Abstract
The aesthetics of clear aligners is a critical factor that can influence patient satisfaction and psychological and social well-being. However, their transparency can be compromised by exposure to staining agents. This study aimed to evaluate the color stability of PET-G aligners following prolonged exposure to common daily substances, including food, tobacco products, and cleaning agents. Flat samples of PET-G (n = 220) were immersed in various solutions, including coffee, tea, Coca-Cola, red wine, a colloidal silver-based disinfectant, nicotine, artificial saliva, cigarette smoke, and mixtures of saliva with smooth, coffee, and nicotine. Immersion times of 10 (n = 110) and 15 days (n = 110) were randomly assigned. Colorimetric assessments were conducted by measuring L*a*b* parameters before and after immersion, and total color change (ΔE) was calculated. Non-parametric statistical tests revealed significant color changes in PET-G samples after both immersion durations, with pairwise comparisons indicating notable differences in ΔE values among groups exposed to different substances, particularly coffee, tea, and Coca-Cola. The findings highlight the psychological and social impact of aligner staining on patient confidence and compliance. Understanding these effects highlights the need for enhanced patient education to improve aligner aesthetics and satisfaction.
Keywords:
clear aligners; PET-G; color stability; daily substances; psychological; social; well-being 1. Introduction
Orthodontics has evolved in response to the increasing demand for aesthetic and comfortable treatment options, leading to the widespread adoption of clear aligners [1,2,3,4,5]. Initially introduced by Kesling in 1946 for fine-tuning tooth positions [6], aligners have undergone significant evolution alongside advancements in digital technology and manufacturing. Modern systems, such as Invisalign, utilize 3D imaging and computer-aided design to produce custom, removable aligners that gradually reposition teeth. Today, clear aligners effectively address a wide range of orthodontic issues, including open bite, deep bite, crowding, and spacing, serving as a modern and efficient alternative to conventional braces [7]. Among the primary materials used in their fabrication, polyurethanes (PUs) and polyesters, particularly polyethylene terephthalate glycol (PET-G), are favored for their biocompatibility, durability, and optical properties [8,9,10]. However, despite their translucency and mechanical advantages, these materials are susceptible to environmental factors such as temperature, humidity, and exposure to food substances, posing significant challenges [11,12]. In particular, the oral environment presents a potential threat to the properties of these materials as microcracks, abrasions, biofilm deposits, and delamination could be created, compromising the mechanical strength and transparency of aligners [13]. While numerous studies have thoroughly examined the physicochemical and biomechanical characteristics of clear aligners [9,14,15,16,17,18], there is a noticeable gap in the literature regarding their long-term color stability and aesthetic performance. Research has shown that daily exposure to staining agents—present in food, beverages, cleaning products, and tobacco—can alter the optical properties of aligners [15]. Tannins (i.e., polyphenolic compounds in tea, coffee, and red wine) cause yellow–brown discoloration and surface roughening due to their acidic nature [19,20,21]. Acidic and pigmented components in carbonated drinks, such as Coca-Cola, also contribute to color changes [22]. Furthermore, nicotine exposure can lead to staining and unpleasant odors due to residue accumulation on the aligner surface [15].
Despite these findings, the long-term impact of such exposures on PET-G aligners remains largely unexplored. Given that the primary appeal of clear aligners is their transparency, any loss of aesthetic quality can have significant psychological and social implications. Patients, particularly adolescents and young adults, often opt for clear aligners due to their discreet appearance, which is associated with enhanced self-esteem, social confidence, and overall treatment satisfaction [23,24,25]. On the other hand, the habit of patients wearing aligners during food consumption exposes the polymer to potential coloring agents, increasing its susceptibility to loss of transparency [26,27]. The aesthetic decline can lead to diminished confidence and dissatisfaction with the treatment [28,29,30]. Considering this, our study aims to bridge the gap by evaluating the colorimetric changes in PET-G aligners following prolonged exposure to commonly encountered substances. By investigating the long-term color stability of these materials, our research provides novel insights that will inform clinical practices, enhance patient education, and ultimately improve treatment outcomes while addressing the psychological and social concerns associated with aligner staining.
2. Materials and Methods
2.1. Preparation and Staining of PET-G Samples
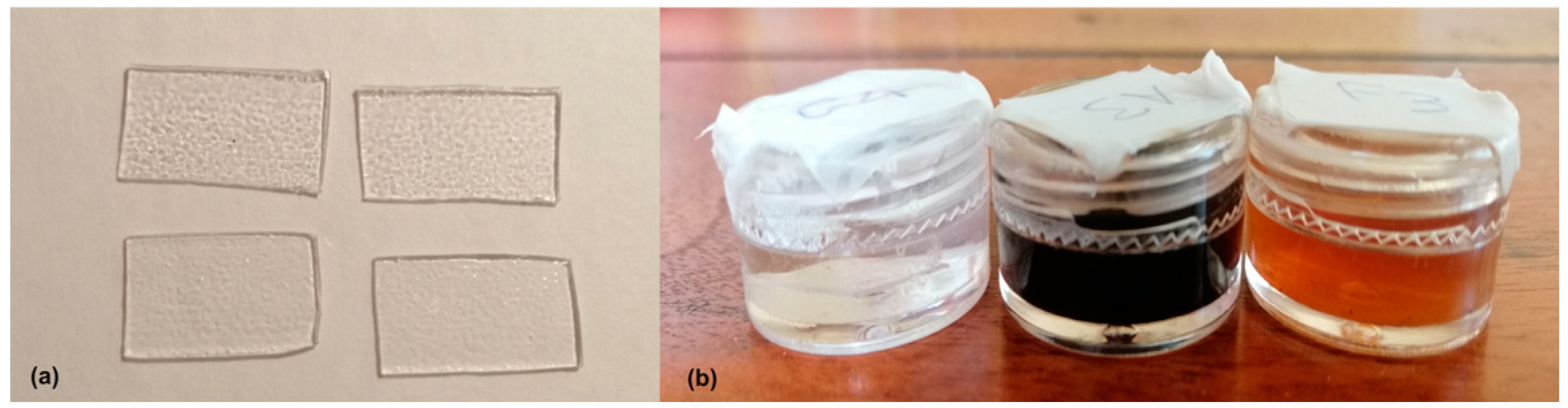
For this investigation, twenty-five sheets of PET-G thermoplastic material (Erkodur, Erkodent Erich Kopp GmbH, Pfalzgrafenweiler, Germany), each measuring 0.8 mm in thickness, and a truncated pyramid-shaped mold for thermoforming were used. Two hundred and twenty samples, measuring 1 × 1.5 cm, were obtained from the horizontal surfaces of the thermoformed models [15]. These samples (Figure 1a) were then immersed in various solutions, including Nescafé Classic coffee, Earl Grey Twinings sugar-free tea, Coca-Cola (Coca-Cola® Company, Atlanta, GA, USA), red wine (San Crispino, Cantine Ronco), colloidal silver disinfectant, nicotine solution, Biotène Oral Balance artificial saliva (GlaxoSmithKline Consumer Healthcare S.p.A.), cigarette smoke and various mixtures of saliva associated with smoking, nicotine, and coffee [15]. Each specimen was placed in a 5 mL plastic container filled with a specific substance and immersed in a water bath at a temperature of 37°. All the samples were rinsed with deionized water (Milli-Q) every 24 h before re-immersion in fresh solution (Figure 1b). Immersion times of 10 (n = 110) and 15 days (n = 110) were randomly assigned. These periods were chosen to simulate mid- to long-term exposure, as these durations approximate the cumulative effect of daily aligner use within a typical two-week wear period. For each immersion group, the samples were further divided into subgroups (n = 11) according to the substance considered.

Figure 1.
Preparation and staining: (a) representative thermoformed PET-G samples obtained from the horizontal surface of the mold; (b) some 5 mL plastic containers filled with the substance considered.
2.2. Colorimetric Evaluation
A flat, rectangular-shaped model was made with a flowable composite resin, A3 Body Shade flowable resin (Filtek Universal Restorative; 3M ESPE, Saint Paul, MN, USA) to serve as a background reference for the color evaluation of the material of PET-G samples. Initially, each sample was placed on the composite model for colorimetric assessment before immersion in the solutions. After 10 and 15 days of immersion, all samples were washed in Milli-Q water, ultrasound for 5 min, and dried properly. The background composite model was reinserted into its original position for colorimetric evaluation after 10 and 15 days. The color changes were detected by a spectrophotometer Easyshade V (Vita Zahnfabrik, Bad Säckingen, Germany) at three time intervals and were measured by the color system of the Commission Internationale de I’Eclairage (CIE) L*a*b* [31]. This system utilizes the following parameters: L* (lightness, with brightness + and darkness −), a* (color scale from red, positive values, to green, negative values), and b* (color scale from yellow, positive values, to blue, negative values). The optical sensor tip of the spectrophotometer was placed vertically on the surface of each sample. All measurements were performed by an investigator unaware of the division into groups to ensure unbiased results in a room with a standardized light source. The total color change () was calculated using the following formula [31]: . , and represented the color difference before and after immersion into solutions (at 10 and 15 days). To perceive the color change at a clinical level, the scale developed by the National Bureau of Standards (NBS) was used, converting the values into NBS units according to the following equation: (Table 1).

Table 1.
Description of color changes from the National Bureau of Standard Units.
2.3. Statistical Analysis
ΔE values for each group were presented as mean ± standard deviation (SD). Non-parametric methods were applied, as the Kolmogorov–Smirnov test did not demonstrate a normal distribution. The Wilcoxon signed-rank test was used to compare the ΔE values before and after immersion in each substance for each time considered (10 and 15 days). The independent-sample Mann–Whitney U test was used to detect differences before and after substance exposure at different times and evaluate the colorimetric stability for each coloring solution between groups. Subsequently, the Kruskal–Wallis test was applied to compare post-exposure ΔE values within groups based on the substance considered. Significance was adjusted based on the Bonferroni correction for multiple comparisons. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 25.0 software (IBM SPSS Statistics, New York, NY, USA) for Windows [32,33].
3. Results
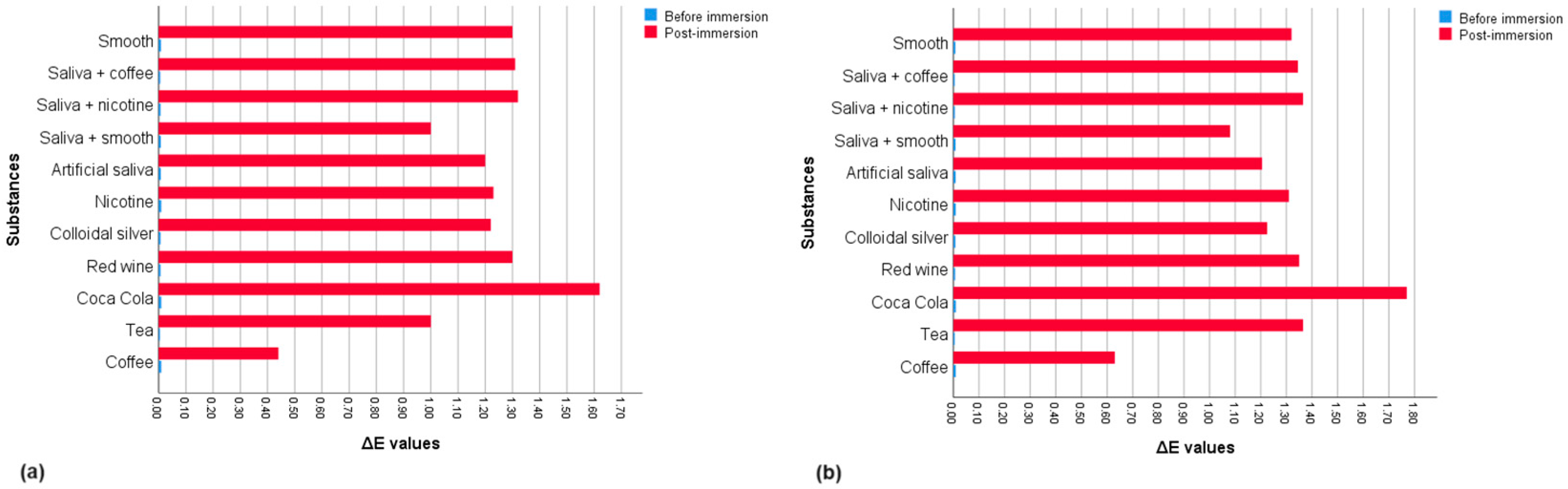
The mean ΔE values and standard deviations (SD) for each group are described in Table 2. Descriptive statistics based on mean values for each group are shown in Figure 2.

Table 2.
ΔE measurements of samples exposed to commonly used solutions at different times.
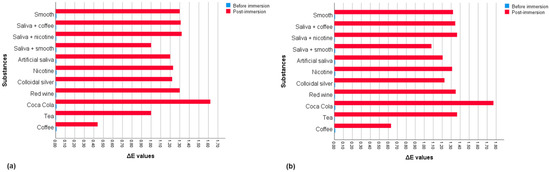
Figure 2.
Mean ΔE values for each group before and after exposure of the substance concerned: (a) up to 10 days; (b) up to 15 days.
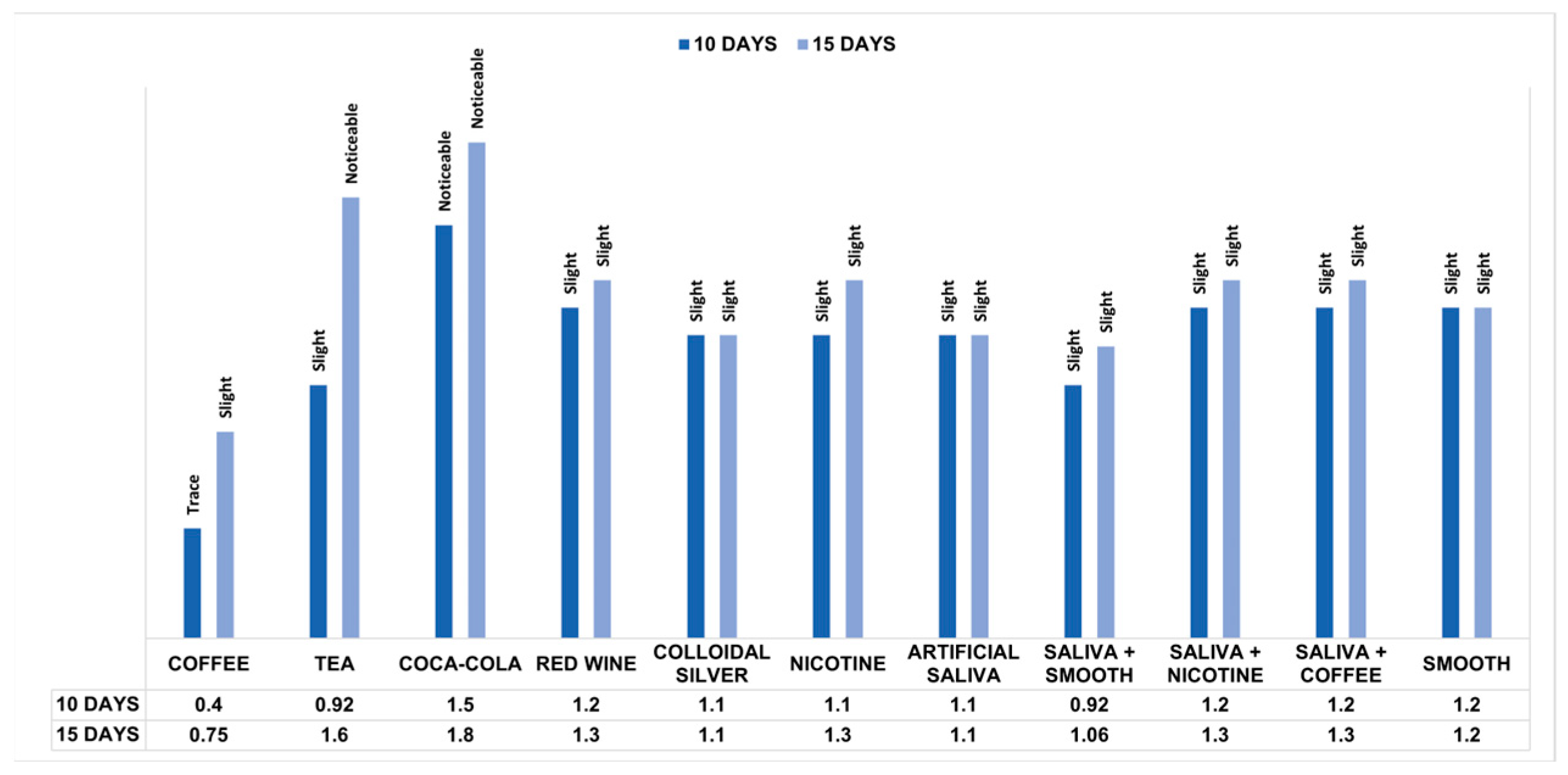
Comparing the mean pre- and post-exposure ΔE values within the groups, the Wilcoxon test showed significant differences for all solutions at 10 and 15 days (Table 2). The Mann–Whitney test found that unlike the pre-immersion measurements (p = 0.930), the distribution of post-immersion ΔE values in the substances changed significantly between groups based on exposure time (p < 0.001). Therefore, the Kruskal–Wallis test, applied only for post-immersion measurements, showed statistically significant differences between groups based on the substances considered for each time. In pairwise comparisons at 10 days, statistically significant differences were found between the ΔE values of the groups exposed to coffee and substances such as colloidal silver (p = 0.037) and nicotine (p = 0.018) and highly significant differences (p < 0.001) between coffee and groups exposed to red wine, smooth, saliva and coffee, saliva and nicotine and Coca Cola. Other significant differences were found between measurements for the groups immersed in Coca-Cola and substances such as artificial saliva (p = 0.003), colloidal silver (p = 0.013), and nicotine (p = 0.026). There were also highly significant differences (p < 0.001) between Coca-Cola and groups exposed to tea and the mixture of saliva and smooth. In pairwise comparisons at 15 days of immersion, statistically significant differences were demonstrated between the ΔE values of the groups exposed to coffee and certain substances such as nicotine (p = 0.003), red wine (p = 0.001), smooth (p = 0.034) and mixtures of saliva and coffee (p = 0.003) and saliva with nicotine (p = 0.003). Other differences were found between measurements for tea with artificial saliva (p = 0.001) and colloidal silver (p = 0.001) and between ΔE values for groups exposed to Coca-Cola and smooth (p = 0.021). In addition, highly significant differences (p < 0.001) were found between groups immersed in tea and those for coffee and the solution of saliva and smooth, as well as between ΔE values for Coca-Cola and substances such as coffee, artificial saliva, colloidal silver, and the mixture of saliva and smooth. The Mann–Whitney test also evaluated the colorimetric stability between the two times considered, demonstrating that the colorimetric changes were highly significant (p < 0.001) between the samples exposed to coffee, tea, and Coca-Cola and significant for red wine (p = 0.043) and nicotine (p = 0.005) (Table 3). Figure 3 shows the NBS rating comparison for each substance based on the exposure time. According to NBS evaluations, PET-G samples showed only slight color changes after 10 days of staining. However, samples exposed to Coca-Cola demonstrated perceptible color changes, while coffee resulted in extremely slight changes. After 15 days of staining, the samples showed slight color changes except those immersed in tea and Coca-Cola, which showed perceivable colorimetric variations.

Table 3.
Comparison of post-immersion ΔE values between groups for each substance based on the immersion period.
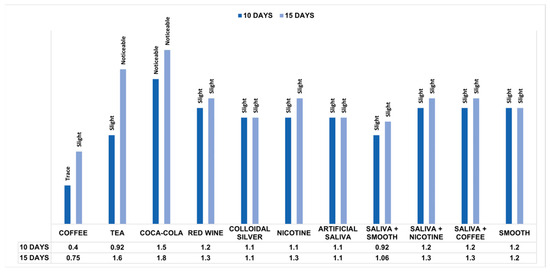
Figure 3.
Colorimetric measurements after 10 and 15 days of immersion into different solutions according to the NBS units.
4. Discussion
Patients often wear clear aligners while eating or drinking due to social discomfort, forgetfulness, or time constraints, despite recommendations to remove them [21,26]. This behavior stresses patient autonomy in decision making by causing the aligners to be exposed to staining agents. Since the primary appeal of these appliances is their transparency, discoloration can significantly impact psychological well-being and social confidence. Previous studies have assessed the effect of various substances on different aligner materials, including Smart Track (multilayer thermoplastic polyurethane with integrated elastomer), Erkodur (PET-G), Essix ACE (copolyester), Essix Plastic, Ghost aligner (PET), and Zendura (polyurethane) [9,34]. Short-term exposure (12 h) typically results in minimal color changes, except for polyurethane-based aligners when exposed to coffee and red wine. However, longer exposure (7 days or more) leads to noticeable staining, particularly in PET-G and multilayer polyurethane aligners when exposed to black tea, coffee, and red wine [21]. Other studies have shown that specific food ingredients, such as turmeric and saffron, can also cause discoloration in aligners made from polyurethane and PET-G [35]. Since aligners are typically worn for about two weeks [34,36], maintaining their transparency throughout this period is essential. Our study contributes to this growing body of evidence by systematically evaluating the color stability of PET-G aligners following prolonged exposure to commonly consumed substances, including food pigments, tobacco products, and cleaning agents. After 10 days of immersion, Coca-Cola caused the most significant color change (ΔE = 1.62 ± 0.10), which was followed by saliva with nicotine (ΔE = 1.32 ± 0.12) and saliva with coffee (ΔE = 1.31 ± 0.12). Nicotine and red wine also caused staining. After 15 days, Coca-Cola (ΔE = 1.92 ± 0.10) and tea (ΔE = 1.73 ± 0.11) determined the most marked color changes, emphasizing their potential to compromise aligner aesthetics over time. It is important to contextualize these ΔE values in terms of clinical significance. The ΔE value serves as an objective measure of color change, with values below approximately 3 or 3.3 generally considered clinically acceptable [37,38], as these variations are often imperceptible or only minimally noticeable to the patient. Although our statistical analyses revealed significant differences in ΔE values between groups and over time, not all differences translate into clinically relevant discoloration. In our case, minor changes (ΔE < 3.0) may be statistically significant yet remain below the perceptibility threshold, thereby maintaining acceptable aesthetic standards for clear aligners. The NBS evaluations provided a practical clinical perspective. After 10 days, PET-G samples exposed to coffee exhibited extremely slight changes, whereas those immersed in Coca-Cola demonstrated perceptible changes. By 15 days, the colorimetric alterations in samples exposed to tea and Coca-Cola were particularly marked, emphasizing their long-term impact on aligner aesthetics. This nuanced interpretation is essential for practitioners, as it emphasizes that while some statistically significant differences may fall within acceptable clinical limits, others could necessitate greater attention in both patient counseling and aligner care recommendations.
This study aims to highlight the broader psychological and social consequences of aligner discoloration. Adolescents susceptible to peer perception could feel embarrassed in social settings, potentially affecting their self-esteem [39,40,41]. Professionals could experience diminished confidence in workplace interactions due to concerns about their appearance [40]. Discoloration can also influence treatment adherence, as patients might wear aligners less frequently to avoid social discomfort, which could prolong treatment duration and affect overall effectiveness [42]. To mitigate these issues and maintain aligner transparency, several preventive measures and cleaning methods can be considered. Patients should be advised to remove their aligners when consuming certain beverages, such as coffee, tea, and Coca-Cola, to minimize direct exposure to staining agents. Additionally, regular cleaning with gentle, non-abrasive agents—such as specialized aligner cleaning solutions or tablets—can help remove surface stains and biofilm without compromising the material of the aligner. Future studies should investigate more effective cleaning solutions and protocols to preserve aligner aesthetics. A personalized approach to aligner care, considering individual lifestyles and dietary habits, could further improve patient confidence, compliance, and overall satisfaction with orthodontic treatment. Nevertheless, several limitations of our study must be acknowledged. First, the relatively small number of PET-G samples used for immersion in various solutions could limit the statistical power and robustness of our findings. Additionally, although our in vitro setup enabled reproducibility and controlled analysis, it does not fully replicate the complexities of the oral environment. Real-world wear conditions are inherently more complex because in everyday use, aligners are subjected to intermittent contact with staining agents, variable exposure durations, and individual differences in oral hygiene practices. Thus, further research is needed to compare in vitro findings with clinical scenarios to fully understand the impact of daily substance exposure on aligner aesthetics. Moreover, the study did not consider other substances commonly consumed by patients, such as colored foods, fruits, and vegetables, which could also affect aligner color stability. Including a broader range of staining agents would provide a more complete understanding of the factors affecting aligner color stability. Finally, the evaluation of PET-G samples exposed to colloidal silver-based cleaning solutions was not compared with other cleaning methods, highlighting the need for additional research to assess the comparative effectiveness of different cleaning regimens on both aligner color stability and material integrity. To address these issues, new polymeric materials with enhanced color stability and durability could be developed through collaboration between materials scientists and dental manufacturers. Moreover, personalized treatment approaches that consider individual patient factors—such as dietary habits, lifestyle behaviors, and oral hygiene practices—should be advocated to provide comprehensive patient education on aligner care and smoking cessation, ultimately minimizing discoloration and optimizing treatment outcomes.
5. Conclusions
Preserving aligner transparency is key to patient confidence and compliance. This study highlights the importance of patient education regarding the risks associated with consuming staining substances while wearing clear aligners. Clinicians should advise patients to remove their aligners during meals and when consuming beverages such as coffee, tea, and Coca-Cola to minimize discoloration. Furthermore, our results underscore the importance of effective cleaning protocols in maintaining aligner transparency, which is essential for both aesthetic appeal and treatment compliance. Lastly, the data can inform manufacturers on potential material modifications to develop stain-resistant polymers, ultimately improving the long-term performance and patient satisfaction of clear aligner treatments.
Author Contributions
Conceptualization, F.N. and A.N.; methodology, F.N. and F.L.; formal analysis, J.L.; investigation, J.L. and A.N.; data curation, F.L.; writing—original draft preparation, F.N.; writing—review and editing, F.N. and A.N.; visualization, J.L.; supervision, A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rosvall, M.D.; Fields, H.W.; Ziuchkovski, J.; Rosenstiel, S.F.; Johnston, W.M. Attractiveness, Acceptability, and Value of Orthodontic Appliances. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 276.e1–276.e12. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Kazaz, R.; Ivgi, I.; Canetti, L.; Bachar, E.; Tsur, B.; Chaushu, S.; Shalish, M. The Impact of Personality on Adult Patients’ Adjustability to Orthodontic Appliances. Angle Orthod. 2012, 83, 76–82. [Google Scholar] [CrossRef]
- Ghafari, J.G. Centennial Inventory: The Changing Face of Orthodontics. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 732–739. [Google Scholar] [CrossRef]
- Vermiglio, G.; Centofanti, A.; Matarese, G.; Militi, A.; Matarese, M.; Arco, A.; Nicita, F.; Cutroneo, G. Human Dental Pulp Tissue during Orthodontic Tooth Movement: An Immunofluorescence Study. J. Funct. Morphol. Kinesiol. 2020, 5, 65. [Google Scholar] [CrossRef]
- Nicita, F.; Salmeri, F.; Runci Anastasi, M.; Aquilio, E.; Lipari, F.; Centofanti, A.; Favaloro, A. Morphological and Three-Dimensional Analysis for the Clinical Reproduction of Orthodontic Attachments: A Preliminary Study. Appl. Sci. 2024, 14, 7963. [Google Scholar] [CrossRef]
- Kesling, H.D. Coordinating the Predetermined Pattern and Tooth Positioner with Conventional Treatment. Am. J. Orthod. Oral. Surg. 1946, 32, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Nshimiyimana, E.; Ubuzima, P.; Mukeshimana, C.; Michelogiannakis, D.; Mbyayingabo, D.; Mugabo, E.; Gakunzi, D.; Ndanga, E.; Mazimpaka, P.; Habumugisha, J. Skeletal and Dental Open Bite Treatment Using Clear Aligners and Orthodontic Miniscrew-Anchored Fixed Appliances in Permanent Dentition: A Systematic Review. J. World Fed. Orthod. 2025, 14, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bai, Y.; Ding, X.; Zhang, Y. Preparation and Characterization of Thermoplastic Materials for Invisible Orthodontics. Dent. Mater. J. 2011, 30, 954–959. [Google Scholar] [CrossRef]
- Daniele, V.; Macera, L.; Taglieri, G.; Di Giambattista, A.; Spagnoli, G.; Massaria, A.; Messori, M.; Quagliarini, E.; Chiappini, G.; Campanella, V.; et al. Thermoplastic Disks Used for Commercial Orthodontic Aligners: Complete Physicochemical and Mechanical Characterization. Materials 2020, 13, 2386. [Google Scholar] [CrossRef]
- Nicita, F.; Calapaj, M.; Alibrandi, S.; Donato, L.; Aquilio, E.; D’Angelo, R.; Sidoti, A. Efficacy of an Experimental Gaseous Ozone-Based Sterilization Method for Clear Aligners. Angle Orthod. 2024. [Google Scholar] [CrossRef]
- Schuster, S.; Eliades, G.; Zinelis, S.; Eliades, T.; Bradley, T.G. Structural Conformation and Leaching from in Vitro Aged and Retrieved Invisalign Appliances. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 725–728. [Google Scholar] [CrossRef]
- Gracco, A.; Mazzoli, A.; Favoni, O.; Conti, C.; Ferraris, P.; Tosi, G.; Guarneri, M.P. Short-Term Chemical and Physical Changes in Invisalign Appliances. Australas. Orthod. J. 2009, 25, 34–40. [Google Scholar] [CrossRef]
- Gerard Bradley, T.; Teske, L.; Eliades, G.; Zinelis, S.; Eliades, T. Do the Mechanical and Chemical Properties of InvisalignTM Appliances Change after Use? A Retrieval Analysis. Eur. J. Orthod. 2016, 38, 27–31. [Google Scholar] [CrossRef]
- Ryokawa, H.; Miyazaki, Y.; Fujishima, A.; Miyazaki, T.; Maki, K. The Mechanical Properties of Dental Thermoplastic Materials in a Simulated Intraoral Environment. Orthod. Waves 2006, 65, 64–72. [Google Scholar] [CrossRef]
- Nicita, F.; D’Amico, C.; Filardi, V.; Spadaro, D.; Aquilio, E.; Mancini, M.; Fiorillo, L. Chemical–Physical Characterization of PET-G-Based Material for Orthodontic Use: Preliminary Evaluation of Micro-Raman Analysis. Eur. J. Dent. 2023. [Google Scholar] [CrossRef]
- Condo’, R.; Pazzini, L.; Cerroni, L.; Pasquantonio, G.; Lagana’, G.; Pecora, A.; Mussi, V.; Rinaldi, A.; Mecheri, B.; Licoccia, S.; et al. Mechanical Properties of “Two Generations” of Teeth Aligners: Change Analysis during Oral Permanence. Dent. Mater. J. 2018, 37, 835–842. [Google Scholar] [CrossRef]
- Barone, S.; Paoli, A.; Neri, P.; Razionale, A.V.; Giannese, M. Mechanical and Geometrical Properties Assessment of Thermoplastic Materials for Biomedical Application. Lect. Notes Mech. Eng. 2017, 437–446. [Google Scholar] [CrossRef]
- Alexandropoulos, A.; Al Jabbari, Y.S.; Zinelis, S.; Eliades, T. Chemical and Mechanical Characteristics of Contemporary Thermoplastic Orthodontic Materials. Aust. Orthod. J. 2015, 31, 165–170. [Google Scholar] [CrossRef]
- Rutkunas, V.; Sabaliauskas, V.; Mizutani, H. Effects of Different Food Colorants and Polishing Techniques on Color Stability of Provisional Prosthetic Materials. Dent. Mater. J. 2010, 29, 167–176. [Google Scholar] [CrossRef]
- Hollis, S.; Eisenbeisz, E.; Versluis, A. Color Stability of Denture Resins after Staining and Exposure to Cleansing Agents. J. Prosthet. Dent. 2015, 114, 709–714. [Google Scholar] [CrossRef]
- Bernard, G.; Rompré, P.; Tavares, J.R.; Montpetit, A. Colorimetric and Spectrophotometric Measurements of Orthodontic Thermoplastic Aligners Exposed to Various Staining Sources and Cleaning Methods. Head. Face Med. 2020, 16, 2. [Google Scholar] [CrossRef]
- Kessler, P.; Türp, J.C. Influence of Coca-Cola on Orthodontic Materials. A Systematic Review. Swiss Dent. J. 2020, 130, 983–993. [Google Scholar] [CrossRef]
- Souza, G.L.N.; de Campos França, E.; de Araújo Lombardi, M.; da Costa, G.C.; da Rocha, N.B.; Abreu, L.G. Impact of Treatment with Orthodontic Aligners on the Oral Health-Related Quality of Life. BMC Oral. Health 2024, 24, 419. [Google Scholar] [CrossRef]
- Jeremiah, H.G.; Bister, D.; Newton, J.T. Social Perceptions of Adults Wearing Orthodontic Appliances: A Cross-Sectional Study. Eur. J. Orthod. 2011, 33, 476–482. [Google Scholar] [CrossRef]
- Jaber, S.T.; Hajeer, M.Y.; Burhan, A.S.; Latifeh, Y. The Effect of Treatment With Clear Aligners Versus Fixed Appliances on Oral Health-Related Quality of Life in Patients With Severe Crowding: A One-Year Follow-Up Randomized Controlled Clinical Trial. Cureus 2022, 14, e25472. [Google Scholar] [CrossRef]
- Tsomos, G.; Ludwig, B.; Grossen, J.; Pazera, P.; Gkantidis, N. Objective Assessment of Patient Compliance with Removable Orthodontic Appliances: A Cross-Sectional Cohort Study. Angle Orthod. 2014, 84, 56–61. [Google Scholar] [CrossRef]
- Lombardo, L.; Martini, M.; Cervinara, F.; Spedicato, G.A.; Oliverio, T.; Siciliani, G. Comparative SEM Analysis of Nine F22 Aligner Cleaning Strategies. Prog. Orthod. 2017, 18. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, Y.; Chen, Y.; Zhao, H.; Wu, M. Evaluation and Comparative Assessment of Clear Aligners and Conventional Appliances on Oral Health-Related Quality of Life in Pediatric Populations: A Cross-Sectional Study. J. Clin. Pediatr. Dent. 2024, 48, 61–67. [Google Scholar] [CrossRef]
- Tania, M.; Veerasankar, S.; Ponniah, H.; Dhayananth, L.X.; Preeti, R.; Missier, M.S. Comparison of Patient Satisfaction between Invisible Appliance and Fixed Orthodontic Appliances - A Systematic Review. J. Pharm. Bioallied Sci. 2024, 16, S1017–S1021. [Google Scholar] [CrossRef]
- Castilhos, J.S.; Gasparello, G.G.; Mota-Júnior, S.L.; Hartmann, G.C.; Miyagusuku, L.F.I.; Pithon, M.M.; Tanaka, O.M. Accessories in Clear Aligner Therapy: Laypeople’s Expectations for Comfort and Satisfaction. J. Dent. Res. Dent. Clin. Dent. Prospects 2024, 18, 102–109. [Google Scholar] [CrossRef]
- Johnston, W.M. Color Measurement in Dentistry. J. Dent. 2009, 37, e2–e6. [Google Scholar] [CrossRef]
- Lo Giudice, G.; Cagidiaco, E.F.; Lo Giudice, R.; Puleio, F.; Nicita, F.; Calapaj, M. Evaluation of Mechanical Properties of a Hollow Endodontic Post by Three Point Test and SEM Analysis: A Pilot Study. Materials 2019, 12, 1983. [Google Scholar] [CrossRef]
- Antonuccio, P.; Pallio, G.; Marini, H.R.; Irrera, N.; Romeo, C.; Puzzolo, D.; Freni, J.; Santoro, G.; Pirrotta, I.; Squadrito, F.; et al. Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium. Int. J. Mol. Sci. 2022, 23, 13144. [Google Scholar] [CrossRef]
- Liu, C.L.; Sun, W.T.; Liao, W.; Lu, W.X.; Li, Q.W.; Jeong, Y.; Liu, J.; Zhao, Z.H. Colour Stabilities of Three Types of Orthodontic Clear Aligners Exposed to Staining Agents. Int. J. Oral. Sci. 2016, 8, 246–253. [Google Scholar] [CrossRef]
- Venkatasubramanian, P.; Jerome, M.S.; Ragunanthanan, L.; Maheshwari, U.; Vijayalakshmi, D. Color Stability of Aligner Materials on Exposure to Indigenous Food Products: An in-Vitro Study. J. Dent. Res. Dent. Clin. Dent. Prospects 2022, 16, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ziuchkovski, J.P.; Fields, H.W.; Johnston, W.M.; Lindsey, D.T. Assessment of Perceived Orthodontic Appliance Attractiveness. Am. J. Orthod. Dentofac. Orthop. 2008, 133, S68–S78. [Google Scholar] [CrossRef]
- Lee, Y.-K. Comparison of CIELAB ΔE* and CIEDE2000 Color-Differences after Polymerization and Thermocycling of Resin Composites. Dent. Mater. 2005, 21, 678–682. [Google Scholar] [CrossRef]
- Pérez, M.d.M.; Saleh, A.; Yebra, A.; Pulgar, R. Study of the Variation between CIELAB Delta E* and CIEDE2000 Color-Differences of Resin Composites. Dent. Mater. J. 2007, 26, 21–28. [Google Scholar] [CrossRef]
- Koaban, A.; Al-Harbi, S.K.; Al-Shehri, A.Z.; Al-Shamri, B.S.; Aburazizah, M.F.; Al-Qahtani, G.H.; Al-Wusaybie, L.H.; Alkhalifa, L.B.; Al-Saad, M.M.; Al-Nehab, A.A.; et al. Current Trends in Pediatric Orthodontics: A Comprehensive Review. Cureus 2024, 16, e68537. [Google Scholar] [CrossRef]
- Adobes-Martin, M.; Montoya-Morcillo, M.-L.; Zhou-Wu, A.; Garcovich, D. Invisalign Treatment from the Patient Perspective: A Twitter Content Analyses. J. Clin. Exp. Dent. 2021, 13, e376–e382. [Google Scholar] [CrossRef]
- Nicita, A.; Fumia, A.; Caparello, C.; Meduri, C.F.; Filippello, P.; Sorrenti, L. Goal Achievement and Academic Dropout Among Italian University Students: The Mediating Role of Academic Burnout. Eur. J. Investig. Heal. Psychol. Educ. 2025, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Alfawzan, A.A. Evaluation of Patient Acceptance and Compliance with Clear Aligners vs. Lingual Braces: A Randomized Trial. J. Pharm. Bioallied Sci. 2024, 16, S546–S548. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).