides in flue gases. Compounds of vanadium, cobalt, nickel, manganese, and copper were used as modifiers. To evaluate their effectiveness, the proposed compositions were tested in an operating installation, and the mass concentration of sulfur oxides was measured at the outlet of the flue gases. Based on the results obtained, it was concluded that additives such as vanadium, nickel, and manganese can achieve a reduction in sulfur oxide emissions of 70–90% [7,8,9].
The qualitative composition of bentonite clays directly depends on their deposits. Bentonites with a high content of calcium and magnesium carbonates have a low adsorption capacity, so chemical modification is used to improve the adsorption properties. Kateryna V. Stepova, Duncan J. Maquarrie, and Ihor M. Krip used solutions of iron (III) chloride and copper(II) chloride for the modification. To do this, bentonite was dissolved in a large amount of water, and a solution of ferric chloride was added in portions. The reaction was carried out until the release of carbon dioxide stopped as a result of the decomposition of carbonates. The modified product was washed and further dried to a constant sample weight. For modification with copper, a solution of copper (II) chloride was used. Considering that the reaction between bentonite and copper chloride solution proceeds very slowly, to intensify the reaction, the mixture was heated to a temperature of 60–70 °C. Testing of the resulting compositions was carried out in laboratory conditions at room temperature and atmospheric pressure. Five grams of modified sorbent was placed in the adsorption column; the granule size was 1–2 mm. Before the experiment, the adsorbent was purged with nitrogen for 30 min to remove adsorbed water and impurities. Next, a mixture of hydrogen sulfide and nitrogen was passed through the adsorbent, and the purified gas was bubbled through the adsorbing solution. The final concentration of sulfides was determined by iodometric titration. The test was stopped when the hydrogen sulfide breakthrough concentration exceeded 20 mg/L [4,10,11].
J.L. Venaruzzo and C. Volzone described an experiment with bentonite clay minerals from Patagonia, Argentina, in their natural state and after acid treatment. Materials with a fraction size of less than 2 microns were used for the experiment. Different concentrations of hydrochloric acid solutions were chosen for the acid modification. The impregnation process was carried out for three hours at the boiling temperature of the solutions. After acid treatment, the compositions were dried and analyzed, and the volume of micropores and the specific surface of the sorption material were determined. The authors stated that the materials increased their adsorption capacity in relation to gaseous media containing sulfur compounds after the treatment [12].
The increased demand for inexpensive and environmentally friendly sorbents has led to the study of the adsorption properties of available natural materials. In addition to bentonite clays, modified forms of zeolites enriched with various metal ions can also be used as natural sorbents. Zeolites have good adsorption properties due to their capacity for ion exchange or special impregnation. These modified forms exhibit highly selective properties in the presence of other interfering components. Zeolites are regenerated thermally, as well as by using various solvents [13]. Also, the modification process can be based on impregnation with special solutions followed by calcination. The method and the anions included have a major influence on the characteristics of zeolite. Experimental studies have shown that zeolite modified with copper ions has a high sorption capacity toward sulfur compounds [14].
To remove sulfur compounds (hydrogen sulfide and mercaptans), sorbents were used, which included Cu(I)Y zeolite, CuCl/MCM-41, and CuCl/SBA-15. Experimental studies have shown that due to their mesoporous structure, these sorbents have a high adsorption capacity. Also, during the experiment, it was revealed that hydrogen sulfide is adsorbed faster than mercaptans. An important advantage of using these sorbents is their ability to be completely regenerated [15].
Recently, several types of inexpensive adsorbents have been developed for cleaning and processing waste from industrial complexes. To increase the adsorption capacity, scientists have proposed the use of combinations of adsorbents. The main criterion for the quantitative proportional mixing of natural components is the specific surface area of the adsorbent. Thus, during experimental studies, scientists determined the optimal ratio between zeolite and bentonite clay [16].
An analysis of literature sources showed that the use of bentonite clays in the preparation of fuel gas is a promising direction. These sorbents have high porosity and low cost, which are important criteria when choosing an adsorbent in industry. Modification and activation of bentonite clays is always accompanied by additional costs and increased costs of the adsorbent preparation process. This paper presents an experimental study on the adsorption efficiency of bentonite clay with a carbon substrate.
3. Materials and Methods
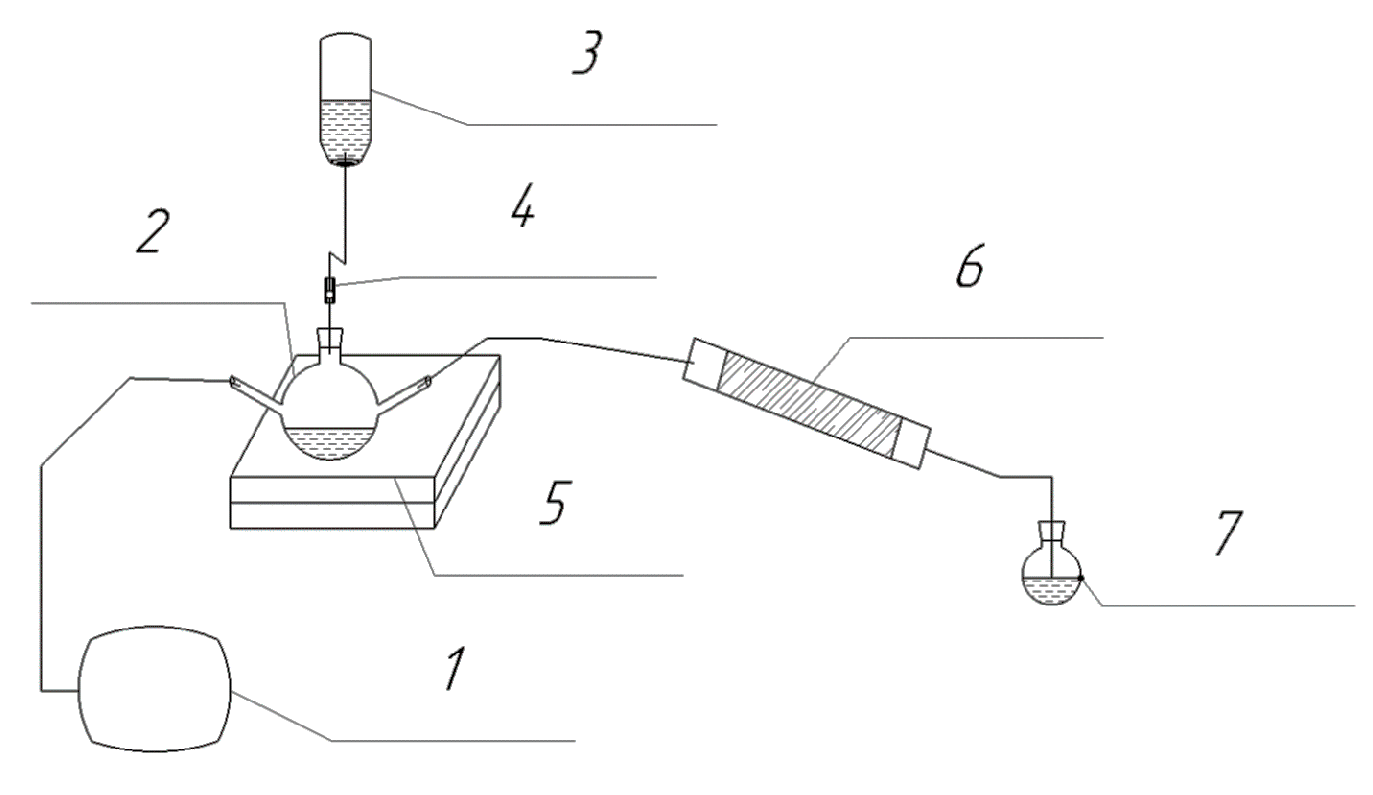
A laboratory experimental setup was assembled to assess the adsorption capacity of sulfur compounds with bentonite clay (Figure 1).
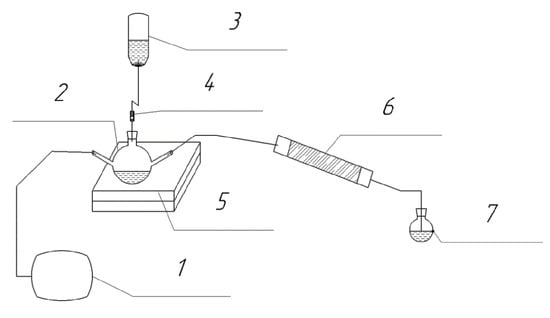
Figure 1.
Laboratory experimental setup: 1—air supply compressor; 2—three-neck flask; 3—container with hydrochloric acid solution; 4—dispenser; 5—magnetic stirrer; 6—adsorber; 7—receiver flask with adsorbing solution.
The laboratory experimental setup includes a compressor, a three-neck flask, an acid dosing system, a magnetic stirrer, a glass adsorber tube, and a receiver flask with an absorption solution (labelled 1–7 in Figure 1). Solutions of hydrochloric acid and sodium sulfide of the required concentrations are pre-prepared. The sodium sulfide solution is poured into a three-neck flask, the air mixture supply system through a compressor is connected to the first outlet, the dispensing system for supplying hydrochloric acid solution is connected to the second outlet, and the third outlet is connected to an adsorber tube. A receiver flask with an adsorbing solution of zinc acetate is installed after the adsorber. The escaped hydrogen sulfide is absorbed by the solution. Next, the amount of breakthrough is determined by the turbidimetric method using spectrophotometric equipment.
The adsorber tube is filled with 80% bentonite clay and 20% activated carbon. This filling method avoids the removal of bentonite from the adsorber. The use of bulk bentonite clay is problematic on an industrial scale. Therefore, before use, the clay is mixed with water and dried at a temperature of 400 °C. Heat treatment makes it possible to obtain porous particles of complex shape. After heat treatment, the composition is activated with a 4% sodium hydroxide solution and dried. Hydrogen sulfide, released by the reaction, is passed through the adsorber to a residual content of no more than 20 mg/m3. This value is standardized in the guidelines for the operation of power plants. After the adsorption properties deteriorate, the composition is regenerated with a 4% sodium hydroxide solution and calcined at 400 °C. This almost completely restores the adsorption capacity after regeneration.
4. Results
After determining the efficiency of adsorption on gases obtained under laboratory conditions, experiments were carried out on real hydrocarbon gas, which is a waste product of the oil industry. The average annual content of sulfur compounds in fuel gas is 1 g/m3. According to the results of a laboratory experiment with fuel gas, the capture capacity of the proposed adsorption composition, comprising 80% bentonite and 20% active carbon activated with a 4% sodium hydroxide solution, is 11.5% of the total mass of the adsorber backfill.
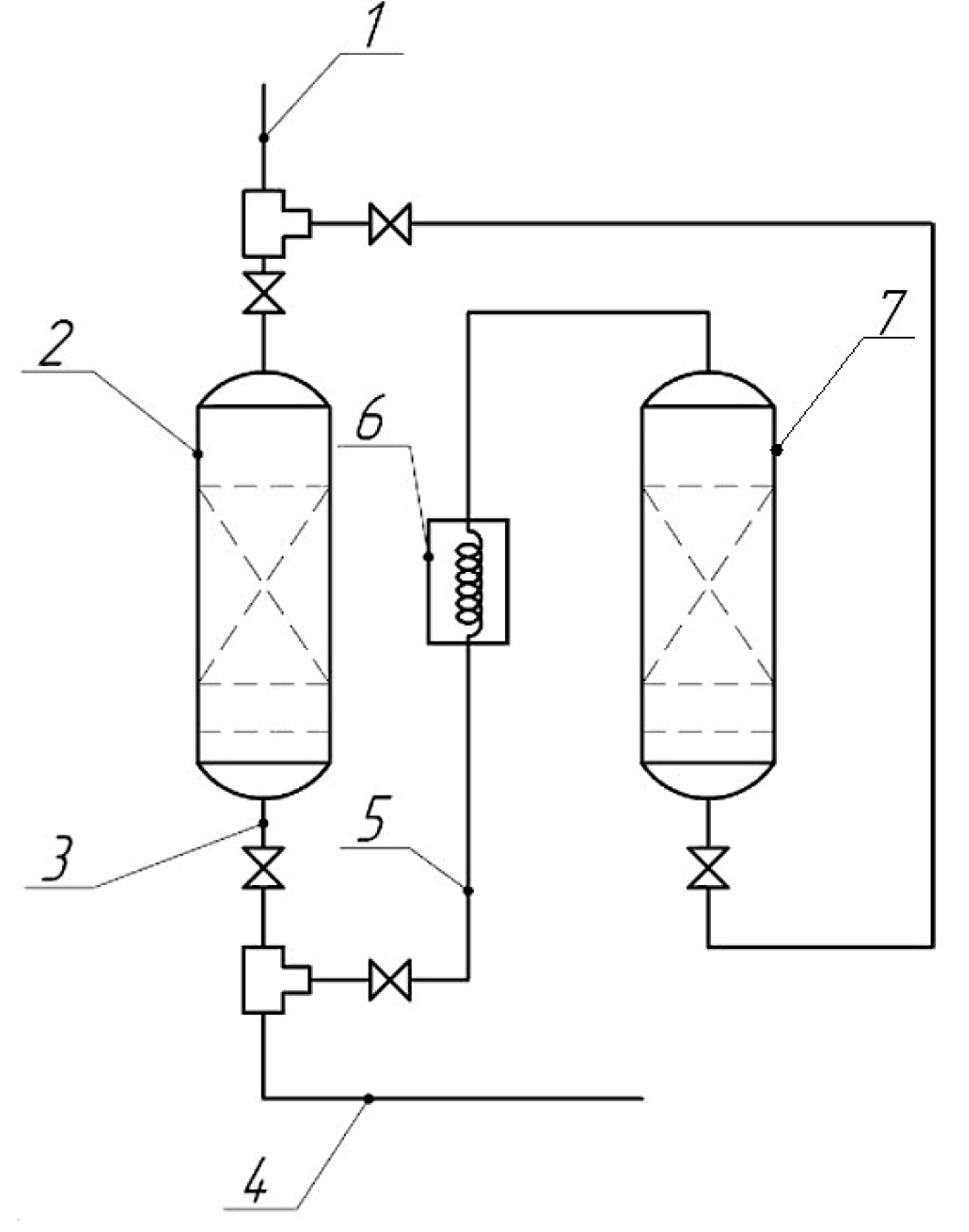
For the purpose of the industrial use of the proposed adsorbent, a technological scheme of an adsorption unit was developed as shown in Figure 2. The technological scheme includes a purified gas supply system and two adsorbers, in one of which (labelled “2”) the adsorption process takes place, while in the other adsorbent (labelled “7”), the regeneration process takes place; purified gas is output via line 3, purified gas is supplied to the power plant via line 4, and the gas is supplied for sorbent regeneration via line 5 through heat exchanger 6 (label numbers as in Figure 2). The composition was regenerated with a 4% sodium hydroxide solution and purged with hot (400 °C) fuel gas purified from sulfur compounds.
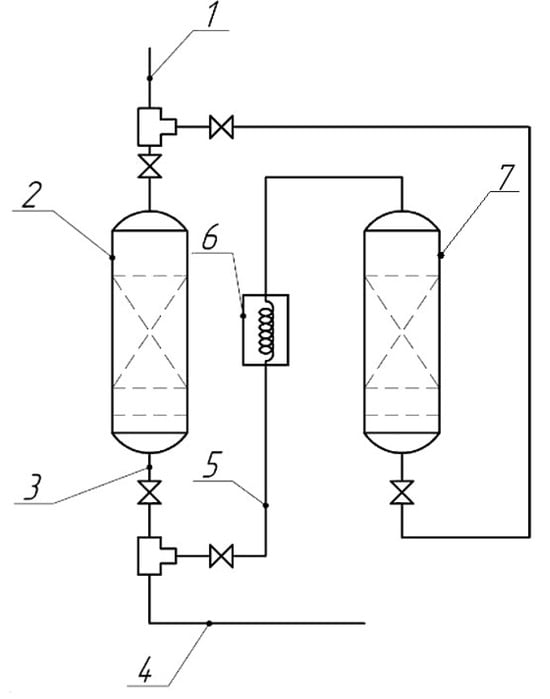
Figure 2.
Adsorption unit: 1—supply of purified gas; 2—adsorber; 3—output of purified gas; 4—gas supply to the power plant; 5—gas supply for adsorbent regeneration; 6—heat exchanger; 7—adsorber for regeneration.
Based on the experimental results, a periodic adsorption unit with a fixed layer of adsorbent was designed to capture hydrogen sulfide from the fuel gas at a flow rate of 0.112 kg/s (586.8 m3/h) for the subsequent supply of purified fuel gas together with natural gas in a ratio of 1:9 to the gas turbine GT-25 with a capacity 25 MW. The initial concentration of hydrogen sulfide in the source gas was 1 g/m3. According to the requirements for gaseous fuel of gas turbines, the mass concentration of hydrogen sulfide should not exceed 20 mg/m3. Bentonite with a substrate of activated carbon in a volume percentage ratio of 80:20 was chosen as an adsorbent. The equivalent diameter of the adsorbent granule was 4.2 mm.
Based on the results of laboratory experiments, the static activity and bulk density of the adsorbent were determined to be 0.069 kg/kg and 321.378 kg/m3, respectively.
The adsorber diameter is predicted as follows:
where V is the volumetric flow rate of the source gas, m3/h; and Wwork is the operating gas speed in the adsorber, calculated by the following formula:
where Wp—permissible fictitious gas speed, m/s; ρb—bulk density of the adsorbent, kg/m3; de—equivalent diameter of granules, m; ρ—air density at adsorption temperature, kg/m3; g—free fall acceleration, m/s2.
To ensure sufficient operating time of the adsorber, the height of the adsorbent layer in the apparatus is assumed to be 1.5 m. The total height of the adsorber is assumed to be 2 m. Additional height is necessary to accommodate the gas distribution device, fittings, and sensors of instrumentation.
The volumetric mass transfer coefficient is predicted as follows:
where Nu is the Nusselt diffusion criterion, determined from expression (5); and D is the diffusion coefficient of the adsorbent in gas under adsorber conditions, m2/s, calculated by the following formula:
where P is the pressure in the system, kgf/cm2; MA, MB are the molar masses of hydrogen sulfide and fuel gas, g/mol; vA and vB are the molar volumes of hydrogen sulfide and fuel gas, cm3/mol; and T is the temperature in the system, K.
where Re—the Reynolds number; ρg—fuel gas density, kg/m3; μg—dynamic gas viscosity coefficient, Pa·s.
The duration of the adsorption process can be estimated as follows:
where H—the height of the adsorbent layer, m; C0—initial concentration of the adsorbed substance, kg/m3; a—static activity of the adsorbent, kg/kg; b—coefficient depending on the content ratio of the adsorbed substance (hydrogen sulfide) in the gas flow leaving the adsorber, kg/m3, to the initial concentration of the adsorbed substance, kg/m3.
The required mass of the sorbent is determined from the following formula:
where K is the safety factor, and G is the mass flow rate of the purified gas, kg/s.
The results obtained are summarized in Table 1.

Table 1.
Calculation results of the adsorption unit.
Taking into account the overall dimensions of the filters, which are a diameter of 0.8 m and a height of 2.5 m, it is necessary to provide a technical area for the process installation. The technology provides two adsorbers, one of which is in reserve, and two adsorbers for regeneration. The operating time without regeneration (filter cycle) is 24 h.
5. Conclusions
Purification of fuel gas from sulfur compounds using natural materials is a promising solution to the problem of the corrosive wear of equipment and the excess content of sulfur oxides in flue gases. The natural material bentonite is environmentally friendly, and its use on an industrial scale is economically feasible. The authors assembled a laboratory setup for testing and determining the adsorption properties of the proposed sorbent based on bentonite and a carbon substrate. The composition was first activated with a 4% sodium hydroxide solution and dried. The adsorption capacity amounted to 11.5% of the total adsorber backfill mass when cleaning oil industry gas hydrocarbon waste from sulfur compounds. After depletion of the adsorption capacity, the composition was calcined and regenerated with an alkali solution; after regeneration, the adsorption capacity was restored almost completely. Based on the obtained results, the adsorption installation was calculated, the main technological parameters were calculated, and a technological scheme of the installation with a regeneration cycle for using petrochemical industry waste as an admixture to the main fuel for a 25 MW gas turbine was presented. It has been shown that to purify fuel gas supplied in a ratio of 1:9 with natural gas as fuel for a gas turbine, it is possible to use an adsorption unit consisting of two adsorbers filled with bentonite with activated carbon weighing 480 kg. The sorption cycle will be 24 h. The use of the proposed adsorbent in industry will reduce the cost of desulfurization, since the main costs will be in the regeneration processes.
Author Contributions
A.V. and A.F. worked on all the tasks, A.F., R.K. and H.B. worked on the literature review, I.I. and A.F. conducted computational studies, I.I. and H.B. performed the supervision, and all authors analyzed the results. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the European Union-NextGenerationEU through the National Recovery and Resilience Plan of the Republic of Bulgaria (project No.: BG-RRP-2.013-0001-C01). It was also funded by the Ministry of Science and Higher Education of the Russian Federation under a project entitled “Study of processes in a fuel cell-gas turbine hybrid power plant” (project code: FZSW-2022-0001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data can be used on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Borah, D.; Nath, H.; Saikia, H. Modification of Bentonite Clay & Its Applications: A Review. Rev. Inorg. Chem. 2022, 42, 265–282. [Google Scholar] [CrossRef]
- Gooneh-Farahani, S.; Anbia, M. A review of advanced methods for ultra-deep desulfurization under mild conditions and the absence of hydrogen. J. Environ. Chem. Eng. 2023, 11, 108997. [Google Scholar] [CrossRef]
- Olszewska, D. Application of modified montmorillonite for desulfurization during the combustion of hard coal. Fuel Process. Technol. 2011, 92, 2412–2419. [Google Scholar] [CrossRef]
- Beloev, H.I.; Filimonova, A.A.; Vlasova, A.Y.; Kamalieva, R.F.; Vinogradov, A.S.; Iliev, I.K. Comparative Analysis of Sorption Materials for a Hybrid Power Plant with a SOFC. In Proceedings of the 2023 4th International Conference on Communications, Information, Electronic and Energy Systems (CIEES), Plovdiv, Bulgaria, 23–25 November 2023; pp. 1–5. [Google Scholar] [CrossRef]
- Stepova, K.V.; Maquarrie, D.J.; Krip, I.M. Modified bentonites as adsorbents of hydrogen sulfide gases. Appl. Clay Sci. 2009, 42, 625–628. [Google Scholar] [CrossRef]
- Alcaraz, M.G.T.; Choi, A.E.S.; Dugos, N.P.; Wan, M.-W. A Review on the Adsorptive Performance of Bentonite on Sulfur Compounds. Chem. Eng. Trans. 2023, 103, 553–558. [Google Scholar] [CrossRef]
- Nguyen-Thanh, D.; Block, K.; Bandosz, T. Adsorption of hydrogen sulfide on montmorillonites modified with iron. Chemosphere 2005, 59, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Beloev, I.; Filimonova, A.; Chichirov, A.; Chichirova, N.; Filimonov, A.; Iliev, I. Utilization of Hydrogen-Containing Gas Waste from Deep Oil Refining at a Hybrid Power Plant with a Solid Oxide Fuel Cell. Eng. Proc. 2024, 60, 5. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, M.; Han, T.; He, Y.; Li, T.; Zhang, J.; Su, Y.; Wu, Y.; Dian, B.; Zong, Y. Removal of hydrogen sulfide in the gas phase by carbide slag modified bentonite. RSC Adv. 2023, 13, 20844–20855. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifah, A.E.I.; Bustam, M.A.; Shariff, A.M.; Murugesan, T. Selective adsorption of CO2 on a regenerable amine-bentonite hybrid adsorbent. Appl. Clay Sci. 2015, 107, 213–219. [Google Scholar] [CrossRef]
- Genbach, A.A.; Olzhabaeva, K.S.; Iliev, I.K. Boiling process in oil coolers on porous elements. Therm. Sci. 2016, 20, 1777–1789. [Google Scholar] [CrossRef]
- Venaruzzo, J.L.; Volzone, C.; Rueda, M.L.; Ortiga, J. Modified bentonitic clay minerals as adsorbents of CO, CO2 and SO2 gases Author links open overlay panel. Microporous Mesoporous Mater. 2002, 56, 73–80. [Google Scholar] [CrossRef]
- Dehghan, R.; Anbia, M. Zeolites for adsorptive desulfurization from fuels: A review. Fuel Process. Technol. 2017, 167, 99–116. [Google Scholar] [CrossRef]
- Zhu, L.; Lv, X.; Tong, S.; Zhang, T.; Song, Y.; Wang, Y.; Hao, Z.; Huang, C.; Xia, D. Modification of zeolite by metal and adsorption desulfurization of organic sulfide in natural gas. J. Nat. Gas Sci. Eng. 2019, 69, 102941. [Google Scholar] [CrossRef]
- Crespo, D.; Qi, G.; Wang, Y.; Yang, F.H.; Yang, R.T. Superior Sorbent for Natural Gas Desulfurization. Ind. Eng. Chem. Res. 2008, 47, 1238–1244. [Google Scholar] [CrossRef]
- Salem, A.; Sene, R.A. Removal of lead from solution by combination of natural zeolite–kaolin–bentonite as a new low-cost adsorbent. Chem. Eng. J. 2011, 174, 619–628. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).