Utilization of Phototrophic Bacteria to Enhance Carbon Sequestration in Rice Paddy †
Abstract
1. Introduction
2. Materials and Methods
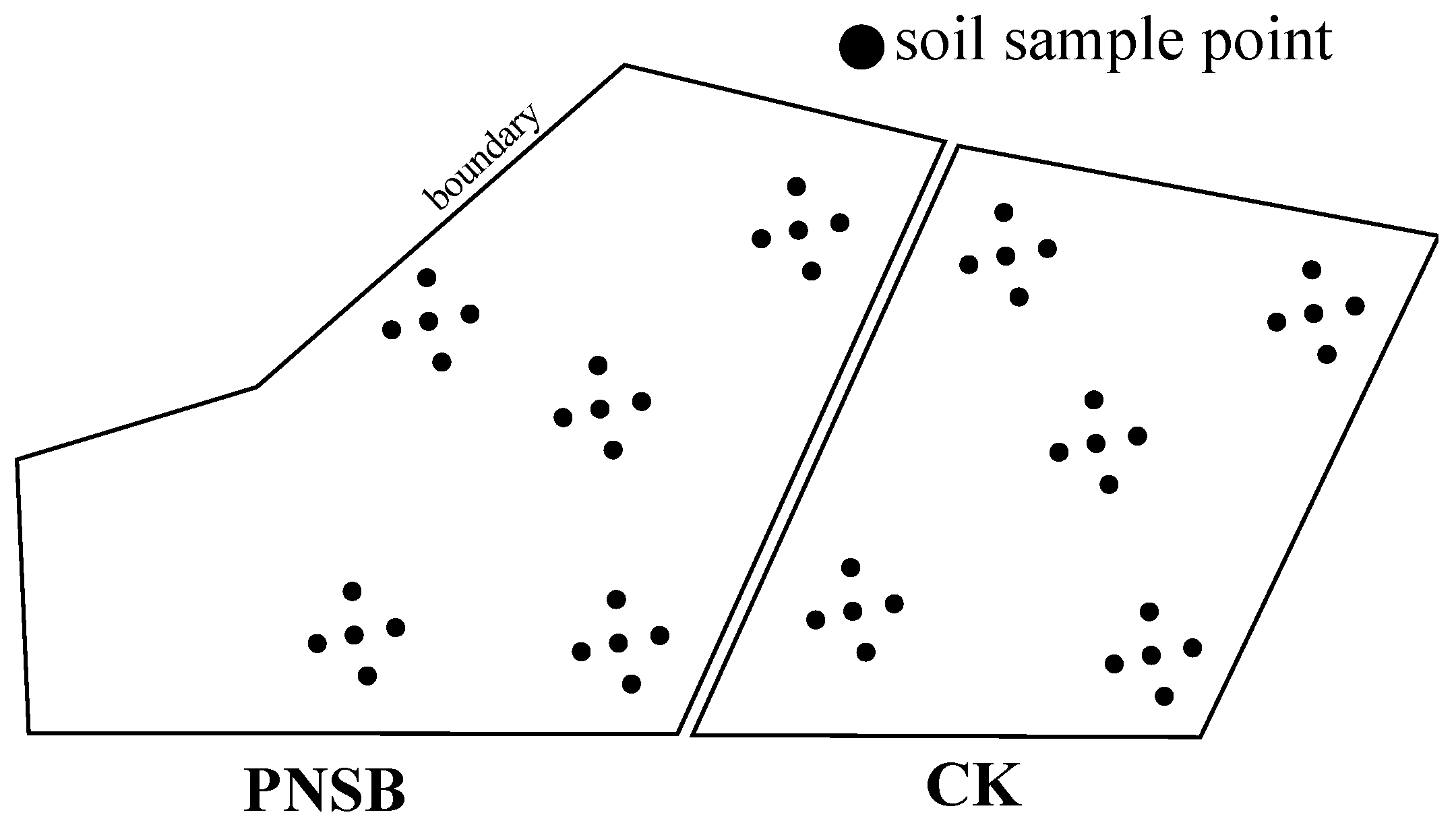
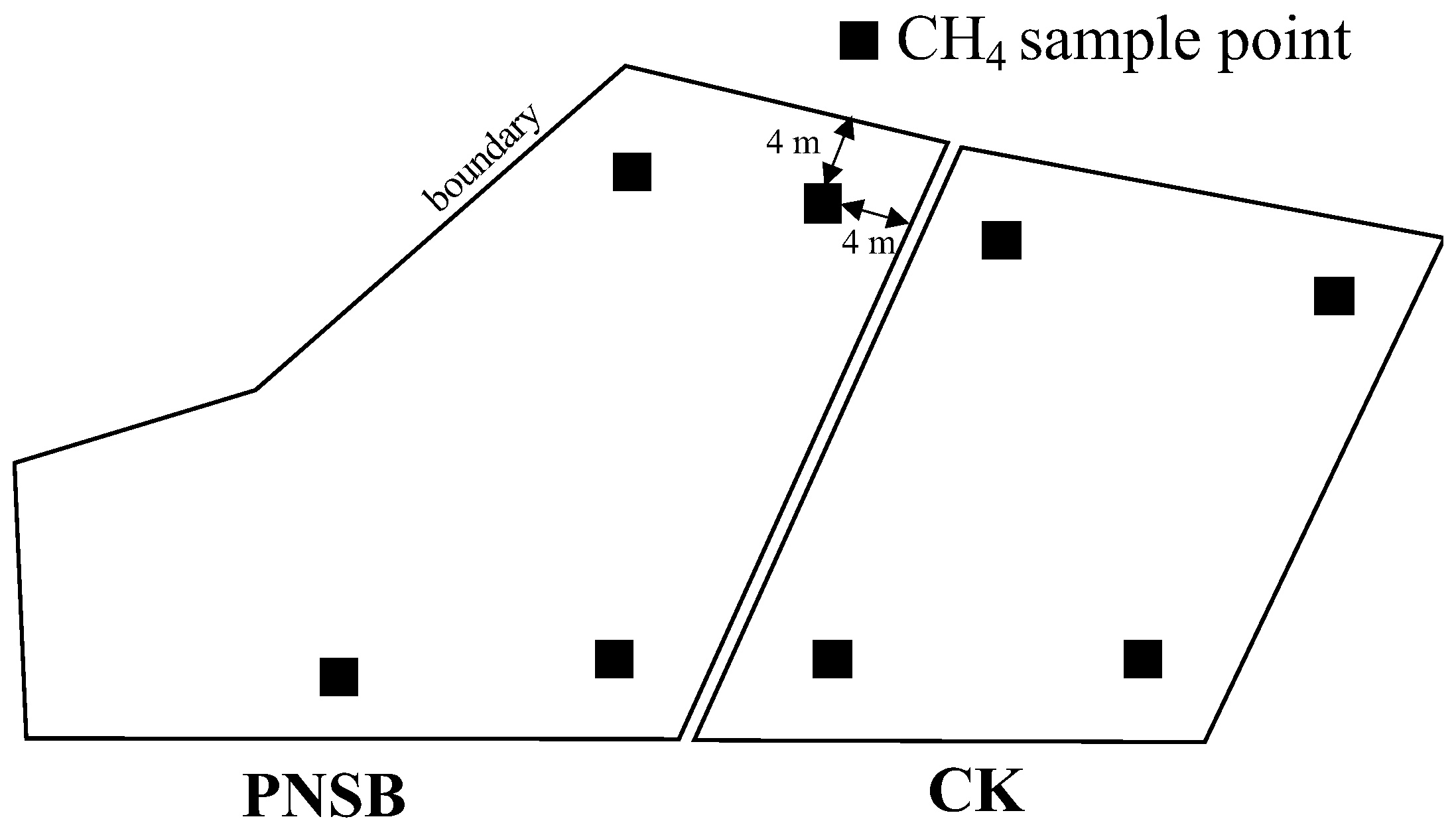
2.1. Field Trial
2.2. Collection and Analysis of Soil Samples
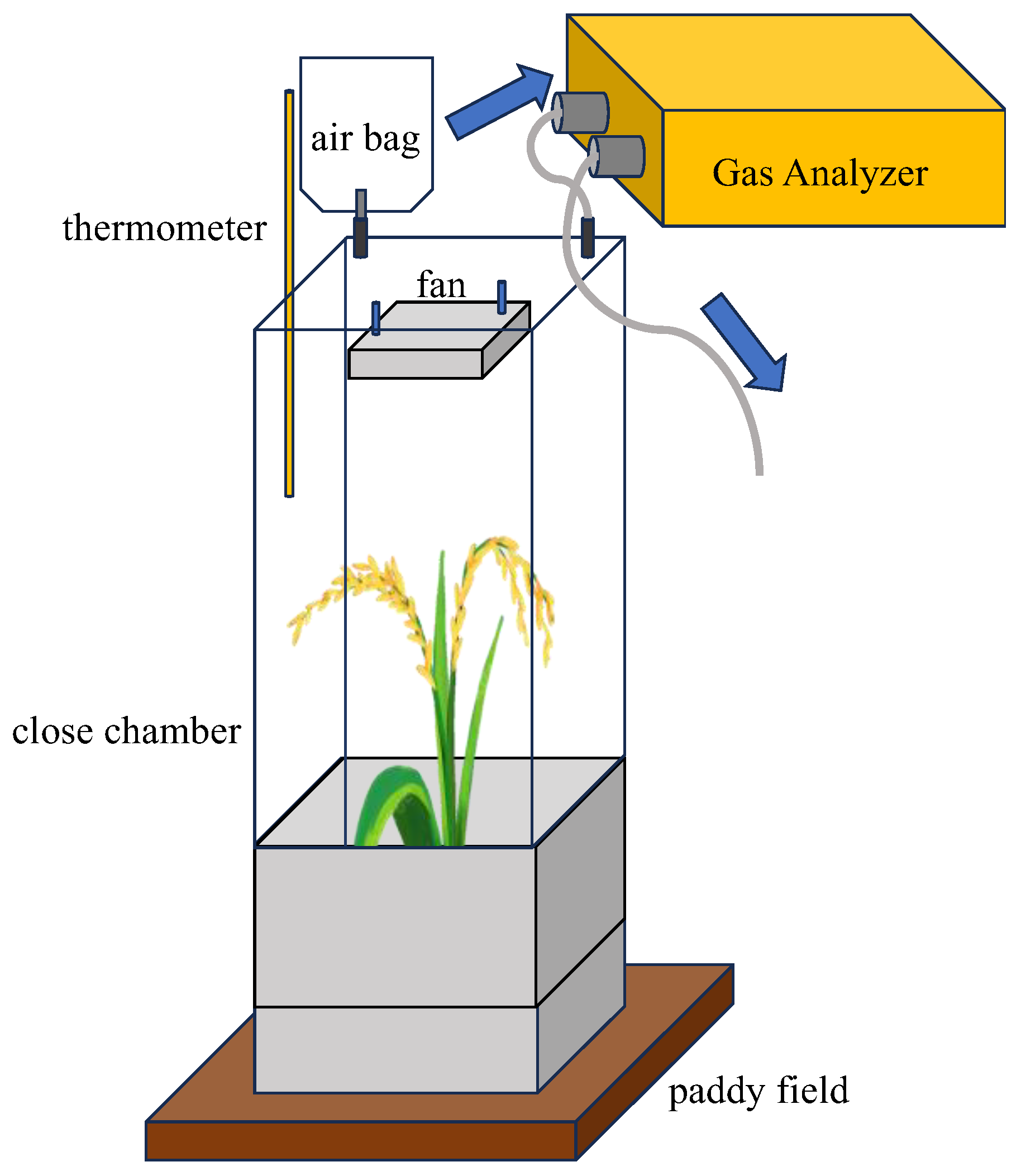
2.3. Collection and Analysis of Gas Samples
2.4. Calculation of Carbon Dioxide Equivalents
2.5. Quantification of Soil Carbon Content (SOC)
2.6. Evaluation of Oryza Sativa Growth
2.7. Analysis of PSNB Content in Soil and Water
2.8. Analysis of PSNB Content by Most Probable Number (MPN) Method
3. Results and Discussion
3.1. SOC
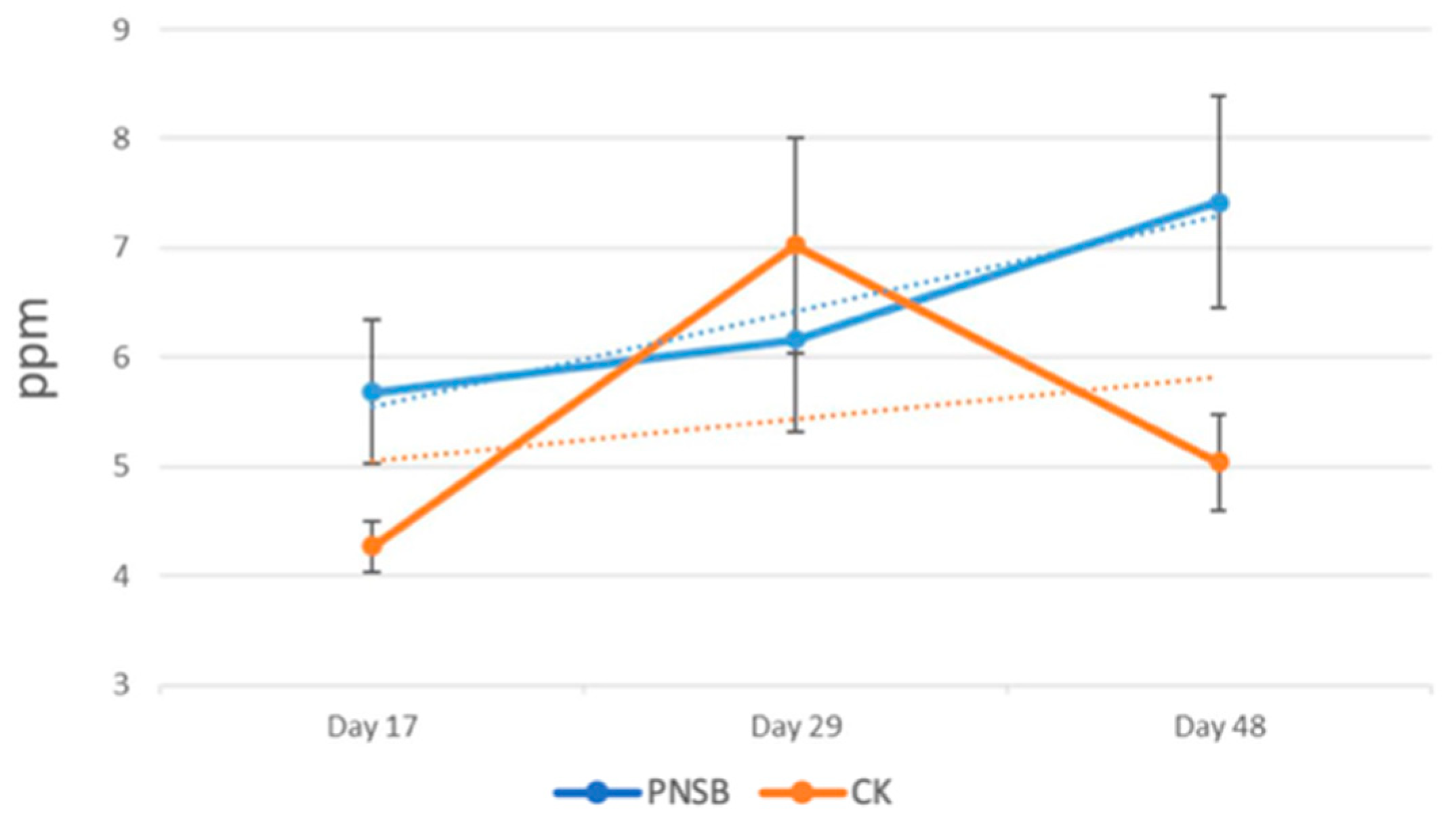
3.2. Analysis of Methane Emissions
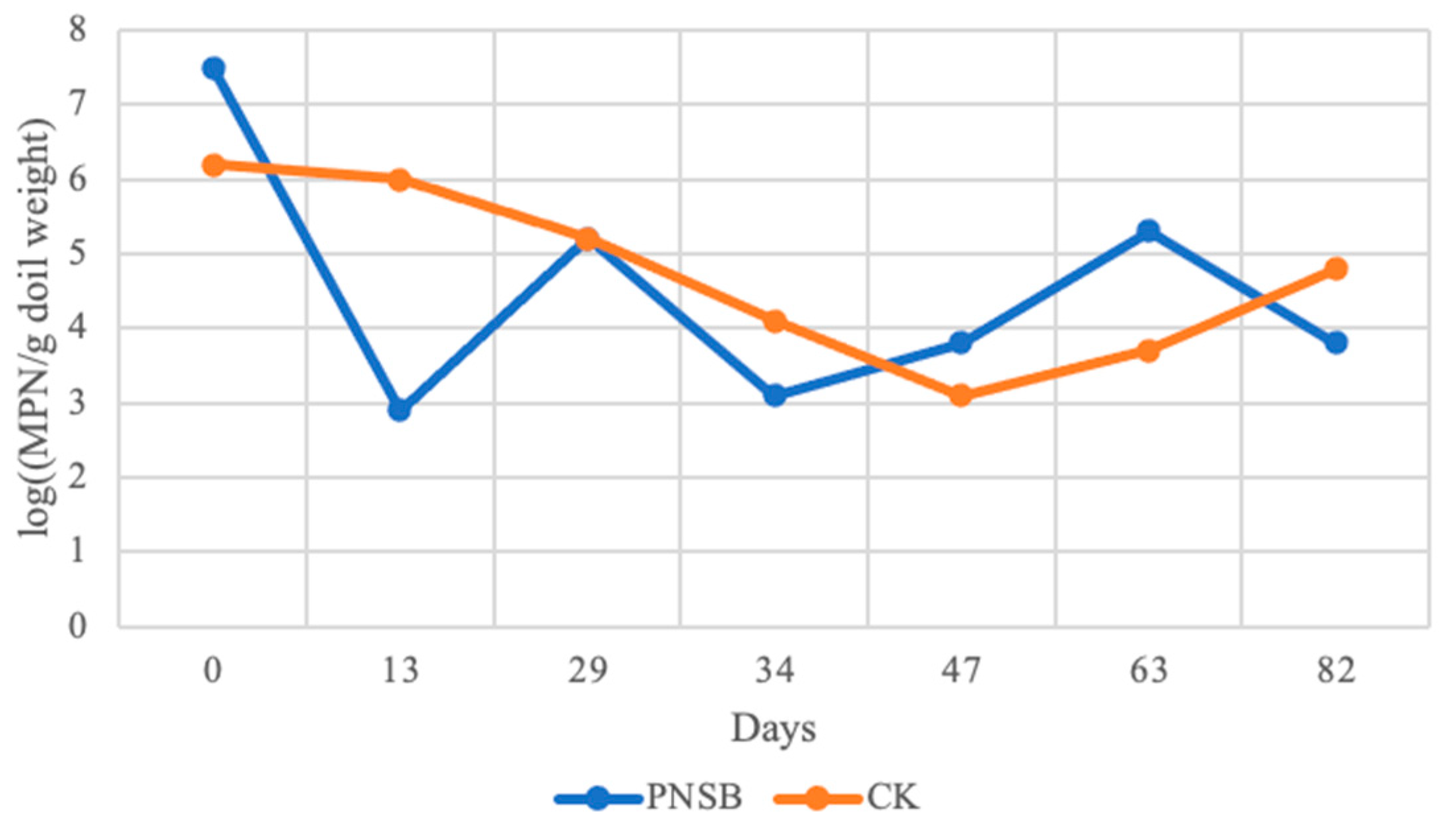
3.3. PNSB in Field Trials
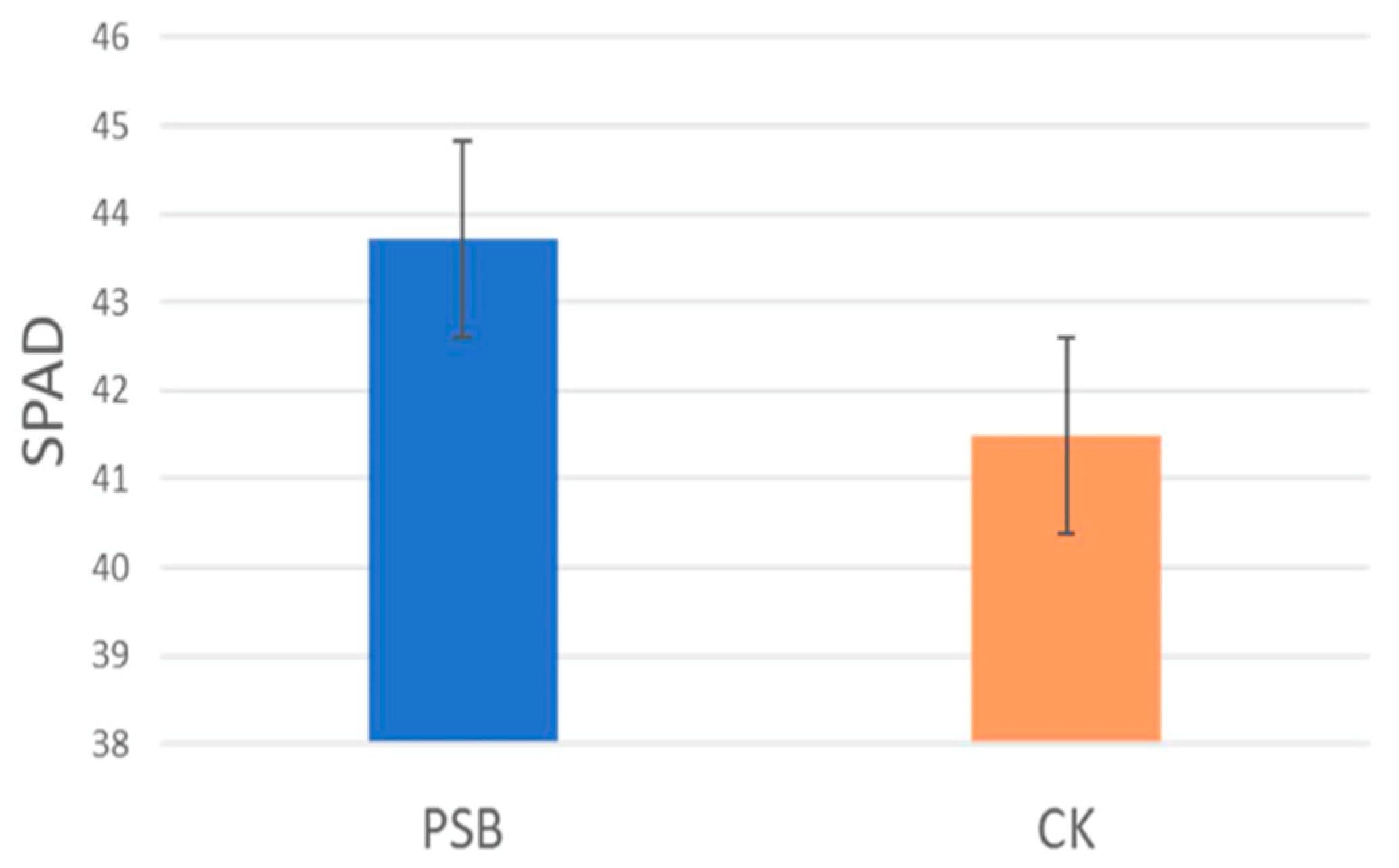
3.4. Evaluation of Oryza Sativa Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shindell, D.T.; Faluvegi, G.; Koch, D.M.; Schmidt, G.A.; Unger, N.; Bauer, S.E. Improved attribution of climate forcing to emissions. Science 2009, 326, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Alexander, L.V.; Allen, S.K.; Bindoff, N.L.; Bréon, F.M.; Church, J.A.; Cubasch, U.; Emori, S.; et al. Technical summary. In Climate Change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 33–115. [Google Scholar]
- Carlson, K.M.; Gerber, J.S.; Mueller, N.D.; Herrero, M.; MacDonald, G.K.; Brauman, K.A.; Havlik, P.; O’Connell, C.S.; Johnson, J.A.; Saatchi, S.; et al. Greenhouse gas emissions intensity of global croplands. Nat. Clim. Chang. 2017, 7, 63–68. [Google Scholar] [CrossRef]
- Wassmann, R.; Aulakh, M.S. The role of rice plants in regulating mechanisms of methane missions. Biol. Fertil. Soils 2000, 31, 20–29. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, T.; van Groenigen, K.J.; Yang, Y.; Wang, P.; Cheng, K.; Zhu, Z.; Wang, J.; Li, Y.; Guggenberger, G.; et al. Rice paddy soils are a quantitatively important carbon store according to a global synthesis. Commun. Earth Environ. 2021, 2, 154. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, C.F.; Guo, C.Q.; Lu, Z.X.; Shi, Y.; Wang, Z.D. Synthesis of GO-modified Cu2O nanosphere and the photocatalytic mechanism of water splitting for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 4007–4016. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Hao, T.; Chen, S. Colonization and Maize Growth Promotion Induced by Phosphate Solubilizing Bacterial Isolates. Int. J. Mol. Sci. 2017, 18, 1253. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jun, W.J.; Shin, D.H.; Cho, H.Y.; Hong, B.S. Effect of Culture Conditions on Production of 5-Aminolevulinic Acid by Recombinant Escherichia coli. Biosci. Biotechnol. Biochem. 2005, 69, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indoleacetic Acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qiu, S.Y. Method for on-Site Measurement of Methane in Paddy Fields. Available online: https://www.hdares.gov.tw/upload/hdares/files/web_structure/13721/02%E6%B0%B4%E7%94%B0.pdf (accessed on 29 August 2024).



| PNSB | Before cultivation | After cultivation | Difference (%) | Total (%) |
| 0–10 cm | 1.433 ± 0.186 | 1.484 ± 0.14 | −0.088 | 0.737 |
| 10–20 cm | 1.623 ± 0.227 | 1.756 ± 0.161 | 0.209 | |
| 20–30 cm | 1.763 ± 0.248 | 2.159 ± 0.141 | 0.615 | |
| Control group | Before cultivation | After cultivation | Difference (%) | Total (%) |
| 0–10 cm | 1.448 ± 0.128 | 1.488 ± 0.051 | 0.003 | 0.209 |
| 10–20 cm | 1.705 ± 0.207 | 1.779 ± 0.09 | 0.172 | |
| 20–30 cm | 2.006 ± 0.154 | 1.779 ± 0.09 | 0.034 |
| PNSB | Before cultivation | After cultivation |
| 0–10 cm | 1.433 ± 0.186 | 1.484 ± 0.14 |
| 10–20 cm | 1.623 ± 0.227 | 1.756 ± 0.161 |
| 20–30 cm | 1.763 ± 0.248 | 2.159 ± 0.141 |
| Control group | Before cultivation | After cultivation |
| 0–10 cm | 1.448 ± 0.128 | 1.488 ± 0.051 |
| 10–20 cm | 1.705 ± 0.207 | 1.779 ± 0.09 |
| 20–30 cm | 2.006 ± 0.154 | 1.779 ± 0.09 |
| Depth | PNSB | Control Group |
|---|---|---|
| 0–10 cm | 0.1959 | 0.1897 |
| 10–20 cm | 0.229 | 0.2012 |
| 20–30 cm | 0.305 | 0.4786 |
| Soil Organic Carbon Content (tons-C/ha) | Amount Sequestered (tons-C/ha) | |||||
|---|---|---|---|---|---|---|
| (A) a | (B) b | (C) c | Total d | |||
| Control group | Before cultivation | 15.564 | 9.530 | 5.717 | 30.812 | 4.89 |
| After cultivation | 15.996 | 12.832 | 6.877 | 35.704 | ||
| PNSB | Before cultivation | 14.301 | 9.825 | 2.929 | 27.055 | 17.45 |
| After cultivation | 13.544 | 14.484 | 16.475 | 44.503 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.K.; Kao, K.-Y.; Kuo, Y.-C.; Liou, R.-M. Utilization of Phototrophic Bacteria to Enhance Carbon Sequestration in Rice Paddy. Eng. Proc. 2024, 74, 30. https://doi.org/10.3390/engproc2024074030
Wang AK, Kao K-Y, Kuo Y-C, Liou R-M. Utilization of Phototrophic Bacteria to Enhance Carbon Sequestration in Rice Paddy. Engineering Proceedings. 2024; 74(1):30. https://doi.org/10.3390/engproc2024074030
Chicago/Turabian StyleWang, Alyssa Katsuyama, Kun-Yi Kao, Yang-Cheng Kuo, and Rey-May Liou. 2024. "Utilization of Phototrophic Bacteria to Enhance Carbon Sequestration in Rice Paddy" Engineering Proceedings 74, no. 1: 30. https://doi.org/10.3390/engproc2024074030
APA StyleWang, A. K., Kao, K.-Y., Kuo, Y.-C., & Liou, R.-M. (2024). Utilization of Phototrophic Bacteria to Enhance Carbon Sequestration in Rice Paddy. Engineering Proceedings, 74(1), 30. https://doi.org/10.3390/engproc2024074030






