MnO Recovered from Alkaline Batteries Functionalized with Ruthenium and Carbon Nanofibers for Supercapacitor Applications †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
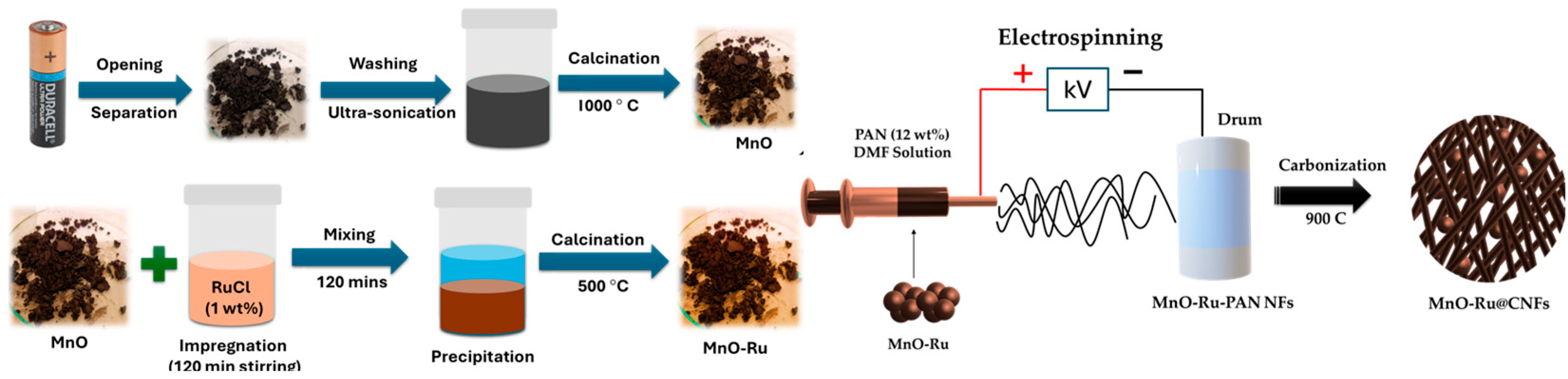
2.2. Preparation of Composite Samples
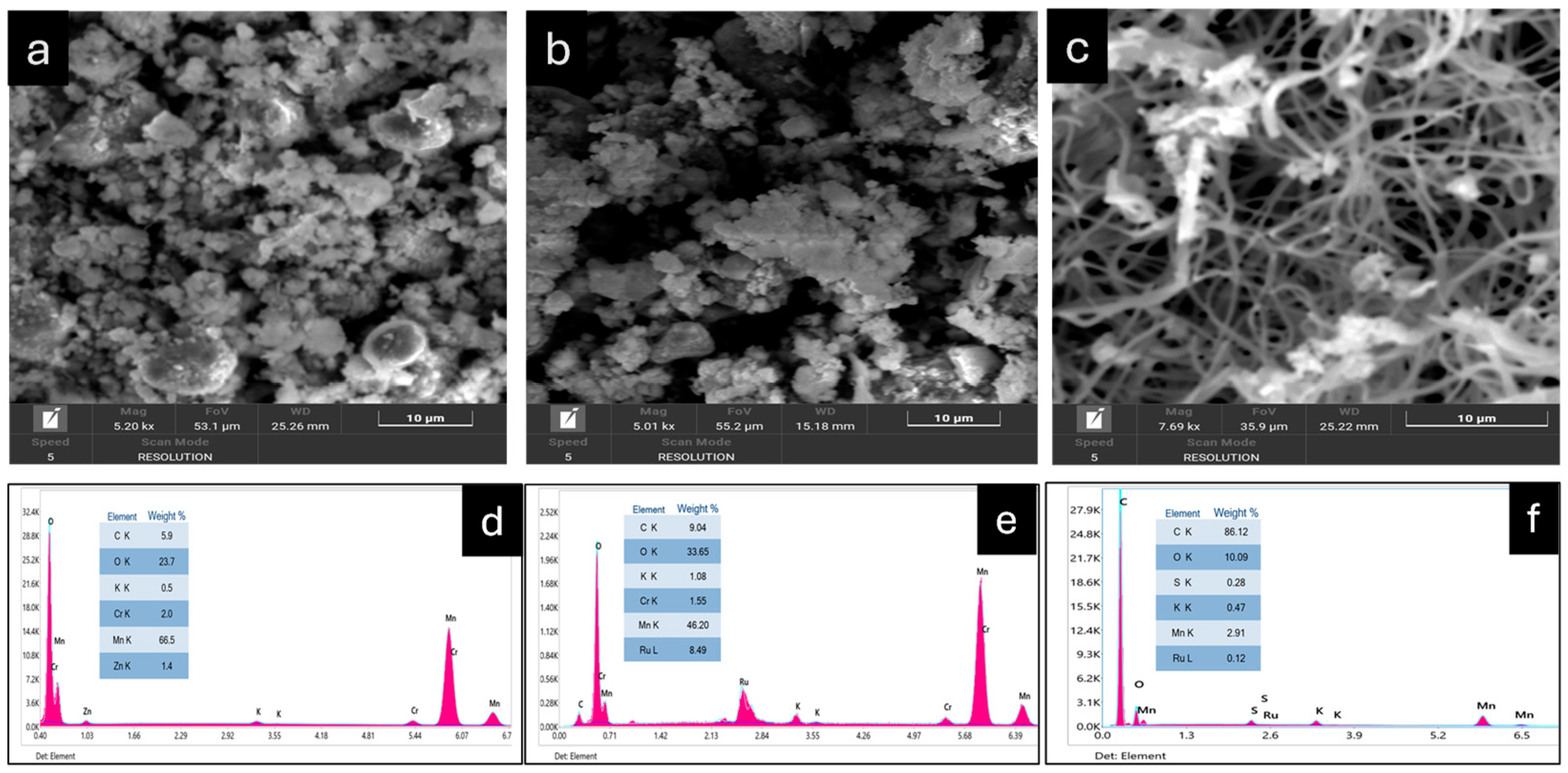
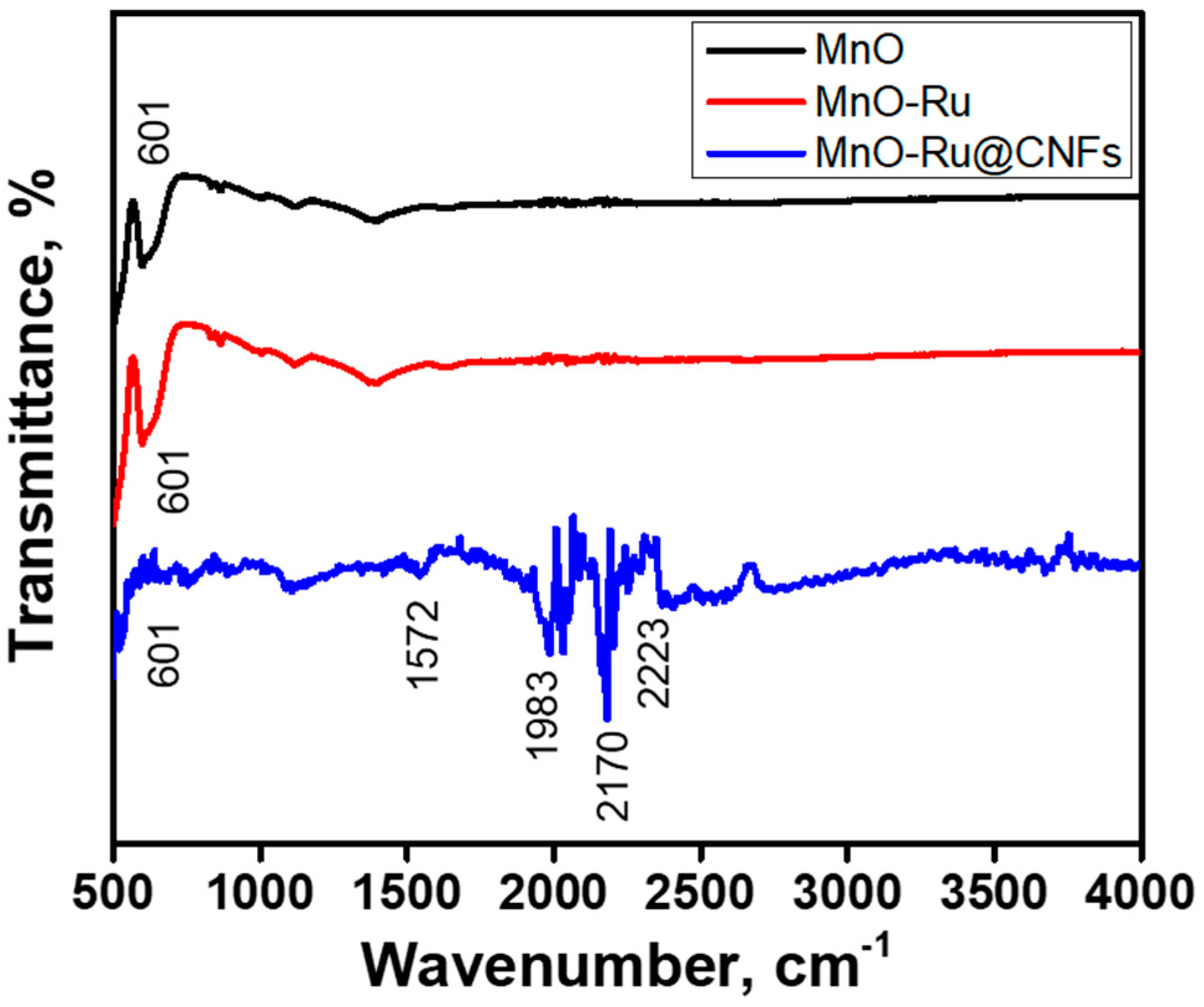
3. Characterization
Electrochemical Measurement
4. Results and Discussion
Electrochemical Measurement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Llorente, J.; Lidtke, A.A.; Hatanaka, K.; Limam, L.; Fajardo, I.; Okuyama, K.-I. In-orbit feasibility demonstration of supercapacitors for space applications. Acta Astronaut. 2020, 174, 294–305. [Google Scholar]
- He, Y.; Wang, Z.; Zhang, Y. The design, test and application on the satellite separation system of space power supply based on graphene supercapacitors. Acta Astronaut. 2021, 186, 259–268. [Google Scholar]
- Uno, M.; Tanaka, K. Accelerated Charge–Discharge Cycling Test and Cycle Life Prediction Model for Supercapacitors in Alternative Battery Applications. IEEE Trans. Ind. Electron. 2011, 59, 4704–4712. [Google Scholar]
- Chin, K.C.; Green, N.W.; Brandon, E.J. Evaluation of supercapacitors for space applications under thermal vacuum conditions. J. Power Sources 2018, 379, 155–159. [Google Scholar] [CrossRef]
- Cherusseri, J.; Kumar, K.S.; Choudhary, N.; Nagaiah, N.; Jung, Y.; Roy, T.; Thomas, J. Novel mesoporous electrode materials for symmetric, asymmetric and hybrid supercapacitors. Nanotechnology 2019, 30, 202001. [Google Scholar]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar]
- Mu, X.; Du, J.; Zhang, Y.; Liang, Z.; Wang, H.; Huang, B.; Zhou, J.; Pan, X.; Zhang, Z.; Xie, E. Construction of hierarchical CNT/rGO-supported MnMoO4 nanosheets on Ni foam for high-performance aqueous hybrid supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 35775–35784. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Zhou, J.; Jiang, F.; Zhang, Z. Hydrothermal synthesis of reduced graphene oxide-modified NiCo2O4 nanowire arrays with enhanced reactivity for supercapacitors. J. Alloy. Compd. 2019, 792, 474–480. [Google Scholar]
- Zhao, X.; Yang, H.; Hou, Y.; Gbologah, L.; Zhu, L.; Wang, Y. Systematically controlled synthesis of rGO/nano-MnOx materials for supercapacitors. Prog. Nat. Sci. Mater. Int. 2019, 29, 504–510. [Google Scholar]
- Dai, Z.; Zhang, C.Y.; Sun, G.W.; Wang, Y.H.; Lu, Z.W.; Zhao, H.; Sun, G.Z.; Gao, X.P.; Lan, W.; Zhang, Z.X.; et al. Highly enhanced electrochemical cycling stabilities of hierarchical partially-embedded MnO/carbon nanofiber composites as supercapacitor electrodes. Mater. Sci. Eng. B 2020, 262, 114684. [Google Scholar]
- Zhou, J.; Zhao, H.; Mu, X.; Chen, J.; Zhang, P.; Wang, Y.; He, Y.; Zhang, Z.; Pan, X.; Xie, E. Importance of polypyrrole in constructing 3D hierarchical carbon nanotube@MnO2 perfect core–shell nanostructures for high-performance flexible supercapacitors. Nanoscale 2015, 7, 14697–14706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, D.; Miao, Z.; Zhang, X.; Chou, S. Research progress in MnO2–carbon based supercapacitor electrode materials. Small 2018, 14, 1702883. [Google Scholar]
- Kulandaivalu, S.; Azahari, M.N.M.; Azman, N.H.N.; Sulaiman, Y. Ultrahigh specific energy of layer by layer polypyrrole/graphene oxide/multi-walled carbon nanotube| polypyrrole/manganese oxide composite for supercapacitor. J. Energy Storage 2020, 28, 101219. [Google Scholar] [CrossRef]
- Ramadan, M.; Abdellah, A.M.; Mohamed, S.G.; Allam, N.K. 3D interconnected binder-free electrospun MnO@C nanofibers for supercapacitor devices. Sci. Rep. 2018, 8, 7988. [Google Scholar] [CrossRef]
- de Souza, C.C.B.M.; de Oliveira, D.C.; Tenório, J.A.S. Characterization of used alkaline batteries powder and analysis of zinc recovery by acid leaching. J. Power Sources 2001, 103, 120–126. [Google Scholar] [CrossRef]
- He, X.-S.; Yu, H.; Xi, B.-D.; Cui, D.-Y.; Pan, H.-W.; Li, D. Difference of contaminant composition between landfill leachates and groundwater and its reasons. Huan Jing Ke Xue 2014, 35, 1399–1406. [Google Scholar]
- Liu, C.; Cui, J.; Jiang, G.; Chen, X.; Wang, L.; Fang, C. Soil heavy metal pollution assessment near the largest landfill of China. Soil Sediment Contam. Int. J. 2013, 22, 390–403. [Google Scholar] [CrossRef]
- Huang, S.S.; Liao, Q.L.; Hua, M.; Wu, X.M.; Bi, K.S.; Yan, C.Y.; Chen, B.; Zhang, X.Y. Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere 2007, 67, 2148–2155. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, B.; Gao, M.; Deng, R.; Zhang, Q. A review on spent Mn-containing Li-ion batteries: Recovery technologies, challenges, and future perspectives. J. Environ. Manag. 2024, 354, 120454. [Google Scholar]
- Farzana, R.; Hassan, K.; Sahajwalla, V. Manganese oxide synthesized from spent Zn-C battery for supercapacitor electrode application. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Warren, R.; Sammoura, F.; Tounsi, F.; Sanghadasa, M.; Lin, L. Highly active ruthenium oxide coating via ALD and electrochemical activation in supercapacitor applications. J. Mater. Chem. A 2015, 3, 15568–15575. [Google Scholar]
- Guo, Y.; Zhu, Z.; Chen, Y.; He, H.; Li, X.; Qin, T.; Wang, Y. High-performance supercapacitors of ruthenium-based nanohybrid compounds. J. Alloy. Compd. 2020, 842, 155798. [Google Scholar] [CrossRef]
- Morag, A.; Shauloff, N.; Maman, N.; Froumin, N.; Ezersky, V.; Jelinek, R. Nickel Alloying Significantly Enhances the Power Density of Ruthenium-Based Supercapacitors. Batter. Supercaps 2020, 3, 946–952. [Google Scholar] [CrossRef]
- Guo, Y.; Zou, X.; Wei, Y.; Shu, L.; Li, A.; Zhang, J.; Wang, R. Synthesis of organic hybrid ruthenium oxide nanoparticles for high-performance supercapacitors. Electrochimica Acta 2023, 443, 141938. [Google Scholar]
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar]
- Xiong, C.; Li, T.; Dang, A.; Zhao, T.; Li, H.; Lv, H. Two-step approach of fabrication of three-dimensional MnO2-graphene-carbon nanotube hybrid as a binder-free supercapacitor electrode. J. Power Sources 2016, 306, 602–610. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Li, K.; Wu, Y.; Liu, H. Optimization of stabilization conditions for electrospun polyacrylonitrile nanofibers. Polym. Degrad. Stab. 2012, 97, 1511–1519. [Google Scholar] [CrossRef]
- Racik, K.M.; Guruprasad, K.; Mahendiran, M.; Madhavan, J.; Maiyalagan, T.; Raj, M.V.A. Enhanced electrochemical performance of MnO2/NiO nanocomposite for supercapacitor electrode with excellent cycling stability. J. Mater. Sci. Mater. Electron. 2019, 30, 5222–5232. [Google Scholar]
- Andrei, R.D.; Marinoiu, A.; Marin, E.; Enache, S.; Carcadea, E. Carbon Nanofibers Production via the Electrospinning Process. Energies 2020, 13, 3029. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Jaafar, J.; Ismail, A.F.; Hasbullah, H.; Abdullah, N.; Ismail, M.S. Preparation and characterization of Polyacrylonitrile/Manganese Dioxides- based Carbon Nanofibers via electrospinning process. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012006. [Google Scholar]
- Rehman, Z.U.; Raza, M.A.; Chishti, U.N.; Hussnain, A.; Maqsood, M.F.; Iqbal, M.J.; Latif, U. Role of Carbon Nanomaterials on Enhancing the Supercapacitive Performance of Manganese Oxide-Based Composite Electrodes. Arab. J. Sci. Eng. 2022, 48, 8371–8386. [Google Scholar]
- Zhao, Y.; Misch, J.; Wang, C.-A. Facile synthesis and characterization of MnO2 nanomaterials as supercapacitor electrode materials. J. Mater. Sci. Mater. Electron. 2016, 27, 5533–5542. [Google Scholar] [CrossRef]
- Ning, P.; Duan, X.; Ju, X.; Lin, X.; Tong, X.; Pan, X.; Wang, T.; Li, Q. Facile synthesis of carbon nanofibers/MnO2 nanosheets as high-performance electrodes for asymmetric supercapacitors. Electrochim. Acta 2016, 210, 754–761. [Google Scholar]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2010, 40, 1697–1721. [Google Scholar]
- Arunachalam, R.; Prataap, R.K.; Raj, R.P.; Mohan, S.; Vijayakumar, J.; Péter, L.; Al Ahmad, M. Pulse electrodeposited RuO2 electrodes for high-performance supercapacitor applications. Surf. Eng. 2019, 35, 102–108. [Google Scholar]
- Zhao, X.; Du, Y.; Li, Y.; Zhang, Q. Encapsulation of manganese oxides nanocrystals in electrospun carbon nanofibers as free-standing electrode for supercapacitors. Ceram. Int. 2015, 41, 7402–7410. [Google Scholar]
- Shaheen, I.; Ahmad, K.S.; Zequine, C.; Gupta, R.K.; Thomas, A.G.; Malik, M.A. Facile ZnO-based nanomaterial and its fabrication as a supercapacitor electrode: Synthesis, characterization and electrochemical studies. RSC Adv. 2021, 11, 23374–23384. [Google Scholar]
- Wang, H.; Shen, C.; Liu, J.; Zhang, W.; Yao, S. Three-dimensional MnCo2O4/graphene composites for supercapacitor with promising electrochemical properties. J. Alloy. Compd. 2019, 792, 122–129. [Google Scholar]
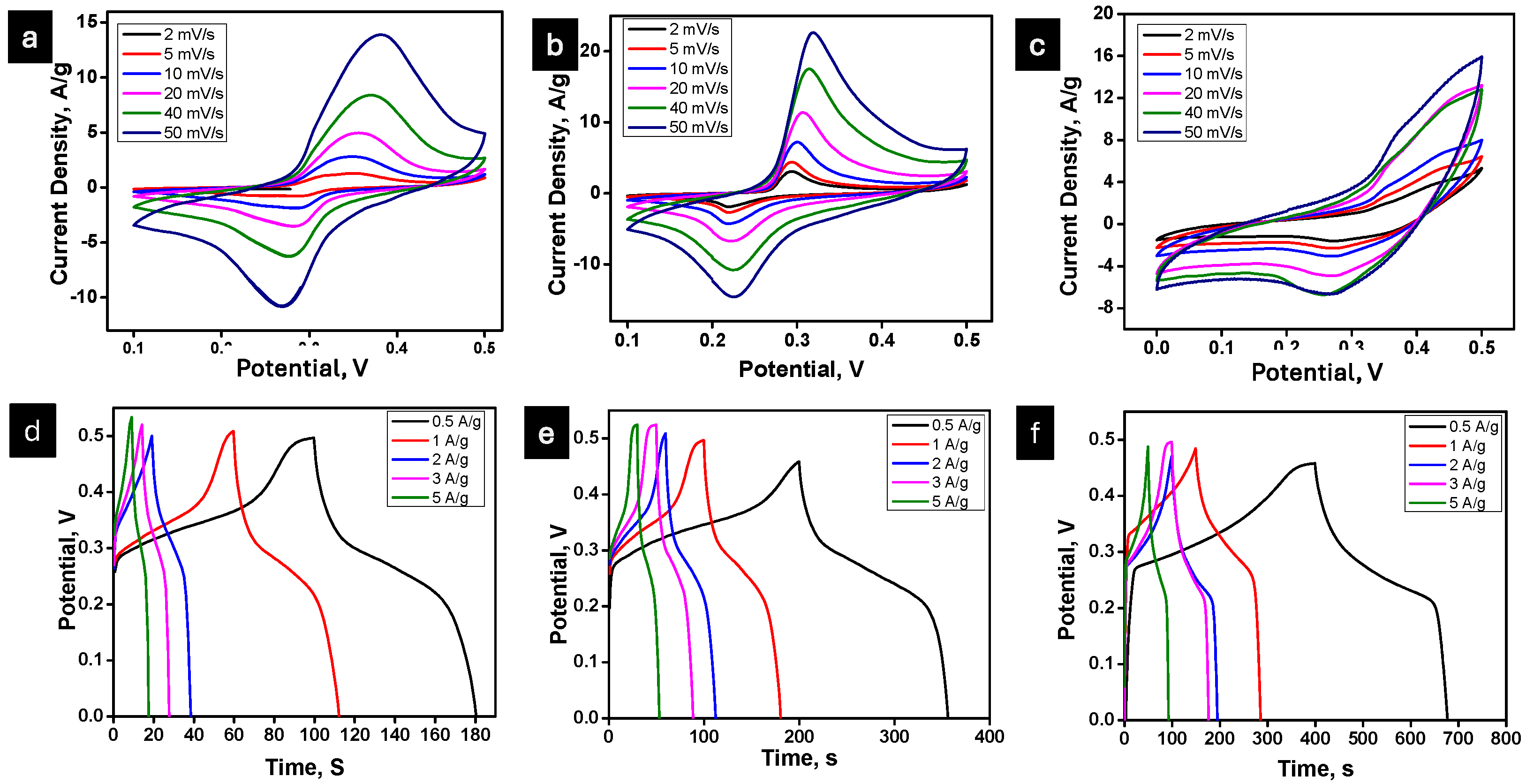
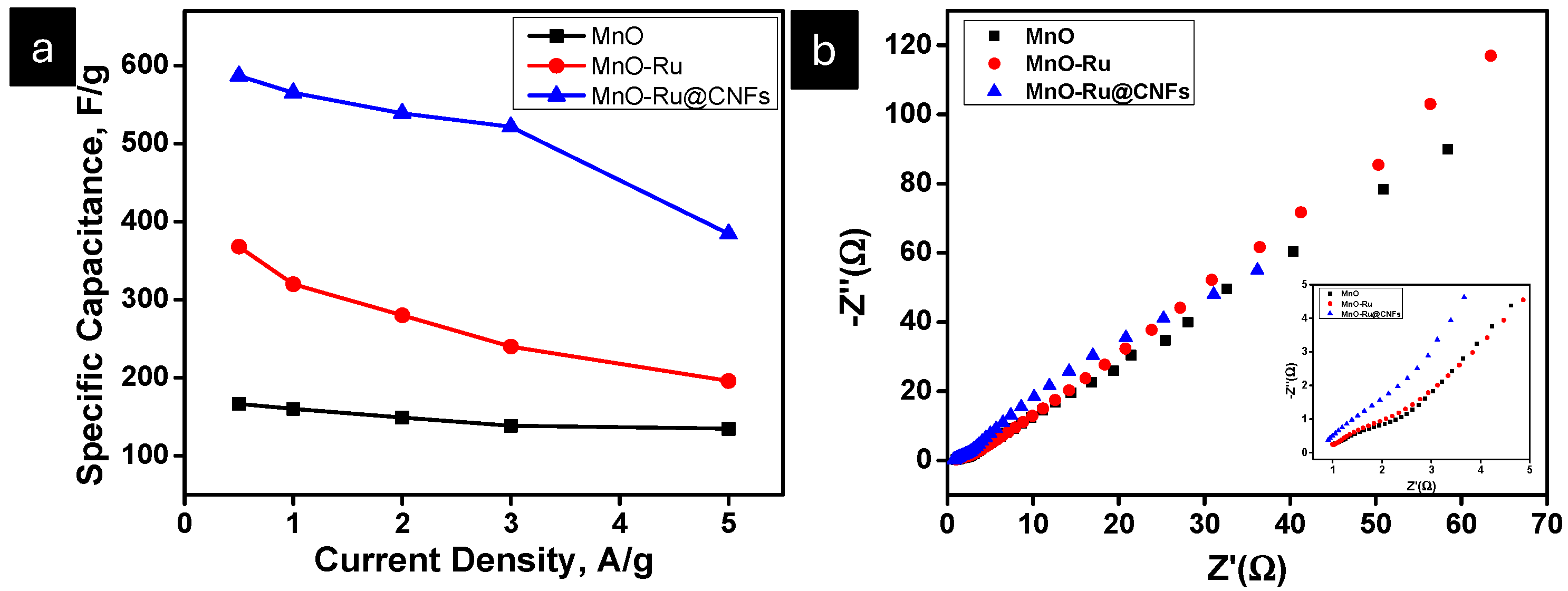
| Specific Capacitance (F/g) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Scan Rate (mV/s) | Current Density (A/g) | |||||||||
| 2 | 5 | 10 | 20 | 40 | 50 | 0.5 | 1 | 2 | 3 | 5 | |
| MnO | 210 | 85 | 72 | 65 | 59 | 51 | 166 | 160 | 149 | 138 | 128 |
| MnO-Ru | 350 | 200 | 120 | 67 | 61 | 53 | 366 | 320 | 300 | 280 | 196 |
| MnO-Ru@CNFs | 688 | 413 | 376 | 157 | 89 | 81 | 586 | 565 | 539 | 521 | 384 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.; Adami, R.; Gallucci, L.; Cirillo, C.; Iuliano, M.; Sesti Osséo, L.; Sarno, M. MnO Recovered from Alkaline Batteries Functionalized with Ruthenium and Carbon Nanofibers for Supercapacitor Applications. Eng. Proc. 2025, 90, 71. https://doi.org/10.3390/engproc2025090071
Khan F, Adami R, Gallucci L, Cirillo C, Iuliano M, Sesti Osséo L, Sarno M. MnO Recovered from Alkaline Batteries Functionalized with Ruthenium and Carbon Nanofibers for Supercapacitor Applications. Engineering Proceedings. 2025; 90(1):71. https://doi.org/10.3390/engproc2025090071
Chicago/Turabian StyleKhan, Faraz, Renata Adami, Luca Gallucci, Claudia Cirillo, Mariagrazia Iuliano, Libero Sesti Osséo, and Maria Sarno. 2025. "MnO Recovered from Alkaline Batteries Functionalized with Ruthenium and Carbon Nanofibers for Supercapacitor Applications" Engineering Proceedings 90, no. 1: 71. https://doi.org/10.3390/engproc2025090071
APA StyleKhan, F., Adami, R., Gallucci, L., Cirillo, C., Iuliano, M., Sesti Osséo, L., & Sarno, M. (2025). MnO Recovered from Alkaline Batteries Functionalized with Ruthenium and Carbon Nanofibers for Supercapacitor Applications. Engineering Proceedings, 90(1), 71. https://doi.org/10.3390/engproc2025090071









