Proteotoxicity and Apical Toxicity of Nicosulfuron to Danio rerio Embryos: A Comprehensive Assessment at Different Temperatures and pH
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Compound
2.2. Fish Maintenance
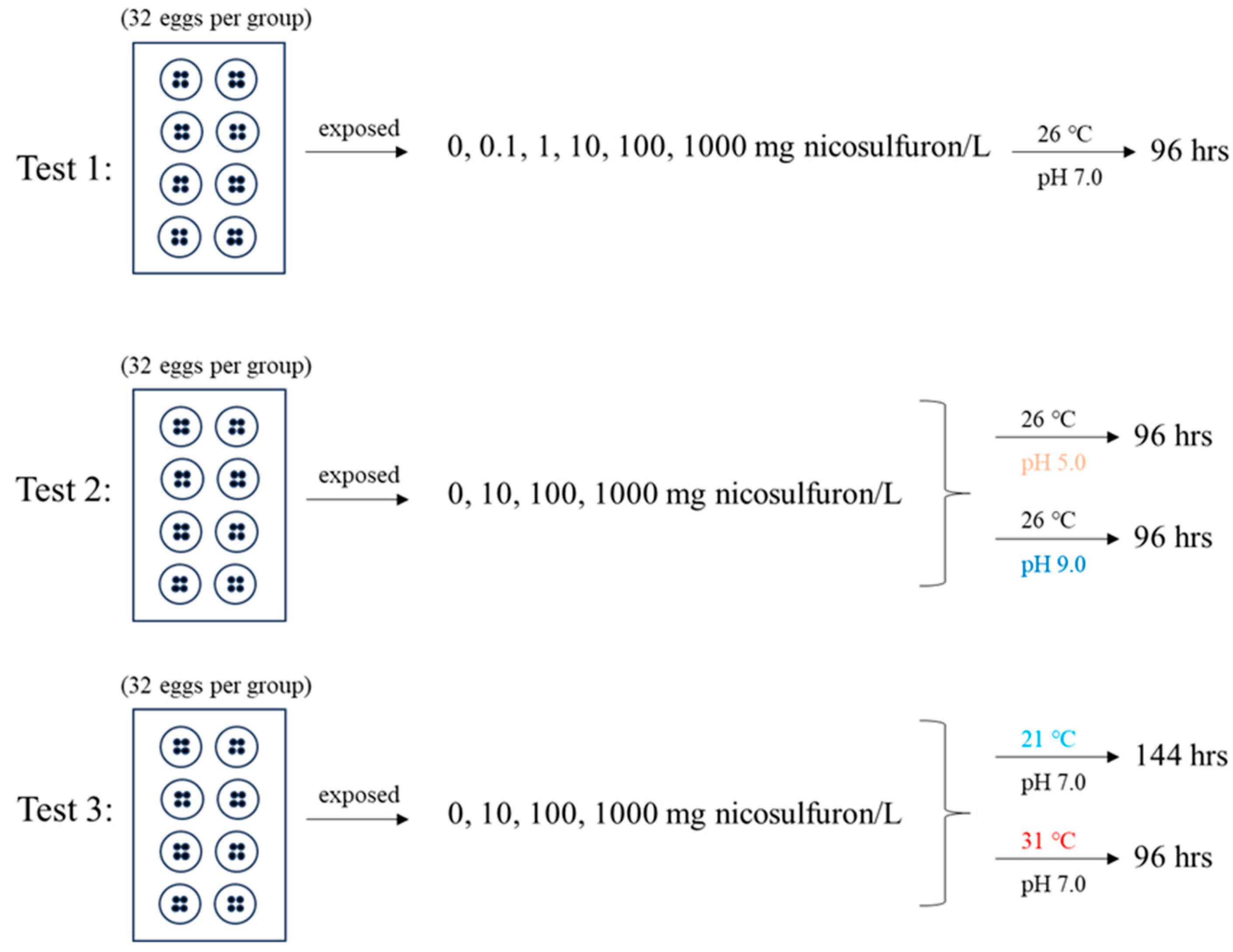
2.3. Fish Embryo Test
2.4. Hsp70 Quantification in Zebrafish Embryos
2.5. Statistic Analysis
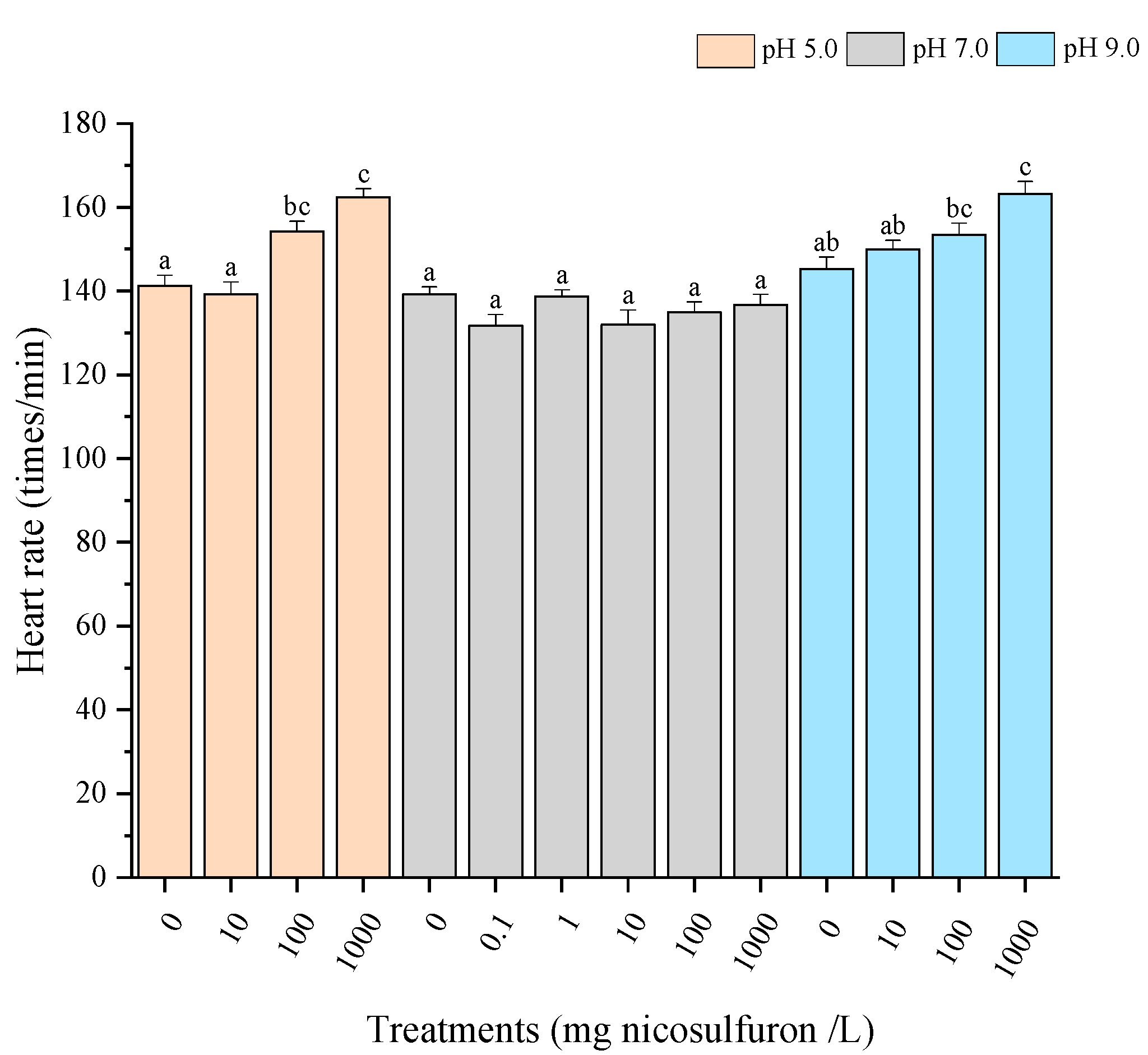
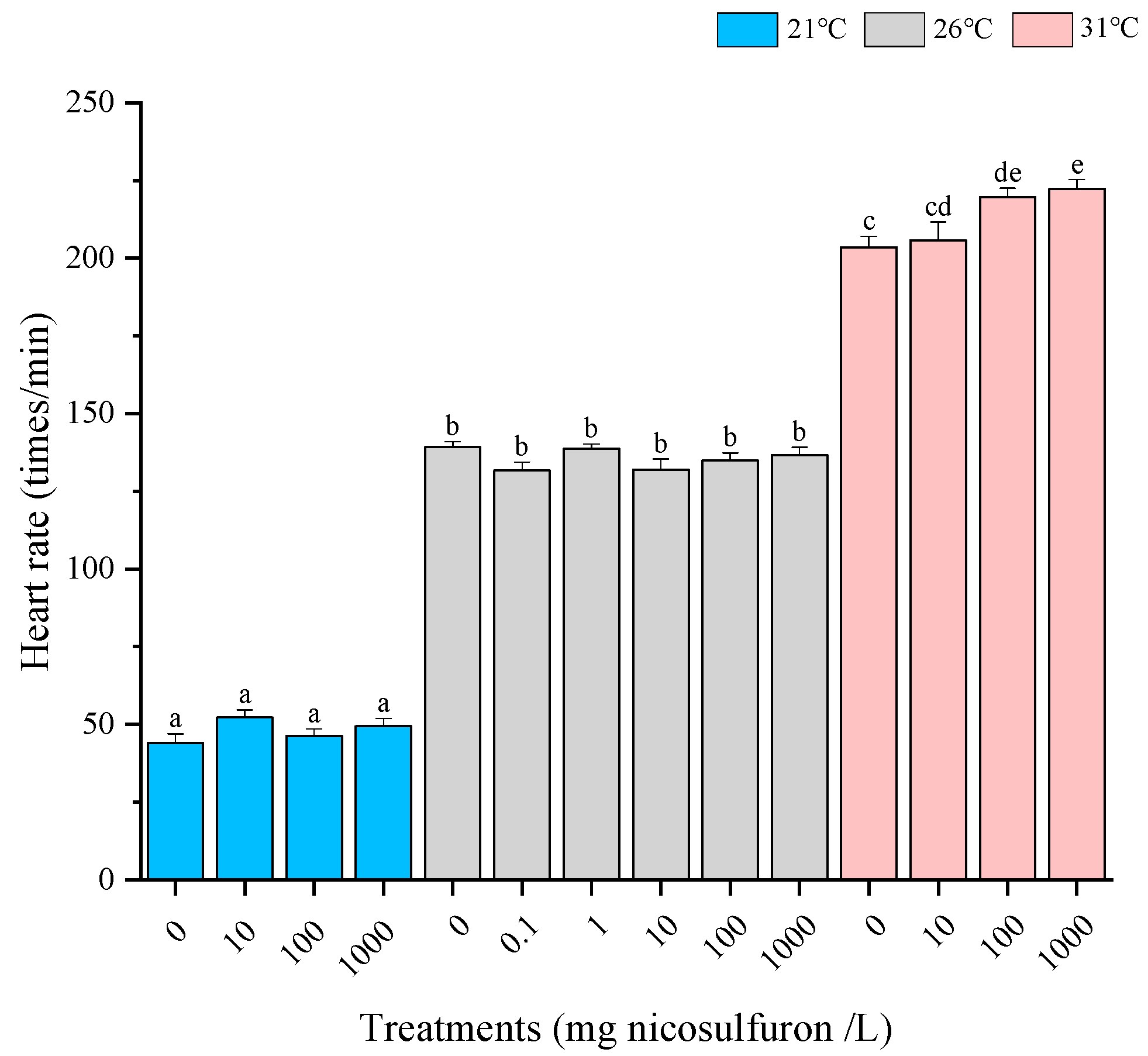
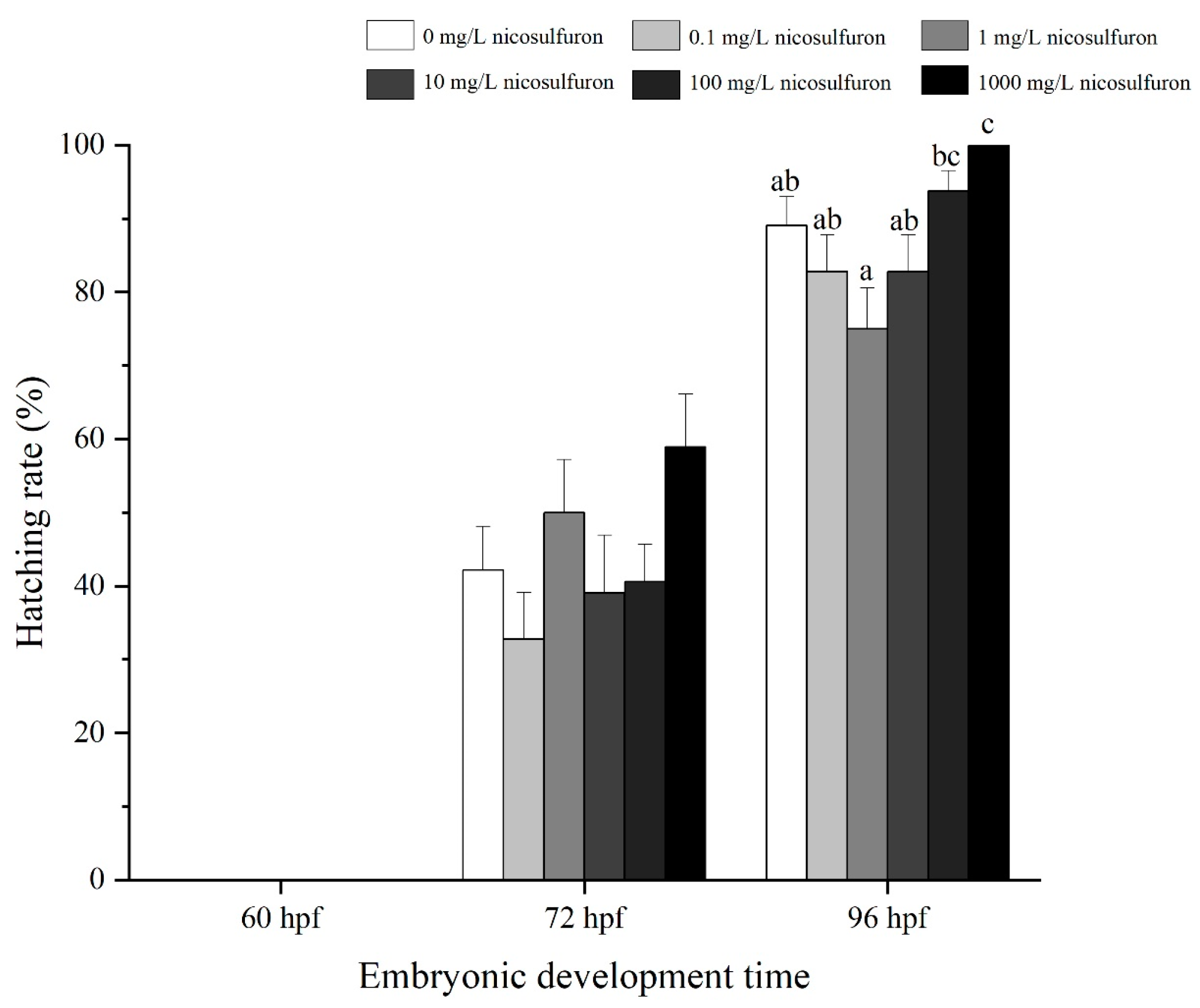
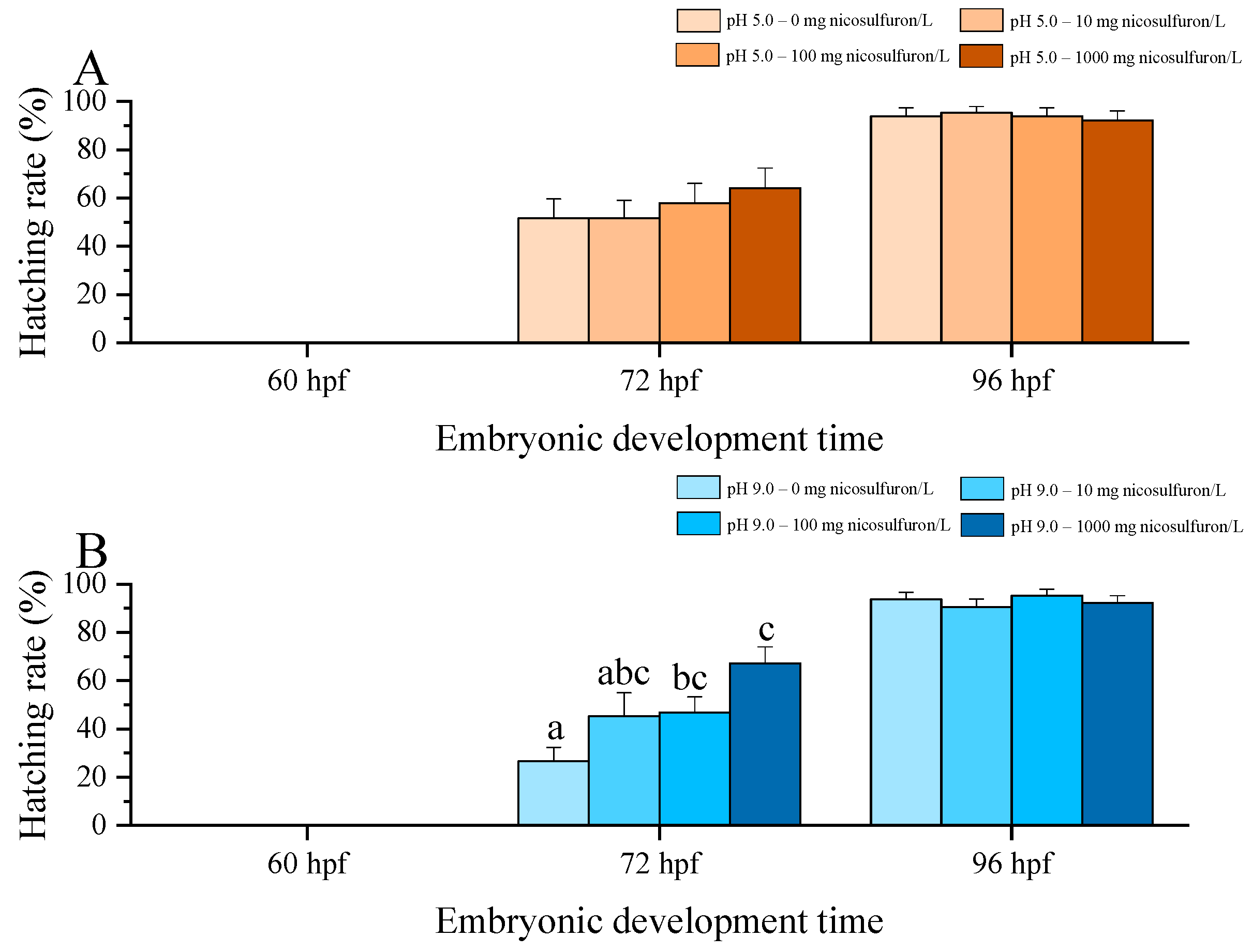
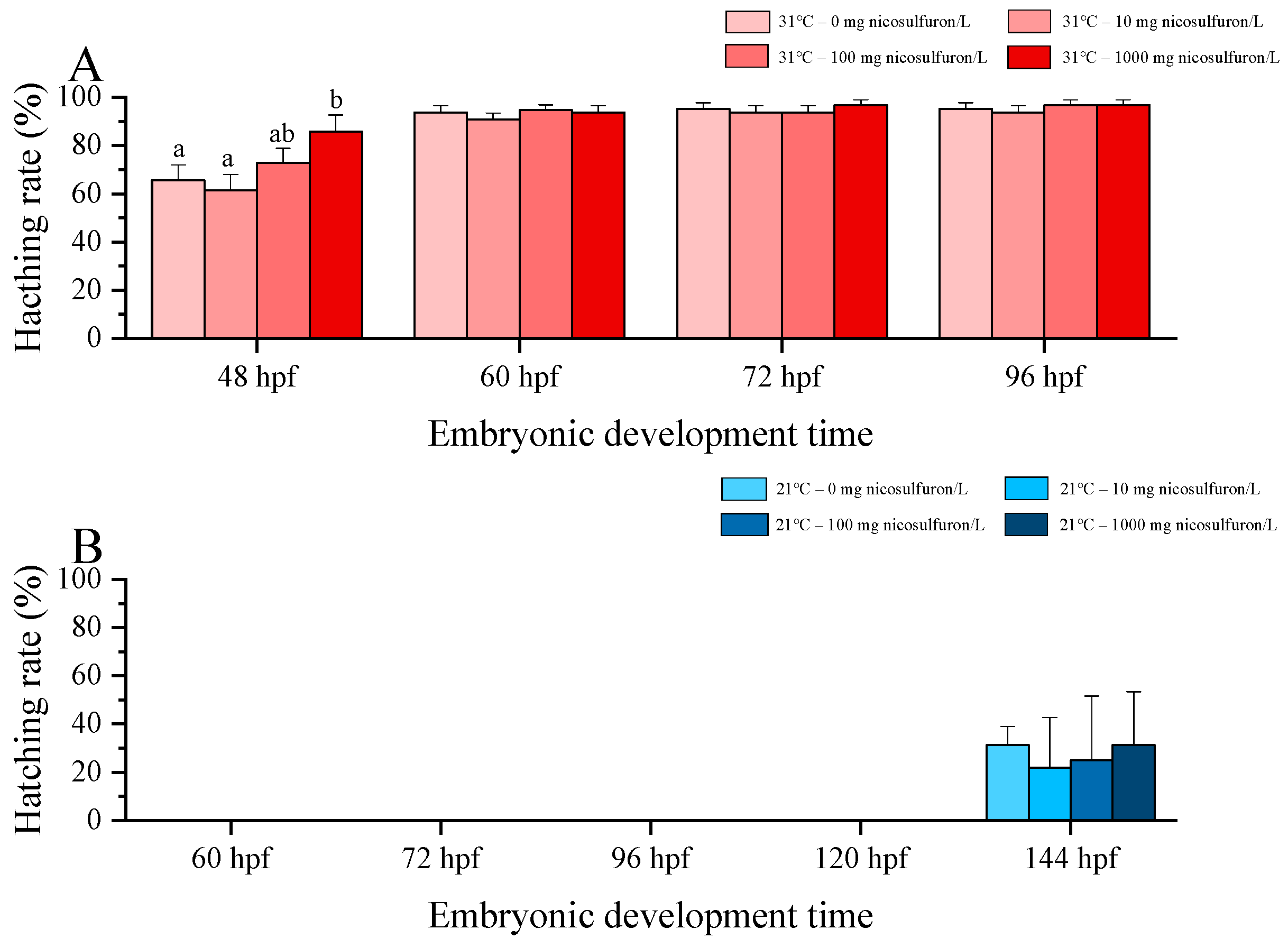
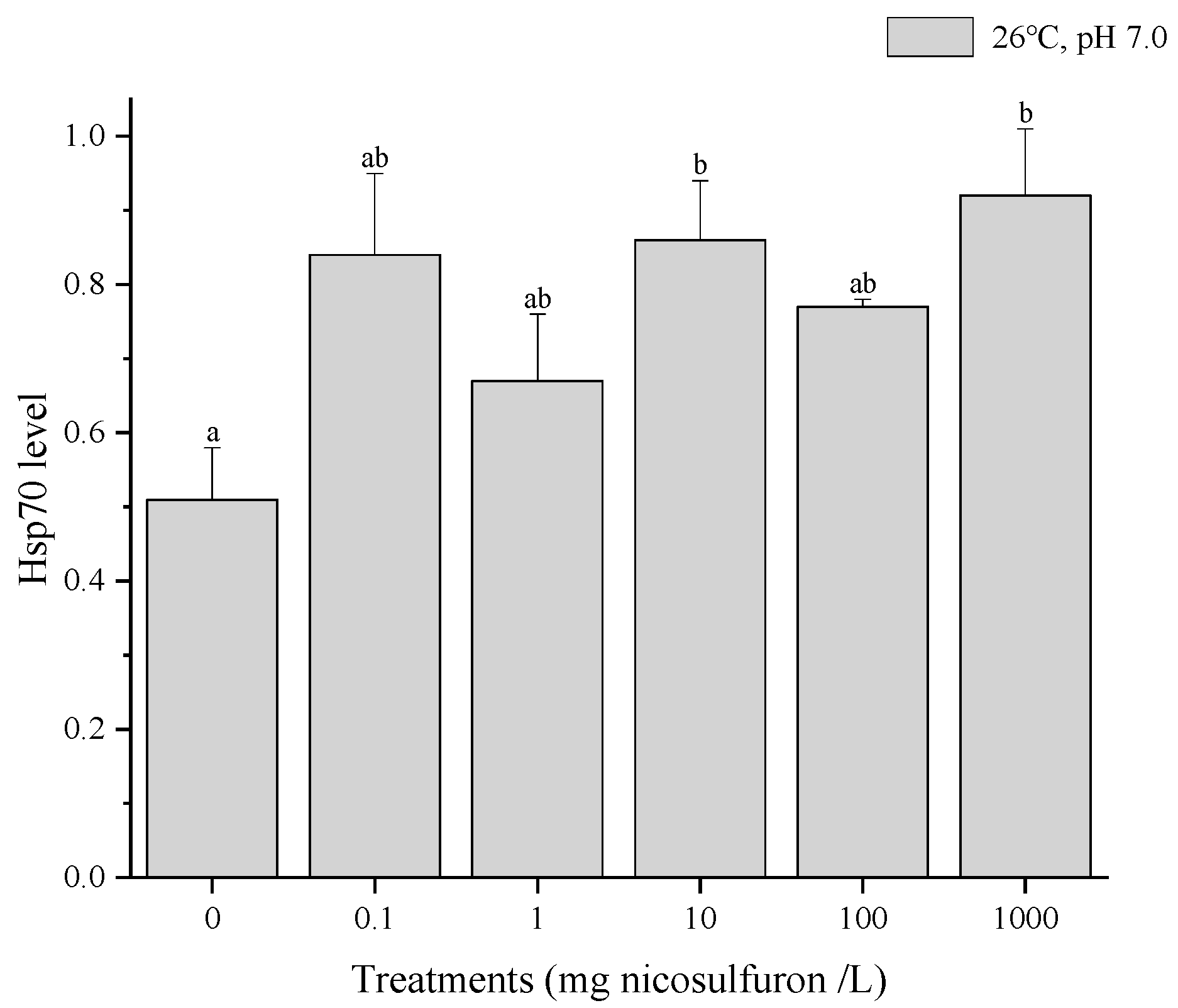
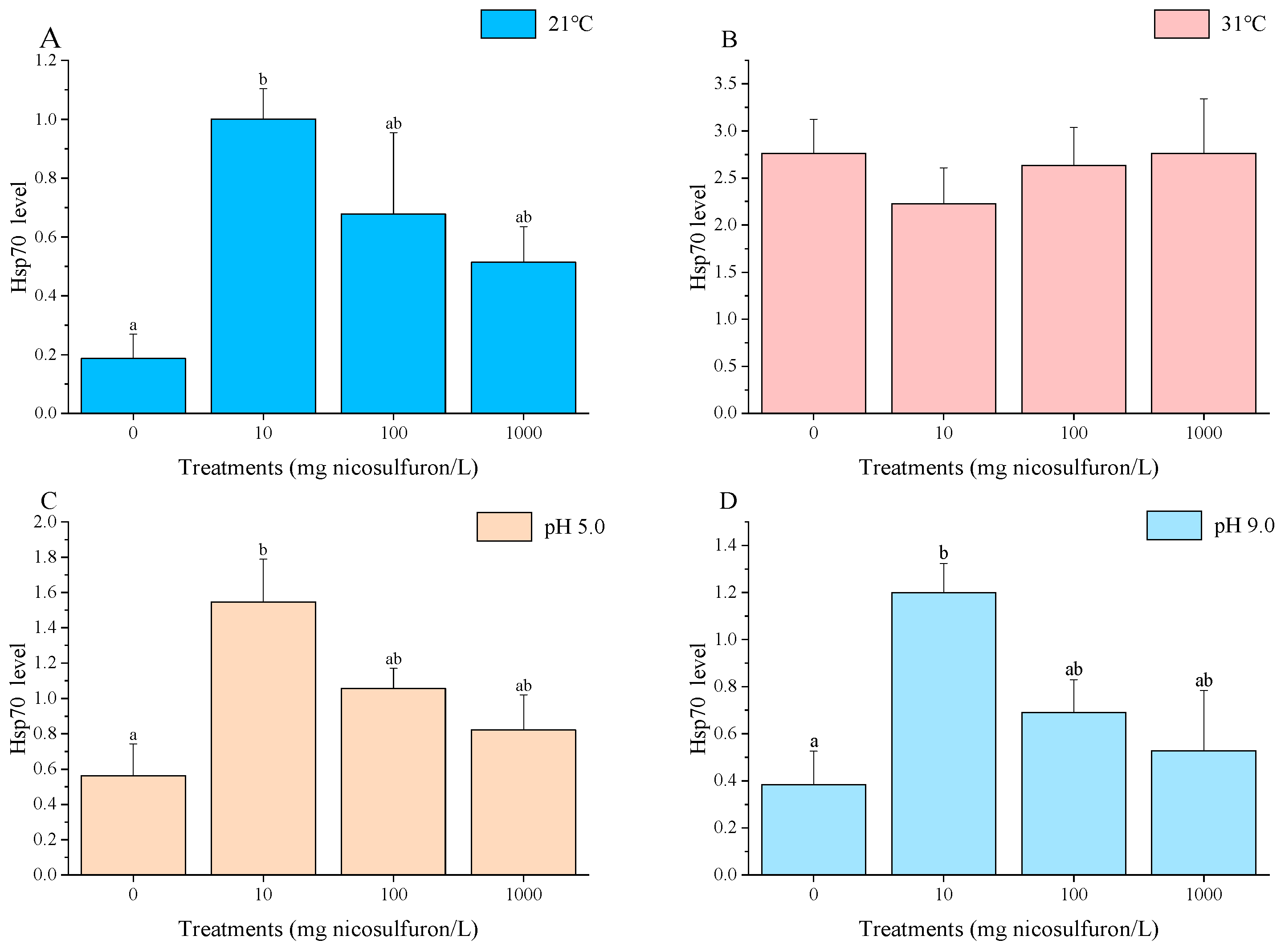
3. Results
4. Discussion
5. Conclusions
- (1)
- Data for the survival of zebrafish embryos exposed to nicosulfuron at different temperatures and pH indicate that nicosulfuron has a low acute toxicity to zebrafish embryos.
- (2)
- Environmental factors (pH and temperature) increased the sublethal toxic effects of nicosulfuron, as evidenced by earlier hatching, elevated heart rate and elevated Hsp70 levels. Although there were no significant differences in survival rates in this experiment, the role of environmental factors is still noteworthy.
- (3)
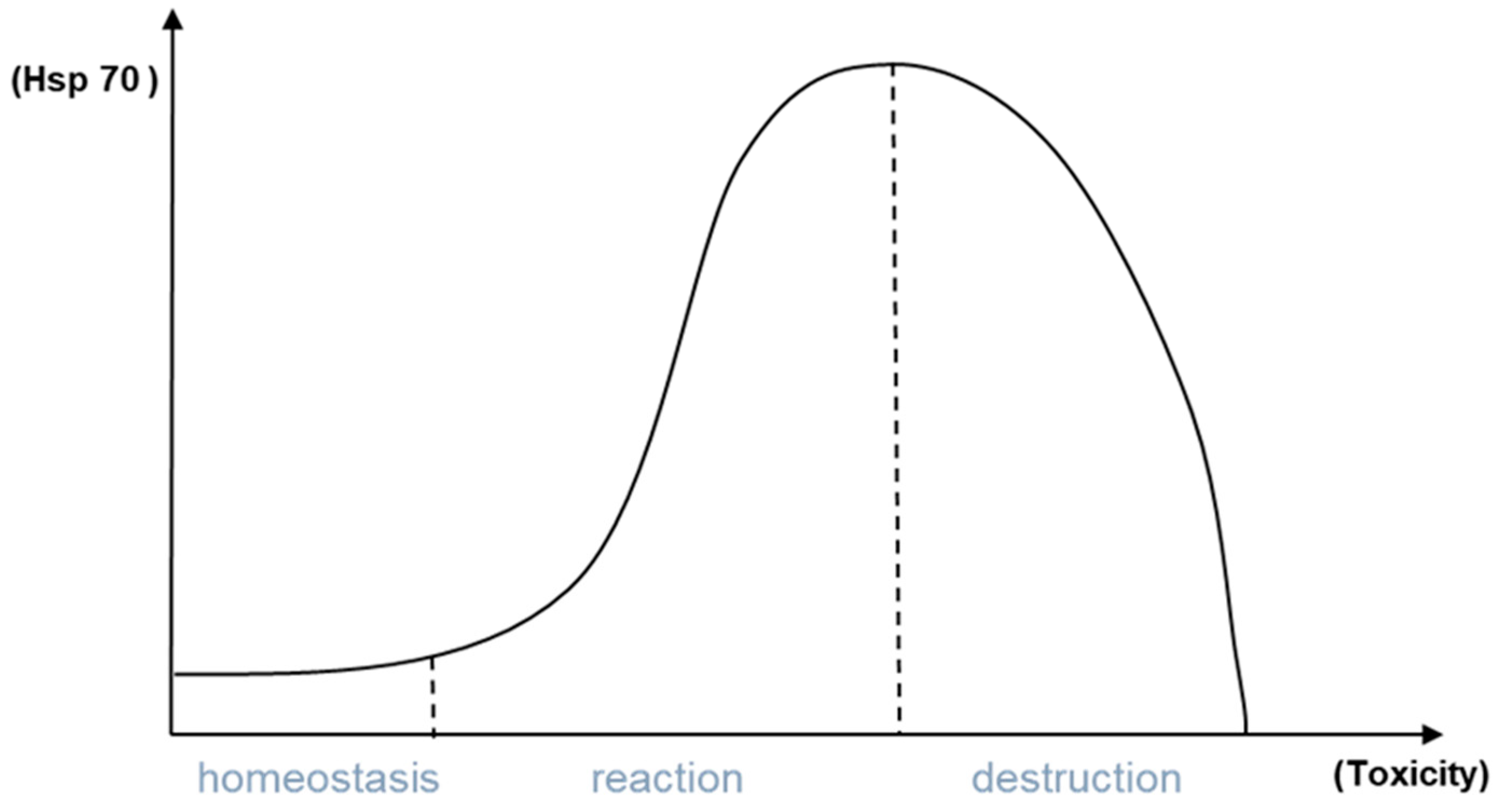
- Hsp70 responses can be regarded as a sensitive biomarker for sublethal chemical stress but lose their indicative power when the organism’s physiology is dominated by a concomitant heat shock.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liess, M.; Liebmann, L.; Vormeier, P.; Weisner, O.; Altenburger, R.; Borchardt, D.; Brack, W.; Chatzinotas, A.; Escher, B.; Foit, K.; et al. Pesticides Are the Dominant Stressors for Vulnerable Insects in Lowland Streams. Water Res. 2021, 201, 117262. [Google Scholar] [CrossRef] [PubMed]
- Mohaupt, V.; Völker, J.; Altenburger, R.; Birk, S.; Kirst, I.; Kühnel, D.; Küster, E.; Semerádová, S.; Šubelj, G.; Whalley, C. Pesticides in European Rivers, Lakes and Groundwaters—Data Assessment. ETC/ICM Technical Report 2020. Available online: https://www.eionet.europa.eu/etcs/etc-icm/products/etc-icm-reports/etc-icm-report-1-2020-pesticides-in-european-rivers-lakes-and-groundwaters-data-assessment (accessed on 31 July 2020).
- Schäffer, A.; Filser, J.; Frische, T.; Gessner, M.; Köck, W.; Kratz, W.; Liess, M.; Nuppenau, E.-A.; Roß-Nickoll, M.; Schäfer, R.; et al. Der Stumme Frühling: Zur Notwendigkeit Eines Umweltverträglichen Pflanzenschutzes; Diskussion/Deutsche Akademie der Naturforscher Leopoldina; Deutsche Akademie der Naturforscher Leopoldina e.V.—Nationale Akademie der Wissenschaften: Halle (Saale), Germany, 2018; ISBN 978-3-8047-3858-4. Available online: https://www.leopoldina.org/uploads/tx_leopublication/2018_Diskussionspapier_Pflanzenschutzmittel.pdf (accessed on 20 May 2018).
- Carles, C.; Bouvier, G.; Lebailly, P.; Baldi, I. Use of Job-Exposure Matrices to Estimate Occupational Exposure to Pesticides: A Review. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Piel, C.; Pouchieu, C.; Carles, C.; Béziat, B.; Boulanger, M.; Bureau, M.; Busson, A.; Grüber, A.; Lecluse, Y.; Migault, L.; et al. Agricultural Exposures to Carbamate Herbicides and Fungicides and Central Nervous System Tumour Incidence in the Cohort AGRICAN. Environ. Int. 2019, 130, 104876. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wei, Z.; Song, J.; Qin, Q.; Yu, K.; Li, G.; Zhang, J.; Wu, W.; Yan, Y. Hydrolysis of Nicosulfuron under Acidic Environment Caused by Oxalate Secretion of a Novel Penicillium Oxalicum Strain YC-WM1. Sci. Rep. 2017, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Bretaud, S.; Toutant, J.-P.; Saglio, P. Effects of Carbofuran, Diuron, and Nicosulfuron on Acetylcholinesterase Activity in Goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2000, 47, 117–124. [Google Scholar] [CrossRef]
- Carles, L.; Rossi, F.; Besse-Hoggan, P.; Blavignac, C.; Leremboure, M.; Artigas, J.; Batisson, I. Nicosulfuron Degradation by an Ascomycete Fungus Isolated from Submerged Alnus Leaf Litter. Front. Microbiol. 2018, 9, 3167. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion Regarding the Peer Review of the Pesticide Risk Assessment of the Active Substance Nicosulfuron. EFSA 2007, 120, 1–91. [Google Scholar] [CrossRef]
- Carvalho, R.N.; Marinov, D.; Loos, R.; Napierska, D.; Chirico, N.; Lettieri, T. Monitoring-Based Exercise: Second Review of the Priority Substances List under the Water Framework Directive. JRC Science for Policy Report 2016. Available online: https://circabc.europa.eu/sd/a/7fe29322-946a-4ead-b3b9-e3b156d0c318/Monitoring-basedExerciseReport_FINALDRAFT_25nov2016.pdf (accessed on 20 January 2016).
- De Lafontaine, Y.; Beauvais, C.; Cessna, A.J.; Gagnon, P.; Hudon, C.; Poissant, L. Sulfonylurea Herbicides in an Agricultural Catchment Basin and Its Adjacent Wetland in the St. Lawrence River Basin. Sci. Total Environ. 2014, 479–480, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moschet, C.; Wittmer, I.; Simovic, J.; Junghans, M.; Piazzoli, A.; Singer, H.; Stamm, C.; Leu, C.; Hollender, J. How a Complete Pesticide Screening Changes the Assessment of Surface Water Quality. Environ. Sci. Technol. 2014, 48, 5423–5432. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Furlong, E.T.; Burkhardt, M.R.; Peter, C.J. Occurrence of Sulfonylurea, Sulfonamide, Imidazolinone, and Other Herbicides in Rivers, Reservoirs and Ground Water in the Midwestern United States, 1998. Sci. Total Environ. 2000, 248, 123–133. [Google Scholar] [CrossRef]
- Cheron, M.; Costantini, D.; Brischoux, F. Nicosulfuron, a Sulfonylurea Herbicide, Alters Embryonic Development and Oxidative Status of Hatchlings at Environmental Concentrations in an Amphibian Species. Ecotoxicol. Environ. Saf. 2022, 232, 113277. [Google Scholar] [CrossRef] [PubMed]
- Hackenberger, D.K.; Stjepanović, N.; Lončarić, Ž.; Hackenberger, B.K. Acute and Subchronic Effects of Three Herbicides on Biomarkers and Reproduction in Earthworm Dendrobaena Veneta. Chemosphere 2018, 208, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Saglio, K.H.; Olsén, S.; Bre, P. Behavioral and Olfactory Responses to Prochloraz, Bentazone, and Nicosulfuron-Contaminated Flows in Goldfish. Arch. Environ. Contam. Toxicol. 2001, 41, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Heugens, E.H.W.; Hendriks, A.J.; Dekker, T.; Straalen, N.M.V.; Admiraal, W. A Review of the Effects of Multiple Stressors on Aquatic Organisms and Analysis of Uncertainty Factors for Use in Risk Assessment. Crit. Rev. Toxicol. 2001, 31, 247–284. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Gong, Z.; Kelly, B.C. Assessing pH-Dependent Toxicity of Fluoxetine in Embryonic Zebrafish Using Mass Spectrometry-Based Metabolomics. Sci. Total Environ. 2019, 650, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Osterauer, R. Temperature-Dependent Effects of the Pesticides Thiacloprid and Diazinon on the Embryonic Development of Zebrafish (Danio rerio). Aquat. Toxicol. 2008, 86, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Köhler, H.-R.; Gräff, T.; Schweizer, M.; Blumhardt, J.; Burkhardt, J.; Ehmann, L.; Hebel, J.; Heid, C.; Kundy, L.; Kuttler, J.; et al. LogD-Based Modelling and ΔlogD as a Proxy for pH-Dependent Action of Ionizable Chemicals Reveal the Relevance of Both Neutral and Ionic Species for Fish Embryotoxicity and Possess Great Potential for Practical Application in the Regulation of Chemicals. Water Res. 2023, 235, 119864. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Von Der Ohe, P.C.; Köhler, H.-R.; Sellier, O.; Junghans, M. Aquatic Thresholds for Ionisable Substances, Such as Diclofenac, Should Consider pH-Specific Differences in Uptake and Toxicity. Sci. Total Environ. 2024, 908, 168222. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The Embryo Test with the Zebrafish Danio rerio—A General Model in Ecotoxicology and Toxicology. ALTEX 2002, 19, 38–48. [Google Scholar]
- Schweizer, M.; Von Der Ohe, P.C.; Gräff, T.; Kühnen, U.; Hebel, J.; Heid, C.; Kundy, L.; Kuttler, J.; Moroff, F.-M.; Schlösinger, A.-F.; et al. Heart Rate as an Early Warning Parameter and Proxy for Subsequent Mortality in Danio Rerio Embryos Exposed to Ionisable Substances. Sci. Total Environ. 2022, 818, 151744. [Google Scholar] [CrossRef]
- Yang, L.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Müller, F.; Strähle, U. Zebrafish Embryos as Models for Embryotoxic and Teratological Effects of Chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Lian, D.; Bai, Y.; Wang, Y.Y.L.; Zhang, D.; Wang, Z.; You, J. The Feasibility of the Zebrafish Embryo as a Promising Alternative for Acute Toxicity Test Using Various Fish Species: A Critical Review. Sci. Total Environ. 2021, 787, 147705. [Google Scholar] [CrossRef]
- Schweizer, M.; Dieterich, A.; Triebskorn, R.; Köhler, H.-R. Drifting Away of a FET Endpoint: The Heart Rate in Danio rerio Embryos Is Extremely Sensitive to Variation in Ambient Temperature. Bull. Environ. Contam. Toxicol. 2017, 99, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Braunbeck, T.; Böttcher, M.; Hollert, H.; Kosmehl, T.; Lammer, E.; Leist, E.; Rudolf, M.; Seitz, N. Towards an Alternative for the Acute Fish LC50 Test in Chemical Assessment: The Fish Embryo Toxicity Test Goes Multi-species—An Update. ALTEX 2005, 22, 87–102. [Google Scholar] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013; Available online: https://www.oecd-ilibrary.org/environment/test-no-236-fish-embryo-acute-toxicity-fet-test_9789264203709-en (accessed on 26 July 2013).
- Schweizer, M.; Brilisauer, K.; Triebskorn, R.; Forchhammer, K.; Köhler, H.-R. How Glyphosate and Its Associated Acidity Affect Early Development in Zebrafish (Danio rerio). PeerJ 2019, 7, e7094. [Google Scholar] [CrossRef] [PubMed]
- Vincze, K.; Graf, K.; Scheil, V.; Köhler, H.-R.; Triebskorn, R. Embryotoxic and Proteotoxic Effects of Water and Sediment from the Neckar River (Southern Germany) to Zebrafish (Danio rerio) Embryos. Environ. Sci. Eur. 2014, 26, 3. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Federal Environment Agency. EQS Data sheet Environmental Quality Standard-Nicosulfuron. Available online: https://webetox.uba.de/webETOX/public/basics/literatur/download.do;jsessionid=0234A4C8381BA06103A08847D06BBBAC?id=19 (accessed on 20 May 2014).
- Tan, K.S.; Zhang, Y.; Liu, L.; Li, S.; Zou, X.; Zeng, W.; Cheng, G.; Wang, D.; Tan, W. Molecular Cloning and Characterization of an Atypical Butyrylcholinesterase-like Protein in Zebrafish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 255, 110590. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E. The Hormetic Response of Heart Rate of Fish Embryos to Contaminants—Implications for Research and Policy. Sci. Total Environ. 2022, 815, 152911. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Bittner, L.; Teixidó, E.; Keddi, I.; Escher, B.I.; Klüver, N. pH-Dependent Uptake and Sublethal Effects of Antihistamines in Zebrafish (Danio rerio) Embryos. Environ. Toxicol. Chem. 2019, 38, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.S.; Henriques, J.F.; Almeida, A.R.; Soares, A.M.V.M.; Scholz, S.; Domingues, I. Zebrafish Embryo Tolerance to Environmental Stress Factors—Concentration–Dose Response Analysis of Oxygen Limitation, pH, and UV-light Irradiation. Environ. Toxicol. Chem. 2017, 36, 682–690. [Google Scholar] [CrossRef]
- Baldisserotto, B. Water pH and Hardness Affect Growth of Freshwater Teleosts. R. Bras. Zootec. 2011, 40, 138–144. [Google Scholar]
- Bolner, K.C.S.; Baldisserotto, B. Water pH and Urinary Excretion in Silver Catfish Rhamdia quelen. J. Fish Biol. 2007, 70, 50–64. [Google Scholar] [CrossRef]
- Westerheide, S.D.; Morimoto, R.I. Heat Shock Response Modulators as Therapeutic Tools for Diseases of Protein Conformation. J. Biol. Chem. 2005, 280, 33097–33100. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, J.; Welsh, J.P.; Manthiram, K.; Swartz, J.R. Comparing the Functional Properties of the Hsp70 Chaperones, DnaK and BiP. Biophys. Chem. 2010, 149, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat Shock Proteins in Toxicology: How Close and How Far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef]
- Hallare, A.; Kosmehl, T.; Schulze, T.; Hollert, H.; Kohler, H.; Triebskorn, R. Assessing Contamination Levels of Laguna Lake Sediments (Philippines) Using a Contact Assay with Zebrafish (Danio rerio) Embryos. Sci. Total Environ. 2005, 347, 254–271. [Google Scholar] [CrossRef]
- Eckwert, H.; Alberti, G.; Kohler, H.-R. The induction of stress proteins (hsp) in Oniscus asellus (Isopoda) as a molecular marker of multiple heavy metal exposure: I. Principles and toxicological assessment. Ecotoxicology 1997, 6, 249–262. [Google Scholar] [CrossRef]
- Mei, Y.; Si, C.; Liu, M.; Qiu, L.; Zheng, M. Investigation of Resistance Levels and Mechanisms to Nicosulfuron Conferred by Non-Target-Site Mechanisms in Large Crabgrass (Digitaria sanguinalis L.) from China. Pestic. Biochem. Physiol. 2017, 141, 84–89. [Google Scholar] [CrossRef]
- Cao, Y.; Lan, Y.; Huang, H.; Wei, S.; Li, X.; Sun, Y.; Wang, R.; Huang, Z. Molecular Characterization of Resistance to Nicosulfuron in Setaria Viridis. Int. J. Mol. Sci. 2023, 24, 7105. [Google Scholar] [CrossRef] [PubMed]
| Properties | |
|---|---|
| Molecular Formula | C15H18N6O6S |
| Molecular Weight | 410.4 g/mol |
| Solubility in water | 7400 mg/L (at pH 7, 25 °C) |
| log Pow (at pH 5) | 0.35 |
| log Pow (at pH 7) | 1.8 |
| log Pow (at pH 9) | −2.0 |
| pKa | 4.60 |
| Endpoints | 12 hpf | 24 hpf | 48 hpf | 60 hpf | 72 hpf | 96 hpf |
|---|---|---|---|---|---|---|
| Mortality | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Developmental Delays 1 | ✓ | |||||
| Heart rate | ✓ | |||||
| Hatching success | ✓ | ✓ | ✓ | |||
| Malformations 2 | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Köhler, H.-R.; Triebskorn, R. Proteotoxicity and Apical Toxicity of Nicosulfuron to Danio rerio Embryos: A Comprehensive Assessment at Different Temperatures and pH. Pollutants 2024, 4, 359-372. https://doi.org/10.3390/pollutants4030025
Li Z, Köhler H-R, Triebskorn R. Proteotoxicity and Apical Toxicity of Nicosulfuron to Danio rerio Embryos: A Comprehensive Assessment at Different Temperatures and pH. Pollutants. 2024; 4(3):359-372. https://doi.org/10.3390/pollutants4030025
Chicago/Turabian StyleLi, Zequn, Heinz-R. Köhler, and Rita Triebskorn. 2024. "Proteotoxicity and Apical Toxicity of Nicosulfuron to Danio rerio Embryos: A Comprehensive Assessment at Different Temperatures and pH" Pollutants 4, no. 3: 359-372. https://doi.org/10.3390/pollutants4030025
APA StyleLi, Z., Köhler, H.-R., & Triebskorn, R. (2024). Proteotoxicity and Apical Toxicity of Nicosulfuron to Danio rerio Embryos: A Comprehensive Assessment at Different Temperatures and pH. Pollutants, 4(3), 359-372. https://doi.org/10.3390/pollutants4030025






