Abstract
The emergence of infectious disease outbreaks and ‘superbugs’ related to pollution combined with climate change is a current problem, not just a future threat. In May 2024, an extreme flood hit the Rio Grando Sul State, southern Brazil, triggering an important leptospirosis outbreak in urban settings with deficient sanitation systems. This and other cases discussed in this article exemplify how extreme weather events exacerbate the consequences of environmental pollution by multiple classes of pathogens in the global scenario of increasing anthropogenic pressures on the environment. A combination of actions to combat climate change and improvements in sanitation systems is essential to mitigate this problem.
1. Introduction
The participation of the environment as an intermediary piece in the puzzle of zoonotic spillover events has been increasingly recognized, with pathogens derived from a reservoir host being released into the environment and then infecting a new host or population [1]. Mounting evidence has indeed shown that pathogens, including antibiotic-resistant bacteria, viruses and parasites derived from humans and other animals, pervasively contaminate the environment, where they can become ‘superbugs’ or spread in new host populations. This can trigger deleterious and unexpected consequences, including potentially untreatable infections and diseases outbreaks [2,3,4]. It is noteworthy that environmental stressors such as pollutants and extreme heat may cause immunosuppression and disrupt the microbiota of reservoir hosts, potentially increasing the shedding of pathogens into the environment [5,6,7]. Also, climate change and related extreme weather events facilitate biodiversity loss and induce changes in the dynamics of disease vectors, which are both factors linked to the increased risk of infectious disease emergence and spread [8,9].
During extreme floods and other water-related weather events (e.g., hurricanes, heavy rainfalls, severe storms), pollution facilitates outbreaks of diseases caused by several pathogens, including viruses (e.g., adenovirus, enterovirus, hepatitis viruses, norovirus, and rotavirus), bacteria (e.g., Vibrio cholerae, Leptospira spp., Escherichia coli, and Salmonella spp.), protozoa/parasites (e.g., Cryptosporidium spp., Giardia lamblia, Schistosoma mansoni, and Leishmania spp.), and fungi (e.g., Aspergillus fumigatus and Scedosporium spp.) [10,11,12,13].
Although the scientific community already recognizes the climate emergency as an urgent and current health problem [14,15,16], many countries, communities, and industry sectors are still slow to apply solutions to curb climate change, suggesting that the consequences of climate change are problems to be faced in a distant future. However, remaining oblivious to such problems is a dangerous behavior. Furthermore, pollution caused by pathogens released into the environment through untreated sewage (among other routes), as well as pollution by chemicals that can accelerate the selection of multi-resistant microorganisms, are problems with medical and ecological importance. In this article, we briefly discuss some points regarding the connections between climate change, pollution, and emerging infectious diseases, highlighting that more than it being a problem to be faced in the future, this is a major ongoing process requiring urgent and bold action.
2. Methodological Notes
In order to carry out a brief review of infectious disease outbreaks and spread of pathogens caused by flood events around the world, searches were performed in the PubMed [17] and Google Scholar [18] databases (in August 2024) to obtain and select examples of flood-related human disease outbreaks (as will be discussed in Section 3). In order to obtain examples of different groups of pathogens, searches were performed using the terms “flood” + “outbreak” in association with “bacteria” or “fungi” or “parasite” or “protozoa” or “virus”. Articles in English were considered eligible, with no limitation regarding the year of publication. Works cited in the reference list of selected articles were also considered eligible. Three examples of each group of pathogens were selected for discussion in this work, valuing the studies that showed robust evidence of an association between the disease outbreak/pathogen spread and the flood event.
This article also compiled information on the 2024 flood-related leptospirosis outbreak observed in Rio Grande do Sul State, southern Brazil (as will be discussed in Section 4.1). Consolidated data on cases of leptospirosis in Rio Grande do Sul in the previous ten years were obtained from the epidemiological situation bulletins made available by the Brazilian Ministry of Health (Confirmed cases and deaths due to leptospirosis in Brazil and Major Regions of Brazil—2000 to 2024) [19].
Data on leptospirosis cases recorded in the Rio Grande do Sul in 2024 were obtained from epidemiological reports made available by the Secretary of Health of the Government of the Rio Grande do Sul State [20]. This is the body responsible for monitoring and disseminating data regarding cases of leptospirosis in Rio Grande do Sul in ‘real time’ (data are updated frequently as case numbers change significantly), including reports on cases related to the 2024 flooding event.
Finally, we highlight that much information about the 2024 extreme flood observed in southern Brazil and the leptospirosis outbreak described in this article express what the authors of this article observed in loco during the flood.
3. Flood-Related Infectious Disease Outbreaks in Different Countries: A Brief Review
Extreme weather events tend to intensify around the world due to climate change, increasing the risk of major floods and inundations in various world regions [21]. Other anthropogenic pressures on the environment exacerbate this problem. The deforestation of riverbeds increases the intensity of floods, as the loss of vegetation favors the water flow and facilitates soil loss [22]. Lack of flood risk management and insufficient urban planning increase the material damage caused by floods, also resulting in more people being affected [23]. Combined, these effects and conditions create serious environmental and public health problems, ranging from psychosocial problems to infectious diseases [24].
Even countries that are not traditionally affected by major floods and downpours are now experiencing these problems more frequently, as evidenced by severe floods observed recently in Germany [25] as well as in other European countries in the last decades [26]. This indicates that the health effects derived from floods will increasingly affect unexpected regions, not just populations in low- and middle-income countries from tropical regions [21]. Understanding the direct and indirect health impacts of floods is essential for establishing risk control and damage mitigation strategies.
Table 1 shows a brief review of infectious disease outbreaks and spread of pathogens caused by flood events around the world [27,28,29,30,31,32,33,34,35,36,37,38]. These examples demonstrate that a variety of pathogens affect human populations during and after floods, causing diseases ranging from mild gastrointestinal problems to more serious conditions that lead to hospitalization and even risk of death [39,40,41].

Table 1.
Examples of infectious disease outbreaks and spread of pathogens caused by flood/water-related events in different countries.
Infections caused during floods may be directly due to contact with contaminated water (e.g., leptospirosis, hepatitis) or, alternatively, some infections occur as a consequence of the environmental and sanitary conditions caused by the flood. For example, homes that remain damp for long periods are susceptible to the proliferation of pathogenic fungi [42], and environmental conditions created by floods can facilitate the dissemination of mosquito-borne diseases [43]. In addition, large-scale floods can impair or lead to the collapse of healthcare services, such as vaccination centers, hospitals, and other types of medical facilities [44], also contributing to the spread of diseases.
As exemplified in Table 1, several risk factors contribute to the spread of infectious diseases/pathogens during and after floods. Poor sanitation, especially deficiencies in sewage collection and treatment systems, and the intense presence of animals that transmit zoonoses in the region affected by the flood are relevant risk factors because they create favorable conditions for contamination of flood and drinking water with pathogens from domestic sewage as well as urine and feces of animals [12,45,46]. During floods, direct contact with flood water, especially of mucous membranes and skin with lacerated wounds, significantly increases the risk of infection [46]. After floods, contact with flood sludge and management of debris contaminated by flood water without personal protective equipment while cleaning houses and streets also creates conditions conducive to infections [47].
During floods, many people lose their homes and are taken in by public shelters. The crowding of many people in these places favors the spread of respiratory diseases, among others [12]. Finally, it is important to highlight that large-scale floods cause biodiversity loss and changes in ecological networks, both factors that act as drivers of zoonotic diseases [1,8]. In other words, in addition to known diseases, as shown in the examples described in Table 1, floods can create favorable conditions for the emergence of new diseases in the human population.
4. Climate Change and Extreme Weather Events Already Favor Multiple Pathogens and ‘Superbugs’
4.1. The 2024 Flood-Related Leptospirosis Outbreak in Rio Grande Do Sul State
Extreme rainfall linked to a combination of atmospheric factors and anthropogenic climate change has recently been observed in southern Brazil [48,49]. This extreme weather event triggered a major flood that affected 2,398,255 people in 478 municipalities in the state of Rio Grande do Sul [50], including Porto Alegre City (Figure 1), the state capital. The rains began at the end of April 2024, but the peak of the flood occurred in May 2024. Until the last data update (10 July 2024), 182 confirmed deaths and 806 injured people were recorded, and 29 people remained missing [50].

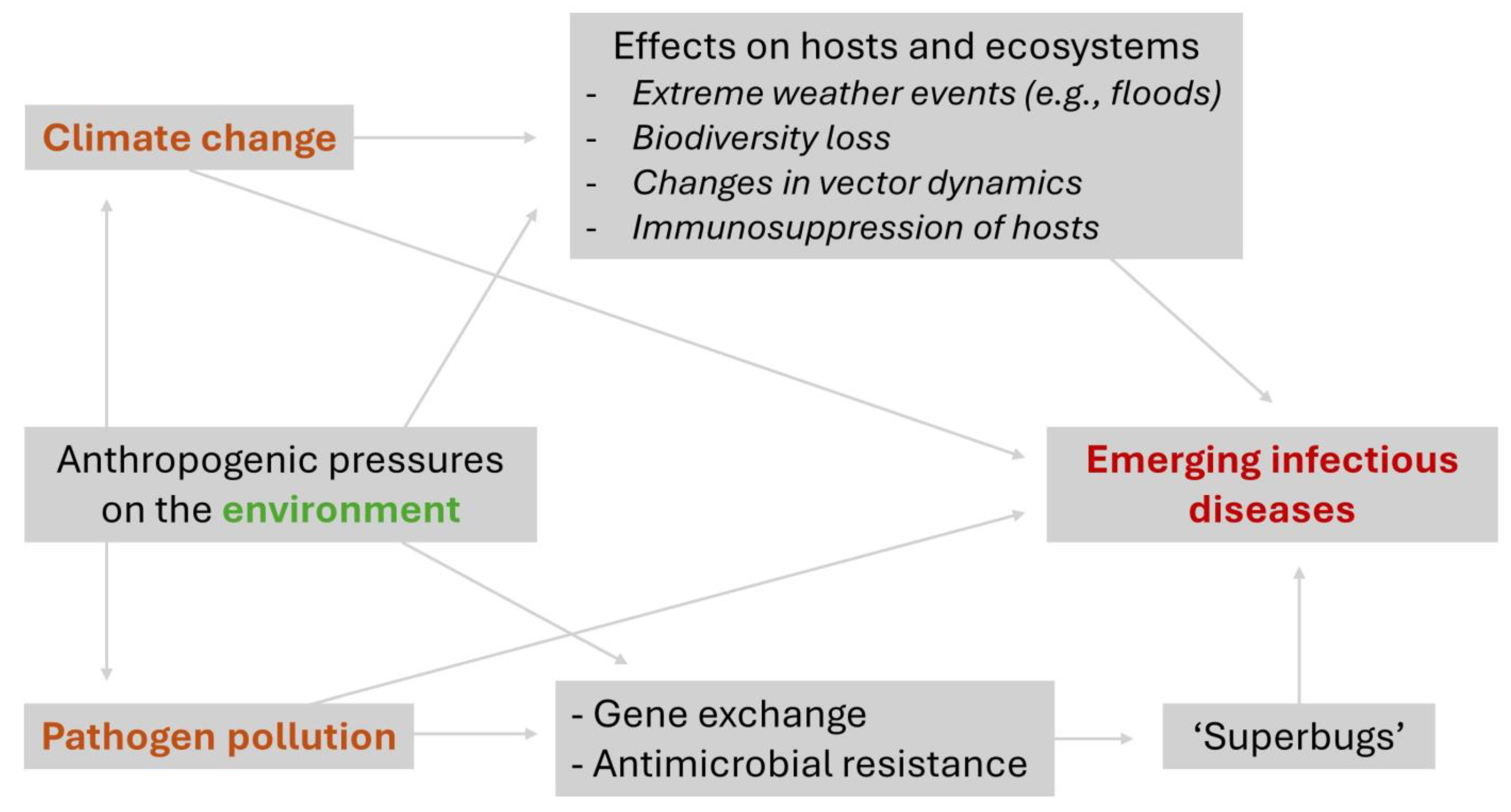
Figure 1.
Flood in Porto Alegre City in May 2024 as a result of extreme rains that hit Rio Grande do Sul State, southern Brazil. The historic district of Porto Alegre (Centro Histórico de Porto Alegre) remained flooded with the water from Guaíba Lake (Lago Guaíba) mixed with sewage for several days (panels (A,B)). It can be seen that the water is quite dark (panel (A)) due to the large amount of sediment and contaminants present in the flood water. A large amount of pollution covered the metropolitan region of Porto Alegre after the flood water receded, as seen by the trash present on the shore of Guaíba Lake (Orla do Guaíba, panels (C,D)). Photo credits: Alexandre Copês.
The infrastructure of Rio Grande do Sul was severely damaged, with many highways and bridges being destroyed, making access to several cities difficult or blocked. In many cases, entry to or exit from many cities was only possible with the help of rescue boats or helicopters, which were often used to transport injured or hospitalized people. The monetary losses caused by the crisis in Rio Grande do Sul was estimated at BRL 62 billion (Brazilian Real), but losses could reach more than BRL 100 billion [51].
The health system was significantly affected in many cities, causing serious problems in the distribution of medicines, performing surgeries and compromising the treatment of chronic patients [52]. Thousands of animals were also affected, including dogs, cats, horses, livestock animals, and native fauna [53].
In addition to various types of trash and debris, the flood water was also polluted by zoonotic pathogens. Contamination of the flood water by urine from animals associated with other risk factors (e.g., poor waste management and direct contact with contaminated water) [45,46,54,55] has caused an outbreak of human leptospirosis in Porto Alegre and other cities in Rio Grande do Sul State [20].
To date (14 August 2024), 725 cases of leptospirosis have been confirmed, with 26 deaths recorded (25 of them directly related to the flood) (Table 2). Another 2844 cases remain under investigation (Table 2) [20]. The number of cases, and possibly deaths, will certainly be higher, as this is an outbreak under investigation. For comparison purposes, in 2024 (in which data represent only seven and a half months), a 65%, 181%, and 306% increase in the number of leptospirosis cases has already been seen, compared to 2023, 2022, and 2021, respectively (Table 2) [19]. Notably, the significant and rapid increase in the number of leptospirosis cases linked to the 2024 flood was widely reported by various news outlets [56,57,58,59,60].

Table 2.
Epidemiological situation of leptospirosis in Rio Grande do Sul State in 2024 * (flood year) and in the previous ten years **.
The classic risk factors for leptospirosis are contact with water contaminated by bacteria of the Leptospira genus, which usually occurs in flood situations in places with the presence of livestock, zoonotic, and domestic animals (e.g., rodents and dogs) associated with poor sanitation. Of note, exposure to contaminated water with skin wounds is an important risk factor for Leptospira infection [45,46,54,55].
During the 2024 flood, a large portion of the population of Rio Grande do Sul came into extensive contact with flood water; this included both people whose homes were invaded by water and people involved in rescue activities and assistance to the affected human and animal populations [61,62]. In addition, many cities in Rio Grande do Sul have deficiencies in sanitation systems, which deteriorates the water quality of Rio Grande do Sul, especially in the Guaíba Lake basin [63]. Only 26.6% of sewage is collected and properly treated in Rio Grande do Sul, according to data from 2022 [64]. In Porto Alegre, the capital of the state, only 55.42% of sewage receives adequate treatment, according to data from 2024 [65]. The epidemiological data presented in Table 2 associated with the risk factors observed in Rio Grande do Sul during the 2024 flood strongly indicate the relationship between this extreme climate event and the disease outbreak. This flood-related leptospirosis outbreak provides an additional example of how climate change facilitates environment-mediated infections in sanitation-deficient settings [66].
4.2. Other Examples of Climate Change-Related Health Problems
Other red lights come from southern Brazil. A study performed in Porto Alegre City by our group indicated that heat waves can have a deleterious effect on non-pathogenic soil nematodes, without affecting populations of soil-transmitted pathogens (e.g., Ascaris spp., Trichuris spp., and hookworms) as intensely [67]. These findings are in line with a series of evidence indicating that anthropogenic disturbances in the environment, including climate change and biodiversity loss, affect the delicate equilibrium among species communities and the intricate web of interactions between humans, non-human animals, and their environments. Such disturbances disrupt ecological chains, creating conditions favorable to the dissemination of pathogenic microorganisms and disease vectors adapted to atrophic environments, thereby facilitating the emergence of infectious disease outbreaks [8].
Climate change also favors the adaptation of microorganisms to new ecological niches and hosts. The emergence of the multi-resistant fungus Candida auris as a human pathogen was potentially facilitated by climate change. Higher temperatures in humid regions would have acted as a pressure for the selection of variants that are thermotolerant to the human temperature. Wild and domestic animals would then have acted as intermediate hosts for the transmission of C. auris from the environment to the human population [68,69,70]. Other fungi species (e.g., Batrachochytrium dendrobatidis, Cryptococcus deuterogattii, and Puccinia striiformis) may have also obtained some benefit from climate change, with detrimental impacts on humans and biodiversity [70]. This phenomenon is intensified when climate change is combined with the release of antifungals for medical and veterinary use into the environment, a pollution problem of increasing importance globally [71].
5. Control of ‘Pathogen Pollution’ Is Urgent
Environmental contamination by domestic, industrial and hospital sewage, as well as residues from agriculture and livestock production, contribute to ‘pathogen pollution’, an expression used both (I) to describe the widespread presence of pathogens in the environment and (II) to refer to the introduction of pathogens into a new host/human population [72] (this second case is also called a ‘spillover event’ [1]). Eventually, ‘spillback events’ can also occur, with the transmission of pathogens from humans to the environment or non-human animal species [1,73]. In any case, pathogen pollution usually occurs due to anthropogenic forces [72].
The widespread release of microorganisms into the environment (from human and other animal populations) indeed facilitates the reinfection of humans and animals [4], especially in urban and rural environments with precarious socio-environmental conditions and poor sanitation infrastructure [72]. This problem is intensified by the widespread discharge of antibiotics into the environment derived from human and veterinary use, which contributes to the selection of multi-resistant bacteria [4,74,75]. Furthermore, we stress that the presence of varied microorganisms in the environment allows for the exchange of genetic information between them, subsequently allowing for the emergence of new pathogen variants [1,76,77]. Climate change and related warmer temperatures add more ‘energy’ to this system, potentially accelerating and intensifying the processes mentioned above.
6. Key Message
Climate change and pathogen pollution are related problems that, if not mitigated, will have increasingly harmful consequences for human and environmental health, facilitating the emergence of new diseases and degrading ecosystems (Figure 2). Examples from Brazil (Figure 1) and other countries make this problem increasingly palpable. To control climate change, countries need to follow international agreements to reduce the release of greenhouse gases into the atmosphere, especially through an energy transition that limits the use of fossil fuels. Improvements in sanitation systems and reducing the use of antimicrobial agents are fundamental measures to control pathogen pollution and, consequently, emerging pathogens. Finally, robust and continuous investments in traditional (e.g., classical epidemiology and entomological surveillance) and next-generation strategies (e.g., AI-assisted tools and genomic vigilance) for pathogen surveillance at human–environment–animal interfaces are needed.
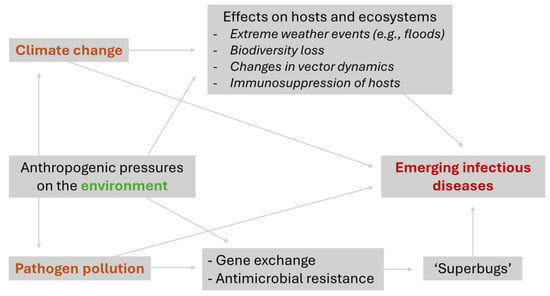
Figure 2.
Connections between climate change, pathogen pollution and emerging infectious diseases.
Author Contributions
Conceptualization, J.H.E.; writing—original draft preparation, J.H.E.; writing—review and editing, M.Z. and J.A.B.C.; visualization, M.Z. and J.H.E.; supervision, J.A.B.C. and J.H.E. All authors have read and agreed to the published version of the manuscript.
Funding
Marina Ziliotto receives a doctoral fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil). José Artur Bogo Chies receives a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (Bolsa de Produtividade em Pesquisa–Nível 1A, CNPq, Brazil) and has research funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES AUXPE 686/2020; Brazil). Joel Henrique Ellwanger receives a postdoctoral fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Conflicts of Interest
Joel Henrique Ellwanger is part of the Topical Advisory Panel of Pollutants and currently act as Guest Editor of this same journal (in a Special Issue unrelated to this manuscript), but did not participate in the review of this article. The authors declare no other conflicts of interest.
References
- Ellwanger, J.H.; Chies, J.A.B. Zoonotic spillover: Understanding basic aspects for better prevention. Genet. Mol. Biol. 2021, 44 (Suppl. S1), e20200355. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, M.J.; Woodford, L.; Quilliam, R.S. Can plastic pollution drive the emergence and dissemination of novel zoonotic diseases? Environ. Res. 2024, 246, 118172. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Ahmed, A.N.; Coulson, T.; Crowther, T.W.; Ejotre, I.; Faust, C.L.; Frick, W.F.; Hudson, P.J.; Kingston, T.; Nameer, P.O.; et al. Ecological countermeasures to prevent pathogen spillover and subsequent pandemics. Nat. Commun. 2024, 15, 2577. [Google Scholar] [CrossRef] [PubMed]
- Rekadwad, B.N. The reverse zoonosis transfer cycle from the human-animal-plant-environment interface: How antibiotic-resistant bacteria from humans threaten the environment and fuel the rise of superbugs. Med. Hypotheses 2024, 186, 111334. [Google Scholar] [CrossRef]
- Martin, L.B.; Hopkins, W.A.; Mydlarz, L.D.; Rohr, J.R. The effects of anthropogenic global changes on immune functions and disease resistance. Ann. N. Y. Acad. Sci. 2010, 1195, 129–148. [Google Scholar] [CrossRef]
- Plowright, R.K.; Reaser, J.K.; Locke, H.; Woodley, S.J.; Patz, J.A.; Becker, D.J.; Oppler, G.; Hudson, P.J.; Tabor, G.M. Land use-induced spillover: A call to action to safeguard environmental, animal, and human health. Lancet Planet Health 2021, 5, e237–e245. [Google Scholar] [CrossRef] [PubMed]
- Kempf, F.; La Ragione, R.; Chirullo, B.; Schouler, C.; Velge, P. Super Shedding in Enteric Pathogens: A Review. Microorganisms 2022, 10, 2101. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Fearnside, P.M.; Ziliotto, M.; Valverde-Villegas, J.M.; Veiga, A.B.G.; Vieira, G.F.; Bach, E.; Cardoso, J.C.; Müller, N.F.D.; Lopes, G.; et al. Synthesizing the connections between environmental disturbances and zoonotic spillover. An. Acad. Bras. Cienc. 2022, 94 (Suppl. S3), e20211530. [Google Scholar] [CrossRef]
- de Souza, W.M.; Weaver, S.C. Effects of climate change and human activities on vector-borne diseases. Nat. Rev. Microbiol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Cann, K.F.; Thomas, D.R.; Salmon, R.L.; Wyn-Jones, A.P.; Kay, D. Extreme water-related weather events and waterborne disease. Epidemiol. Infect. 2013, 141, 671–686. [Google Scholar] [CrossRef]
- Benedict, K.; Park, B.J. Invasive fungal infections after natural disasters. Emerg. Infect. Dis. 2014, 20, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A.; Sabzevari, S.; Hashemi, S.A. Impacts of flood on health of Iranian population: Infectious diseases with an emphasis on parasitic infections. Parasite Epidemiol. Control 2020, 9, e00144. [Google Scholar] [CrossRef]
- Seidel, D.; Wurster, S.; Jenks, J.D.; Sati, H.; Gangneux, J.P.; Egger, M.; Alastruey-Izquierdo, A.; Ford, N.P.; Chowdhary, A.; Sprute, R.; et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe 2024, 5, e594–e605. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yu, P.; Abramson, M.J.; Johnston, F.H.; Samet, J.M.; Bell, M.L.; Haines, A.; Ebi, K.L.; Li, S.; Guo, Y. Wildfires, Global Climate Change, and Human Health. N. Engl. J. Med. 2020, 383, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.; Di Napoli, C.; Drummond, P.; Green, C.; Kennard, H.; Lampard, P.; Scamman, D.; Arnell, N.; Ayeb-Karlsson, S.; Ford, L.B.; et al. The 2022 report of the Lancet Countdown on health and climate change: Health at the mercy of fossil fuels. Lancet 2022, 400, 1619–1654. [Google Scholar] [CrossRef] [PubMed]
- WHO—World Health Organization. Climate Change. Available online: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (accessed on 18 June 2024).
- National Library of Medicine, National Center for Biotechnology Information. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 16 August 2024).
- Google Scholar. Available online: https://scholar.google.com/ (accessed on 16 August 2024).
- Brasil, Ministério da Saúde. Leptospirose, Situação Epidemiológica. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/l/leptospirose/situacao-epidemiologica (accessed on 20 August 2024).
- Secretaria da Saúde; Governo do Estado do Rio Grande do Sul. Leptospirose. Available online: https://saude.rs.gov.br/leptospirose (accessed on 20 August 2024).
- Hirabayashi, Y.; Mahendran, R.; Koirala, S.; Konoshima, L.; Yamazaki, D.; Watanabe, S.; Kim, H.; Kanae, S. Global flood risk under climate change. Nat. Clim. Chang. 2013, 3, 816–821. [Google Scholar] [CrossRef]
- De la Paix, M.J.; Lanhai, L.; Xi, C.; Ahmed, S.; Varenyam, A. Soil degradation and altered flood risk as a consequence of deforestation. Land Degrad. Dev. 2013, 24, 478–485. [Google Scholar] [CrossRef]
- Tingsanchali, T. Urban flood disaster management. Procedia Eng. 2012, 32, 25–37. [Google Scholar] [CrossRef]
- Du, W.; FitzGerald, G.J.; Clark, M.; Hou, X.Y. Health impacts of floods. Prehosp. Disaster Med. 2010, 25, 265–272. [Google Scholar] [CrossRef]
- Fekete, A.; Sandholz, S. Here Comes the Flood, but Not Failure? Lessons to Learn after the Heavy Rain and Pluvial Floods in Germany 2021. Water 2021, 13, 3016. [Google Scholar] [CrossRef]
- Barredo, J.I. Major flood disasters in Europe: 1950–2005. Nat. Hazards 2007, 42, 125–148. [Google Scholar] [CrossRef]
- Agampodi, S.B.; Dahanayaka, N.J.; Bandaranayaka, A.K.; Perera, M.; Priyankara, S.; Weerawansa, P.; Matthias, M.A.; Vinetz, J.M. Regional differences of leptospirosis in Sri Lanka: Observations from a flood-associated outbreak in 2011. PLoS Negl. Trop. Dis. 2014, 8, e2626. [Google Scholar] [CrossRef] [PubMed]
- Dura, G.; Pándics, T.; Kádár, M.; Krisztalovics, K.; Kiss, Z.; Bodnár, J.; Asztalos, A.; Papp, E. Environmental health aspects of drinking water-borne outbreak due to karst flooding: Case study. J. Water Health 2010, 8, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Sur, D.; Dutta, P.; Nair, G.B.; Bhattacharya, S.K. Severe cholera outbreak following floods in a northern district of West Bengal. Indian J. Med. Res. 2000, 112, 178–182. [Google Scholar]
- Tibuhwa, D.D. Moulds Menaces in Flood Ravaged Homes: A Case Study of Dar Es Salaam City Tanzania. J. Biol. Life Sci. 2016, 7, 110–121. [Google Scholar] [CrossRef][Green Version]
- Davies, B.W.; Smith, J.M.; Hink, E.M.; Durairaj, V.D. Increased Incidence of Rhino-Orbital-Cerebral Mucormycosis After Colorado Flooding. Ophthalmic Plast. Reconstr. Surg. 2017, 33 (Suppl. S1), S148–S151. [Google Scholar] [CrossRef]
- Toda, M.; Williams, S.; Jackson, B.R.; Wurster, S.; Serpa, J.A.; Nigo, M.; Grimes, C.Z.; Atmar, R.L.; Chiller, T.M.; Ostrosky-Zeichner, L.; et al. Invasive Mold Infections Following Hurricane Harvey-Houston, Texas. Open Forum Infect. Dis. 2023, 10, ofad093. [Google Scholar] [CrossRef]
- Gertler, M.; Dürr, M.; Renner, P.; Poppert, S.; Askar, M.; Breidenbach, J.; Frank, C.; Preußel, K.; Schielke, A.; Werber, D.; et al. Outbreak of Cryptosporidium hominis following river flooding in the city of Halle (Saale), Germany, August 2013. BMC Infect. Dis. 2015, 15, 88. [Google Scholar] [CrossRef]
- Elsanousi, Y.E.A.; Elmahi, A.S.; Pereira, I.; Debacker, M. Impact of the 2013 Floods on the Incidence of Malaria in Almanagil Locality, Gezira State, Sudan. PLoS Curr. 2018, 10, ecurrents.dis.8267b8917b47bc12ff3a712fe4589fe1. [Google Scholar] [CrossRef]
- Meier, P.A.; Mathers, W.D.; Sutphin, J.E.; Folberg, R.; Hwang, T.; Wenzel, R.P. An epidemic of presumed Acanthamoeba keratitis that followed regional flooding. Results of a case-control investigation. Arch. Ophthalmol. 1998, 116, 1090–1094. [Google Scholar] [CrossRef]
- Caillouët, K.A.; Michaels, S.R.; Xiong, X.; Foppa, I.; Wesson, D.M. Increase in West Nile neuroinvasive disease after Hurricane Katrina. Emerg. Infect. Dis. 2008, 14, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Juyal, D.; Sharma, M.; Kotian, S.; Negi, V.; Sharma, N. An outbreak of hepatitis A virus among children in a flood rescue camp: A post-disaster catastrophe. Indian J. Med. Microbiol. 2016, 34, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Yee, E.L.; Palacio, H.; Atmar, R.L.; Shah, U.; Kilborn, C.; Faul, M.; Gavagan, T.E.; Feigin, R.D.; Versalovic, J.; Neill, F.H.; et al. Widespread outbreak of norovirus gastroenteritis among evacuees of Hurricane Katrina residing in a large “megashelter” in Houston, Texas: Lessons learned for prevention. Clin. Infect. Dis. 2007, 44, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Wright, H.; Harris, P.N.A. Health Risks of Flood Disasters. Clin. Infect. Dis. 2018, 67, 1450–1454. [Google Scholar] [CrossRef]
- Saatchi, M.; Khankeh, H.R.; Shojafard, J.; Barzanji, A.; Ranjbar, M.; Nazari, N.; Mahmodi, M.A.; Ahmadi, S.; Farrokhi, M. Communicable diseases outbreaks after natural disasters: A systematic scoping review for incidence, risk factors and recommendations. Prog. Disaster Sci. 2024, 23, 100334. [Google Scholar] [CrossRef]
- Suhr, F.; Steinert, J.I. Epidemiology of floods in sub-Saharan Africa: A systematic review of health outcomes. BMC Public Health 2022, 22, 268. [Google Scholar] [CrossRef]
- Precha, N.; Kliengchuay, W.; Woo, C.; Yamamoto, N.; Tantrakarnapa, K. Fungal Assemblages on Indoor Surfaces with Visible Mold Growth in Homes after the 2016 Flood Disaster in Thailand. Appl. Sci. 2020, 10, 5322. [Google Scholar] [CrossRef]
- Coalson, J.E.; Anderson, E.J.; Santos, E.M.; Madera Garcia, V.; Romine, J.K.; Dominguez, B.; Richard, D.M.; Little, A.C.; Hayden, M.H.; Ernst, K.C. The Complex Epidemiological Relationship between Flooding Events and Human Outbreaks of Mosquito-Borne Diseases: A Scoping Review. Environ. Health Perspect. 2021, 129, 96002. [Google Scholar] [CrossRef]
- Salam, A.; Wireko, A.A.; Jiffry, R.; Ng, J.C.; Patel, H.; Zahid, M.J.; Mehta, A.; Huang, H.; Abdul-Rahman, T.; Isik, A. The impact of natural disasters on healthcare and surgical services in low- and middle-income countries. Ann. Med. Surg. 2023, 85, 3774–3777. [Google Scholar] [CrossRef]
- Mwachui, M.A.; Crump, L.; Hartskeerl, R.; Zinsstag, J.; Hattendorf, J. Environmental and Behavioural Determinants of Leptospirosis Transmission: A Systematic Review. PLoS Negl. Trop Dis. 2015, 9, e0003843. [Google Scholar] [CrossRef]
- Naing, C.; Reid, S.A.; Aye, S.N.; Htet, N.H.; Ambu, S. Risk factors for human leptospirosis following flooding: A meta-analysis of observational studies. PLoS ONE 2019, 14, e0217643. [Google Scholar] [CrossRef]
- Brennan, T.; Cole, G.; Stephens, B. Report to the U.S. Environmental Protection Agency on Guidance Documents to Safely Clean, Decontaminate, and Reoccupy Flood-Damaged Houses; U.S. Environmental Protection Agency: Washington, DC, USA, 2018.
- Martins-Filho, P.R.; Croda, J.; Araújo, A.A.S.; Correia, D.; Quintans-Júnior, L.J. Catastrophic Floods in Rio Grande do Sul, Brazil: The Need for Public Health Responses to Potential Infectious Disease Outbreaks. Rev. Soc. Bras. Med. Trop. 2024, 57, e006032024. [Google Scholar] [CrossRef]
- Zhong, R.; Andreoni, M. Deadly Floods in Brazil Were Worsened by Climate Change, Study Finds. The New York Times. Available online: https://www.nytimes.com/2024/06/03/climate/brazil-floods-climate-change.html (accessed on 22 July 2024).
- Secretaria da Comunicação; Governo do Estado do Rio Grande do Sul. Balanço das enchentes no RS-10/7, 11h, Situação nos Municípios. Available online: https://sosenchentes.rs.gov.br/situacao-nos-municipios (accessed on 20 August 2024).
- Watanabe, M.; Otta, L.A. Valor International, Brazil Floods: Rio Grande do Sul Estimates Losses of R$62bn. Available online: https://valorinternational.globo.com/economy/news/2024/06/10/brazil-floods-rio-grande-do-sul-estimates-losses-of-r62bn.ghtml (accessed on 19 August 2024).
- Machado, G.P. Floods in south Brazil: More than an environmental crisis. Lancet 2024, 404, 24–25. [Google Scholar] [CrossRef]
- Rodrigues, A. Mais de 11 mil Animais Afetados pelas Enchentes no RS Foram Resgatados. Agência Brasil. Available online: https://agenciabrasil.ebc.com.br/geral/noticia/2024-05/mais-de-11-mil-animais-afetados-pelas-enchentes-no-rs-foram-resgatados#:~:text=Segundo%20a%20secretaria%20estadual%20do,ilhada%2C%20em%20Canoas%2C%20e%20cujo (accessed on 22 July 2024).
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Goarant, C. Leptospirosis: Risk factors and management challenges in developing countries. Res. Rep. Trop. Med. 2016, 7, 49–62. [Google Scholar] [CrossRef]
- BBC News Brasil. Quais os Sintomas da Leptospirose, que está Deixando Mortos no RS após Inundações. Available online: https://www.bbc.com/portuguese/articles/cx887d4gp23o (accessed on 16 August 2024).
- Faleiro, F. Correio do Povo, Enchente Aumenta Notificações de Leptospirose e Governo do RS muda Estratégia Contra Doença. Available online: https://www.correiodopovo.com.br/not%C3%ADcias/sa%C3%BAde/enchente-aumenta-notifica%C3%A7%C3%B5es-de-leptospirose-e-governo-do-rs-muda-estrat%C3%A9gia-contra-doen%C3%A7a-1.1498556 (accessed on 16 August 2024).
- g1 RS. Sobe para 17 Número de Mortes por Leptospirose Após Cheias no RS. Available online: https://g1.globo.com/rs/rio-grande-do-sul/noticia/2024/06/11/sobe-para-17-numero-de-mortes-por-leptospirose-apos-cheias-no-rs.ghtml (accessed on 16 August 2024).
- Laboissière, P. Agência Brasil, Rio Grande do Sul Confirma 25 Mortes por Leptospirose. Available online: https://agenciabrasil.ebc.com.br/saude/noticia/2024-07/rio-grande-do-sul-confirma-25-mortes-por-leptospirose (accessed on 16 August 2024).
- Sul 21. RS já tem 7 Mortes por Leptospirose Relacionadas às Enchentes. Available online: https://sul21.com.br/noticias/saude/2024/05/rs-ja-tem-7-mortes-por-leptospirose-relacionadas-as-enchentes/ (accessed on 16 August 2024).
- Ferreira, M. Brasil de Fato, Population Organizes to Save Lost Animals from the Flood in Rio Grande do Sul’s Capital City. Available online: https://www.brasildefato.com.br/2024/05/10/population-organize-to-save-lost-animals-from-the-flood-in-rio-grande-do-sul-s-capital-city (accessed on 19 August 2024).
- Wells, I. BBC, Inside the Dangerous Rescue for Brazil Flood Victims. Available online: https://www.bbc.com/news/articles/c103lel2p5yo (accessed on 19 August 2024).
- Pescke, I.K.; Perez, K.J.; de Lara, D.M. Se não agora, quando? Água e saneamento como ODS da Agenda 2030 e a realidade no Rio Grande do Sul (Brasil). Revbea 2022, 17, 433–451. [Google Scholar] [CrossRef]
- Instituto Trata Brasil. Rio Grande do Sul Pouco Evolui no Saneamento Básico e Condições Ainda São Precárias. Available online: https://tratabrasil.org.br/rio-grande-do-sul-pouco-evolui-no-saneamento-basico-e-condicoes-ainda-sao-precarias/ (accessed on 16 August 2024).
- Instituto Trata Brasil. Ranking do Saneamento 2024. Available online: https://tratabrasil.org.br/ranking-do-saneamento-2024/ (accessed on 19 August 2024).
- Ziliotto, M.; Chies, J.A.B.; Ellwanger, J.H. Environmental Sanitation in Porto Alegre City, Brazil: A Basic Step towards Sustainable Development. Sustainability 2024, 16, 2672. [Google Scholar] [CrossRef]
- Ziliotto, M.; Ellwanger, J.H.; Chies, J.A.B. Soil-transmitted parasites and non-pathogenic nematodes in different regions of Porto Alegre city, Brazil: A comparison between winter and summer. Parasitologia 2024, 4, 1–14. [Google Scholar] [CrossRef]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the emergence of Candida auris: Climate change, azoles, swamps, and birds. mBio 2019, 10, e01397-19. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Candida auris emergence as a consequence of climate change: Impacts on Americas and the need to contain greenhouse gas emissions. Lancet Reg. Health Am. 2022, 11, 100250. [Google Scholar] [CrossRef]
- Nnadi, N.E.; Carter, D.A. Climate change and the emergence of fungal pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef]
- Monapathi, M.E.; Oguegbulu, J.C.; Adogo, L.; Klink, M.; Okoli, B.; Mtunzi, F.; Modise, J.S. Pharmaceutical Pollution: Azole Antifungal Drugs and Resistance of Opportunistic Pathogenic Yeasts in Wastewater and Environmental Water. Appl. Environ. Soil Sci. 2021, 2021, 9985398. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Pathogen pollution: Viral diseases associated with poor sanitation in Brazil. Hygiene 2023, 3, 441–449. [Google Scholar] [CrossRef]
- Campos, R.K.; Rossi, S.L.; Tesh, R.B.; Weaver, S.C. Zoonotic mosquito-borne arboviruses: Spillover, spillback, and realistic mitigation strategies. Sci. Transl. Med. 2023, 15, eadj2166. [Google Scholar] [CrossRef]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean–Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Aminov, R.I. Horizontal gene exchange in environmental microbiota. Front. Microbiol. 2011, 2, 158. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).