Strategy of Phytoremediation for Sustainable Use of Arsenic-Rich Farmland †
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site
2.2. Soil Properties
2.3. Planting Experiment
3. Results
3.1. Soil Properties and Arsenic Concentration
3.2. Pteris vittata L.
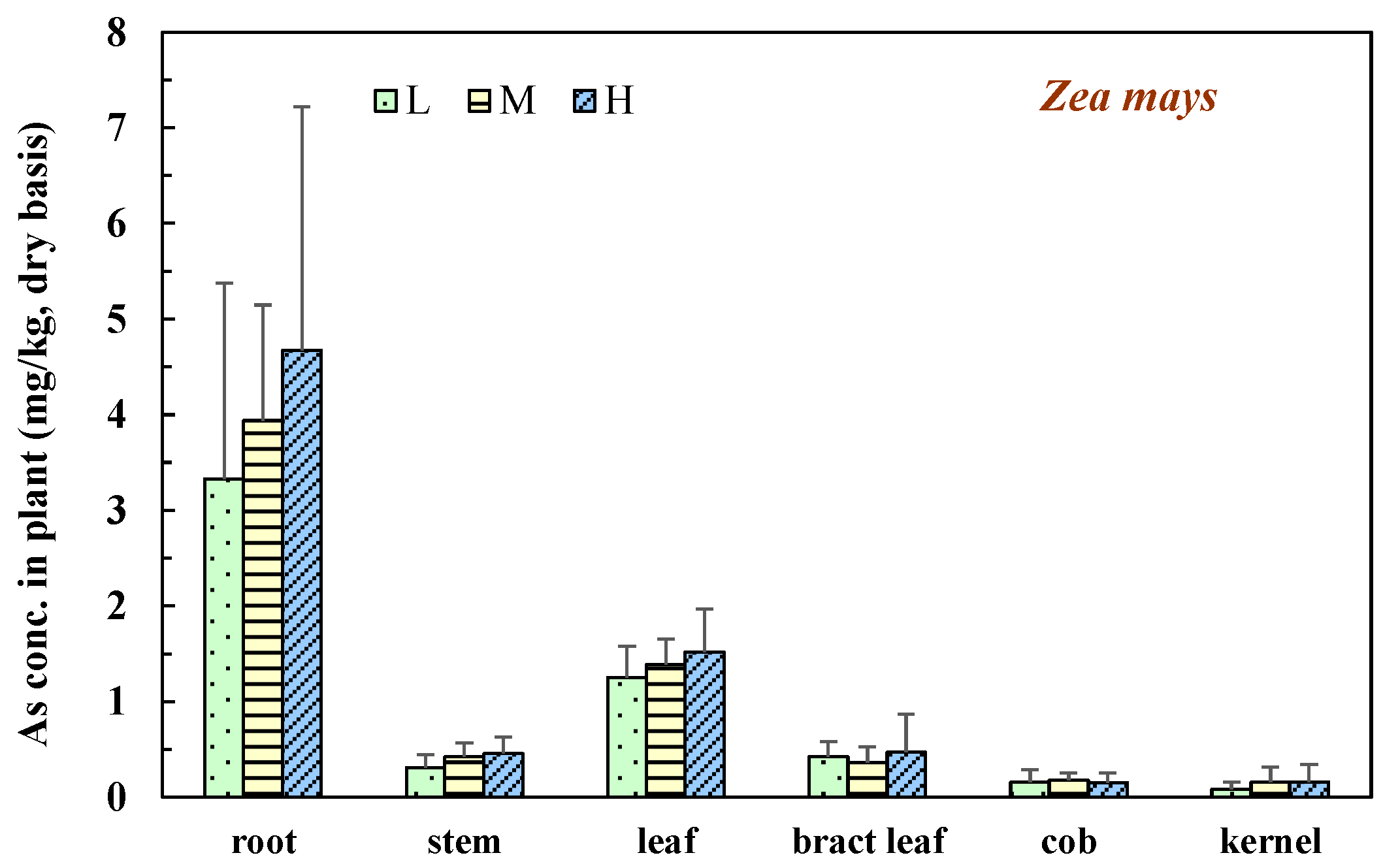
3.3. Zea mays L.
3.4. Rotala rotundifolia
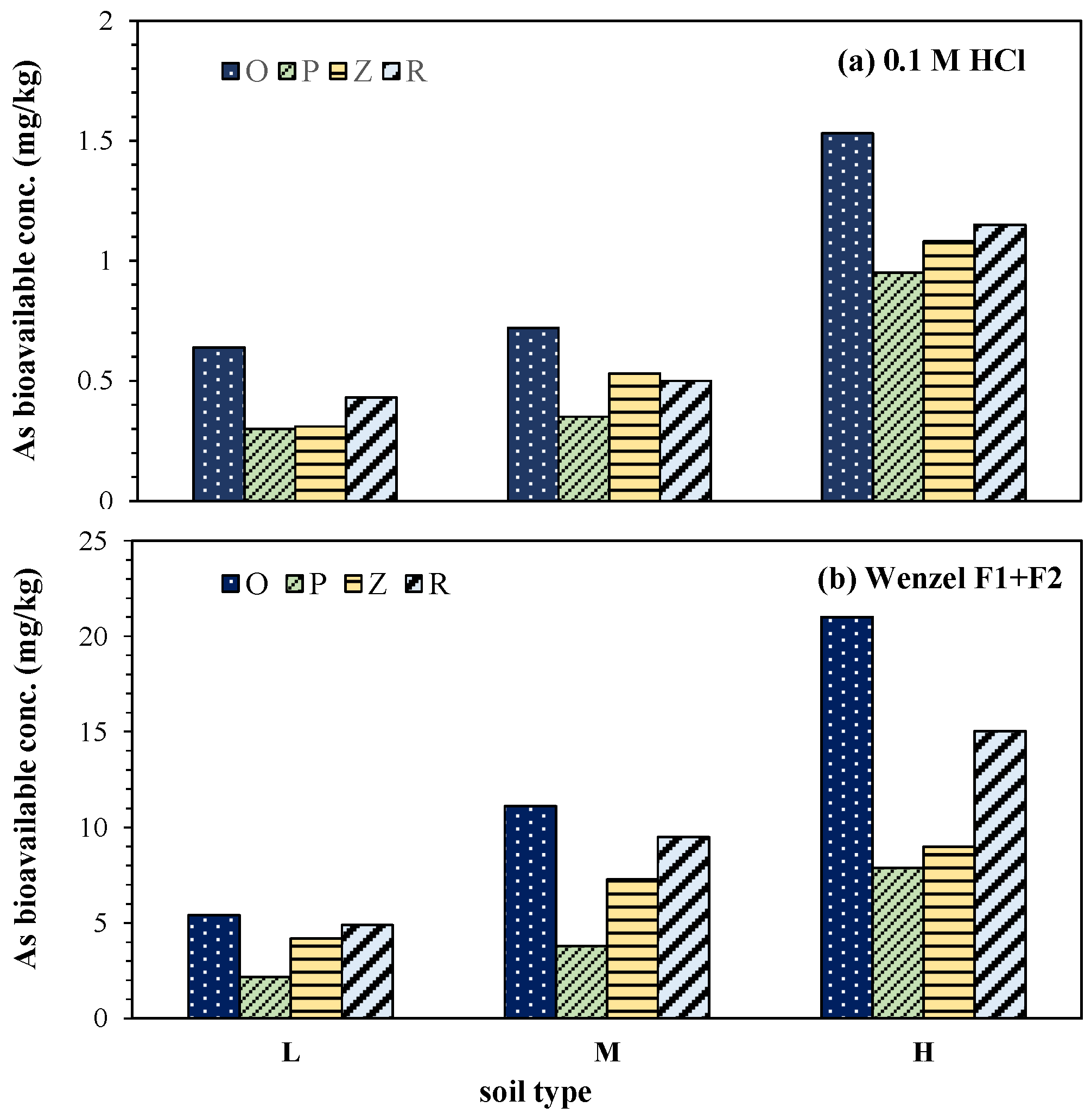
3.5. Changes in the Bioavailable Arsenic Concentration in the Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of drinking-water by arsenic Bangladesh: A public health emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Arsenic uptake and accumulation mechanisms in rice species. Plants 2020, 9, 129. [Google Scholar] [CrossRef]
- Liu, C.W.; Wu, M.Z. Geochemical, mineralogical and statistical characteristics of arsenic in groundwater of the Lanyang Plain, Taiwan. J. Hydrol. 2019, 577, 123975. [Google Scholar] [CrossRef]
- World Health Organization. Arsenic. Geneva 2018. WHO Fact Sheet. Available online: https://www.who.int/en/news-room/fact-sheets/detail/arsenic (accessed on 18 April 2024).
- Taiwan FDA. Sanitation Standard for Contaminants and Toxins in Food. 2021. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0040138 (accessed on 18 April 2024).
- Dradrach, A.; Karczewska, A.; Szopka, K. Arsenic uptake by two tolerant grass species: Holcus lanatus and Agrostis capillaris growing in soils contaminated by historical mining. Plants 2020, 9, 980. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernandez-Allica, J.; Becerril, J.M.; Amezaga, I.; Albizu, I.; Onaindia, M.; Garbisu, C. Chelate-enhanced phytoremediation of soils polluted with heavy metals. Rev. Environ. Sci. Bio-Technol. 2004, 3, 55–70. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.S.; Hernandez-Allica, J.; Becerril, J.M.; Amaral-Sobrinho, N.; Mazur, N.; Garbisu, C. Chelate-induced phytoextraction of metal polluted soils with Brachiaria decumbens. Chemosphere 2006, 65, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Stevanović, B.; Mitrović, M.; Pavlović, P. Phytoremediation potential, photosynthetic and antioxidant response to arsenic-induced stress of Dactylis glomerata L. sown on fly ash deposits. Plants 2020, 9, 657. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Srivastava1, M.; Santos, J.; Srivastava, P.; Ma, L.Q. Comparison of arsenic accumulation in 18 fern species and four Pteris vittata accessions. Bioresour. Technol. 2010, 101, 2691–2699. [Google Scholar] [CrossRef]
- Jiang, Y.; Lei, M.; Duanc, L.; Longhurst, P. Integrating phytoremediation with biomass valorisation and critical element recovery: A UK contaminated land perspective. Biomass Bioenergy 2015, 83, 328–339. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Saha, U.K. Arsenic hyperaccumulating fern: Implications for remediation of arsenic contaminated soils. Geoderma 2016, 284, 132–143. [Google Scholar] [CrossRef]
- Cantamessa, S.; Massa, N.; Gamalero, E.; Berta, G. Phytoremediation of a highly arsenic polluted site, using Pteris vittata L. and arbuscular mycorrhizal fungi. Plants 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Zemanová, V.; Pavlíková, D.; Hnilička, F.; Pavlík, M. Arsenic toxicity-induced physiological and metabolic changes in the shoots of Pteris cretica and Spinacia oleracea. Plants 2021, 10, 2009. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.B.; Zhang, B.C.; Huang, Z.C.; Liu, Y.R.; Zheng, Y.M.; Lei, M.; Liao, X.Y.; Piao, S.J. Geographical distribution and characteristics of habitat of As-hyperaccumulator Pteris vittata L. in China. Geogr. Res. 2005, 24, 825–833. [Google Scholar]
- Kertulis-Tartar, G.M.; Ma, L.Q.; Tu, C.; Chirenje, T. Phytoremediation of an arsenic-contaminated site using Pteris vittata L.: A two-year study. Int. J. Phytoremediation 2006, 8, 311–322. [Google Scholar] [CrossRef]
- Liu, Y.R.; Chen, T.B.; Huang, Z.C.; Liao, X.Y. As hyperaccumulation of Pteris vittata L. as influenced by As concentrations in soils of contaminated fields. Environ. Sci. 2005, 26, 181–186. [Google Scholar]
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 2021, 10, 2254. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W. Arsenic transformation in the soil-rhizosphere-plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Takahashi, Y.; Minamikawa, R.; Hattori, K.H. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol. 2004, 38, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Inskeep, W.P.; McDermott, T.R.; Fendorf, S. Arsenic (V)/(III) Cycling in Soils and Natural Waters: Chemical and Microbiological Processes, Environmental Chemistry of Arsenic; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Alvarado, S.; Guédez, M.; Lué-Merú, M.P.; Nelson, G.; Alvaro, A.; Jesús, A.C.; Gyula, Z. Arsenic removal from waters by bioremediation with the aquatic plants Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Bioresour. Technol. 2008, 99, 8436–8844. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Wu, P.; Zhang, M.; Zhang, C.P.; Han, Z.W. Accumulation characteristics of Sb and As in cattail growing in antimony mine tailings. Chin. J. Ecol. 2015, 34, 2645–2649. [Google Scholar]
- Chen, T.; Bao, N.Y.; Du, C.X.; Wang, Y.; Liu, Y.G. Effects of exogenous phosphorus input on growth and arsenic accumulation in Typha angustifolia L. contaminated by arsenic. J. South. Agric. 2020, 51, 2104–2112. [Google Scholar]
- Lin, S.C.; Zhao, F.J.; Huang, W.D.; Shyu, G.S.; Yao, P.H.; Chang, T.K. The relationship between arsenic species in paddy soils and arsenic content of rice tissues at Guandu plain. J. Taiwan Agric. Eng. 2008, 54, 60–70. [Google Scholar]
- Marbaniang, D.; Chaturvedi, S.S. Laboratory study of arsenic uptake and phytoremediation potential of three aquatic macrophytes of meghalaya, India. Int. J. Sci. Technol. Res. 2014, 3, 47–53. [Google Scholar]
- Brunettia, G.; Farrag, K.; Rovirae, P.S.; Nigrof, F.; Senesia, N. Greenhouse and field studies on Cr, Cu, Pb and Zn phytoextraction by Brassica napus from contaminated soils in the Apulia region, Southern Italy. Geoderma 2011, 160, 517–523. [Google Scholar] [CrossRef]
- Wittersa, N.; Mendelsohnb, R.; Van Passela, S.; Van Slyckenc, S.; Weyensa, N.; Schreursa, E.; Meersc, E.; Tackc, F.; Vanheusdena, B.; Vangronsveld, J. Phytoremediation, a sustainable remediation technology? II: Economic assessment of CO2 abatement through the use of phytoremediation crops for renewable energy production. Biomass Bioenergy 2012, 39, 470–477. [Google Scholar] [CrossRef]
- Meers, E.; Slycken, S.V.; Adriaenseb, K.; Ruttens, A.; Vangronsveld, J.; Laing, G.D.; Witters, N.; Thewys, T.; Tack, F.M.G. The use of bio-energy crops (Zea mays) for ‘phytoattenuation’ of heavy metals on moderately contaminated soils: A field experiment. Chemosphere 2010, 78, 35–41. [Google Scholar] [CrossRef]
- Mi, Y.H.; Lei, M.; Li, Q.W.; Chen, L.; Du, L.J.; Deng, X.X.; Yang, X.K.; Zhang, W.B. Phytoremediation and human health risk of farmland polluted by heavy metal in Southern Yunnan Mining Area. Ecol. Environ. 2016, 25, 864–871. [Google Scholar]
- Lukacova, Z.; Svubova, R.; Selvekova, P.; Hensel, K. The effect of plasma activated water on Maize (Zea mays L.) under arsenic stress. Plants 2021, 10, 1899. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Huang, C.Y.; Lin, Y.C.; Lin, S.C.; Chen, K.L. Phytoremediation of lead using corn in contaminated agricultural land—An in situ study and benefit assessment. Ecotoxicol. Environ. Saf. 2015, 111, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]



| Properties | Unit | L | M | H | |
|---|---|---|---|---|---|
| As concentration | mg/kg | 34.1 ± 5.0 | 68.9 ± 9.4 | 105.5 ± 3.3 | |
| pH | - | 6.58 | 6.61 | 6.81 | |
| Conductivity | μS/cm | 245 | 162 | 269 | |
| Organic matter | % | 3.23 | 3.22 | 3.06 | |
| Bioavailable As concentration | 0.1 M HCl | mg/kg | 0.64 | 0.72 | 1.61 |
| Wenzel F1 + F2 | mg/kg | 5.4 | 11.1 | 21.0 | |
| Soil Type | Average Biomass (g Per Plant, Dry Basis) | As Removal Amount (mg) | |||
|---|---|---|---|---|---|
| Underground | Aboveground | Total | Per Plant | Per m2 | |
| PL | 20.4 ± 8.6 | 135.6 ± 29.6 | 156.0 | 7.63 | 63.61 |
| PM | 14.7 ± 5.7 | 120.4 ± 34.1 | 135.1 | 14.27 | 118.93 |
| PH | 17.2 ± 4.9 | 91.2 ± 29.1 | 108.4 | 24.20 | 201.68 |
| Soil Type | Average Biomass (g Per Plant, Dry Basis) | As Removal Amount (mg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | B.Leaf | Cob | Kernel | Total | Per Plant | Per m2 | |
| ZL | 60.4 | 121.3 | 61.0 | 58.0 | 59.2 | 178.8 | 538.7 | 0.36 | 6.05 |
| ZM | 59.0 | 134.3 | 58.4 | 67.9 | 63.7 | 127.4 | 510.7 | 0.43 | 7.09 |
| ZH | 58.7 | 148.6 | 57.0 | 62.5 | 60.7 | 143.4 | 530.8 | 0.49 | 8.16 |
| Soil Type | Average Biomass (g/m2, Dry Basis) | As Removal Amount (mg/m2) | ||||
|---|---|---|---|---|---|---|
| Root | Shoot | Total | Root | Shoot | Total | |
| GL | 3.9 ± 0.3 | 134.9 ± 23.0 | 138.8 | 1.144 | 7.732 | 8.877 |
| GM | 3.4 ± 0.4 | 172.2 ± 13.1 | 175.6 | 0.225 | 0.969 | 1.194 |
| GH | 7.1 ± 1.3 | 181.8 ± 39.8 | 188.9 | 0.302 | 0.680 | 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Lin, M.-S.; Cheng, P.-C.; Huang, C.-Y.; Cheng, S.-F. Strategy of Phytoremediation for Sustainable Use of Arsenic-Rich Farmland. Eng. Proc. 2024, 74, 8. https://doi.org/10.3390/engproc2024074008
Chen C-C, Lin M-S, Cheng P-C, Huang C-Y, Cheng S-F. Strategy of Phytoremediation for Sustainable Use of Arsenic-Rich Farmland. Engineering Proceedings. 2024; 74(1):8. https://doi.org/10.3390/engproc2024074008
Chicago/Turabian StyleChen, Chang-Chao, Min-Siou Lin, Pei-Cheng Cheng, Chin-Yuan Huang, and Shu-Fen Cheng. 2024. "Strategy of Phytoremediation for Sustainable Use of Arsenic-Rich Farmland" Engineering Proceedings 74, no. 1: 8. https://doi.org/10.3390/engproc2024074008
APA StyleChen, C.-C., Lin, M.-S., Cheng, P.-C., Huang, C.-Y., & Cheng, S.-F. (2024). Strategy of Phytoremediation for Sustainable Use of Arsenic-Rich Farmland. Engineering Proceedings, 74(1), 8. https://doi.org/10.3390/engproc2024074008





