Phosphate Treatment by Five Onsite Wastewater Systems in a Nutrient Sensitive Watershed
Abstract
:1. Introduction
2. Materials and Methods
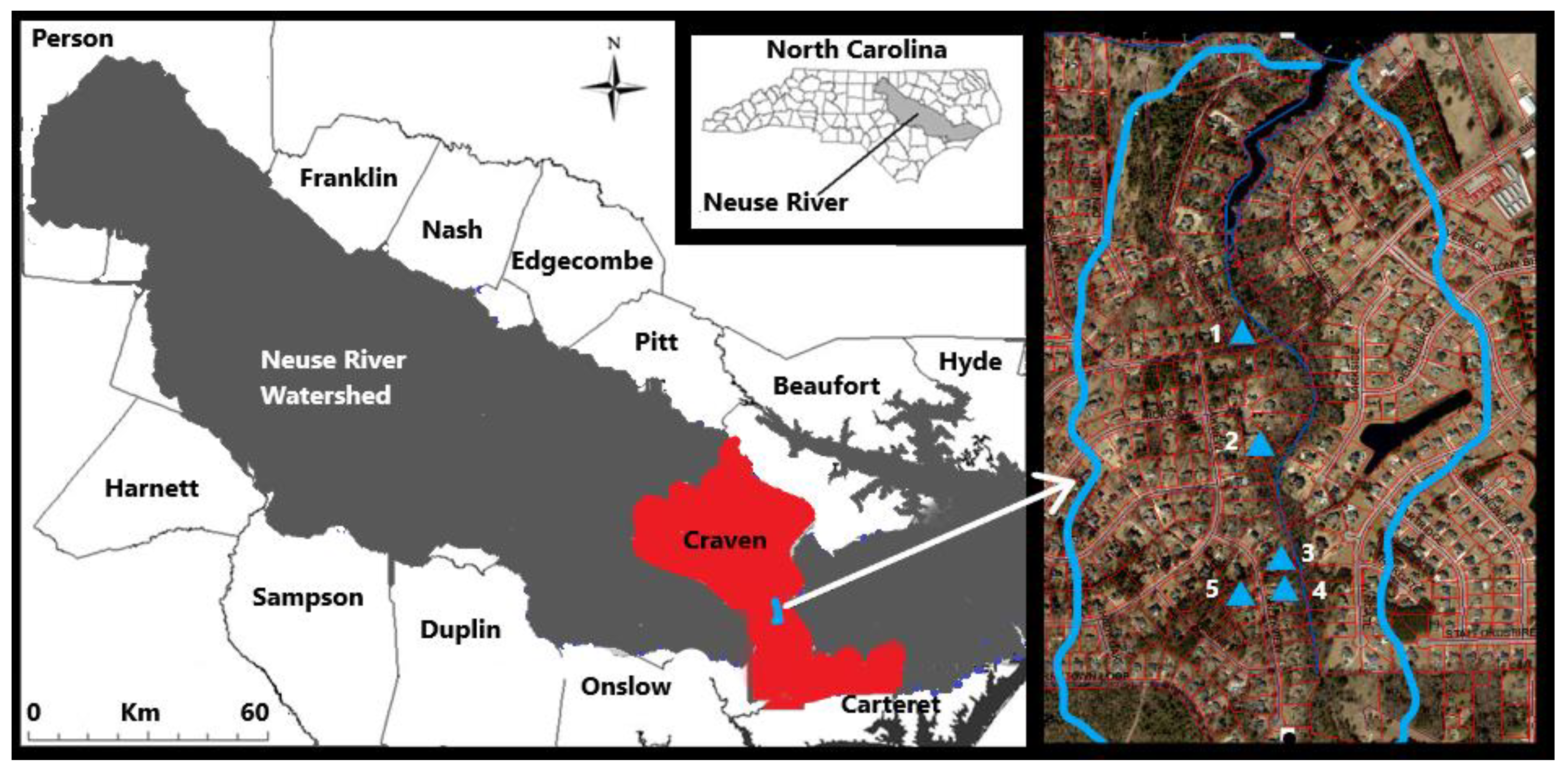
2.1. Study Location
2.2. Groundwater Monitoring Infrastructure
2.3. Bromide Tracer
2.4. Measurements and Sampling
2.5. Statistical Analyses
3. Results and Discussion
3.1. Hydrology and Soils
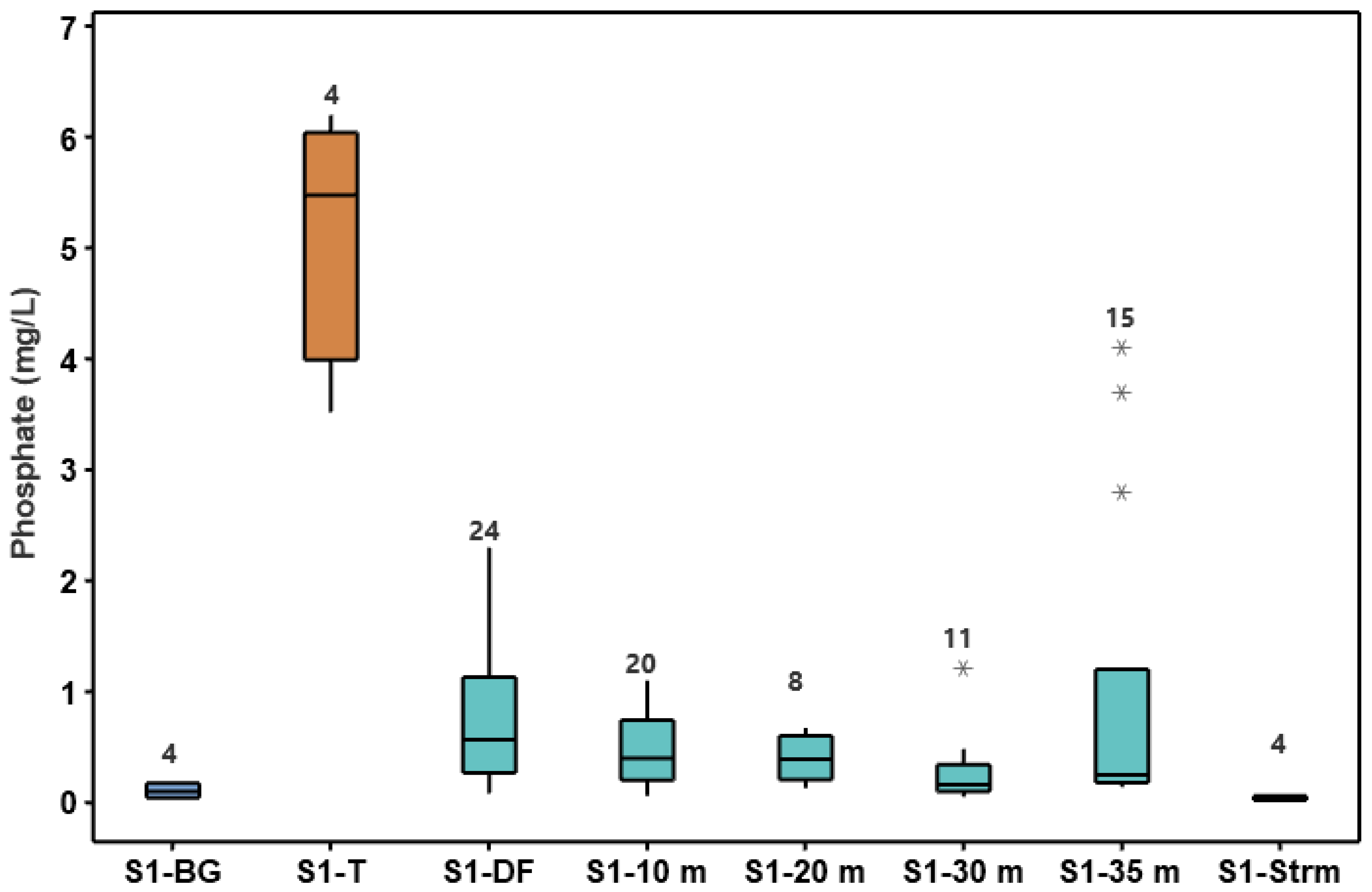
3.2. Phosphorus Treatment Efficiency by Onsite Wastewater Systems
3.3. Phosphorus Treatment by Adsorption
3.4. Soil Texture and Phosphorus Treatment
3.5. Wastewater Loading and Phosphorus Treatment
3.6. Phosphorus Treatment by Mineral Precipitation
3.7. Physical and Chemical Properties of Groundwater
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing Phosphorus to Curb Lake Eutrophication is a Success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef]
- Paerl, H.W. Controlling Eutrophication along the Freshwater-Marine Continuum: Dual Nutrient (N and P) Reductions are Essential. Estuaries Coasts 2009, 32, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology: Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.; Johnson, M.-V.V.; Morton, S.L.; Perkins, D.A.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J. Are Harmful Algal Blooms Becoming the Greatest Inland Water Quality Threat to Public Health and Aquatic Ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999; pp. 86–153. [Google Scholar]
- North Carolina Department of Environmental Quality. Neuse River Nutrient Strategy. 2022. Available online: https://deq.nc.gov/about/divisions/water-resources/planning/nonpoint-source-management/nutrient-strategies/neuse (accessed on 3 March 2022).
- Hoffman, F.A.; Bishop, J.W. Impacts of Phosphate Detergent Ban on Concentrations of Phosphorus in the James River, Virginia. Wat. Res. 1995, 29, 1425–1426. [Google Scholar] [CrossRef]
- Schellenger, F.L.; Hellweger, F.L. Phosphorus Loading from Onsite Wastewater Systems to a Lake (At Long Time Scales). Lake Reserv. Manag. 2019, 35, 90–101. [Google Scholar] [CrossRef]
- Lebo, M.E.; Paerl, H.W.; Peierls, B.L. Evaluation of Progress in Achieving TMDL Mandated Nitrogen Reductions in the Neuse River Basin, North Carolina. Environ. Manag. 2012, 49, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Lusk, M.; Toor, G.S.; Obreza, T. Onsite Sewage Treatment and Disposal Systems: Phosphorus. Florida Cooperative Extension Service SL349. Available online: https://edis.ifas.ufl.edu/publication/SS551 (accessed on 6 March 2022).
- Bowes, M.J.; Gozzard, E.; Johnson, A.C.; Scarlett, P.M.; Roberts, C.; Read, D.S.; Armstrong, L.K.; Harman, S.A.; Wickman, H.D. Spatial and Temporal Changes in Chlorophyll-a Concentrations in the River Thames Basin, UK: Are Phosphorus Concentrations Beginning to Limit Phytoplankton Biomass? Sci. Total Environ. 2012, 426, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, C.P.; Anderson-Evans, E.; O’Driscoll, M.; Manda, A.; Iverson, G. Comparison of Phosphorus Concentrations in Coastal Plain Watersheds Served by Onsite Wastewater Treatment Systems and a Municipal Sewer Treatment System. Water Air Soil Pollut. 2015, 226, 2259. [Google Scholar] [CrossRef]
- Iverson, G.; O’Driscoll, M.; Humphrey Jr, C.; Manda, A.; Anderson-Evans, E. Wastewater Nitrogen Contributions to Coastal Plain Watersheds, NC, USA. Water Air Soil Pollut. 2015, 226, 325. [Google Scholar] [CrossRef]
- Humphrey, C.P.; O’Driscoll, M.A.; Deal, N.; Lindbo, D. Fate and Transport of Phosphate from an On-Site Wastewater System in Beaufort County, North Carolina. J. Environ. Health 2014, 76, 28–33. [Google Scholar] [PubMed]
- US EPA. Onsite Wastewater Treatment Systems Manual. 2002. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/2004_07_07_septics_septic_2002_osdm_all.pdf (accessed on 6 March 2022).
- Iverson, G.; Humphrey, C.P., Jr.; O’Driscoll, M.; Jernigan, J.; Serozi, B.; Sanderford, C. Quantifying Total Phosphorus and Heavy Metals in Residential Septage. Appl. Sci. 2022, 12, 3336. [Google Scholar] [CrossRef]
- Robertson, W.D.; Blowes, D.W. Major Ion and Trace Metal Geochemistry of an Acidic Septic-System Plume in Silt. Ground Water 1995, 33, 275–283. [Google Scholar] [CrossRef]
- Lusk, M.G.; Toor, G.S.; Yang, Y.-Y.; Mechtensimer, S.; De, M.; Obreza, T.A. A Review of the Fate and Transport of Nitrogen, Phosphorus, Pathogens, and Trace Organic Chemicals in Septic Systems. Crit. Rev. Environ. Sci. Technol. 2017, 47, 455–541. [Google Scholar] [CrossRef]
- Karathanasis, A.D.; Mueller, T.G.; Boone, B.; Thompson, Y.L. Nutrient Removal from Septic Effluents as Affected by Soil Thickness and Texture. J. Water Health 2006, 4, 177–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, L.; O’Luanaigh, N.; Johnston, P.; Misstear, B.; O’Suilleabhain, C. Nutrient Loading on Subsoils from On-Site Wastewater Effluent, Comparing Septic Tank and Secondary Treatment Systems. Water Res. 2009, 43, 2739–2749. [Google Scholar] [CrossRef]
- Zanini, L.; Robertson, W.D.; Ptacek, C.J.; Schiff, S.L.; Mayer, T. Phosphorus Characterization in Sediments Impacted by Septic Effluent at Four Sites in Central Canada. J. Contam. Hydrol. 1998, 33, 405–429. [Google Scholar] [CrossRef]
- Reay, W.G. Septic Tank Impacts on Groundwater Quality and Nearshore Sediment Nutrient Flux. Groundwater 2004, 42, 1079–1089. [Google Scholar] [CrossRef]
- Humphrey, C.; Serozi, B.; Iverson, G.; Jernigan, J.; Pradhan, S.; O’Driscoll, M.; Bean, E. Phosphate Treatment by Onsite Wastewater Systems in Nutrient-Sensitive Watersheds of North Carolina’s Piedmont. Water Sci. Technol. 2016, 74, 1527–1538. [Google Scholar] [CrossRef]
- Corbett, D.R.; Dillon, K.; Burnett, W.; Schaefer, G. The Spatial Variability of Nitrogen and Phosphorus Concentration in a Sand Aquifer Influenced by Onsite Sewage Treatment and Disposal Systems: A Case Study on St. George Island, Florida. Environ. Pollut. 2002, 117, 337–345. [Google Scholar] [CrossRef]
- Daniels, R.B.; Buol, S.W.; Kleiss, H.J.; Ditzler, C.A. Soil Systems in North Carolina. Technical Bulletin 314; North Carolina State University, Soil Science Department: Raleigh, NC, USA, 1999; pp. 1–6. [Google Scholar]
- United States Geologic Survey StreamStats. Available online: https://streamstats.usgs.gov/ss/ (accessed on 16 February 2022).
- United States Climate Data. New Bern, North Carolina. Available online: https://www.usclimatedata.com/climate/new-bern/north-carolina/united-states/usnc0484 (accessed on 15 February 2022).
- Winner, M.D.; Coble, R.W. Hydrogeologic Framework of the North Carolina Coastal Plain. U.S. Geological Survey Professional Paper 1404-1; United States Government Printing Office: Washington, DC, USA, 1996. [Google Scholar]
- Domenico, P.A.; Schwartz, W. Physical and Chemical Hydrogeology, 2nd ed.; Wiley: New York, NY, USA, 1998; pp. 36–115. [Google Scholar]
- United States Department of Agriculture, Natural Resources Conservation Service. Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 14 February 2022).
- Harman, J.; Robertson, W.D.; Cherry, J.A.; Zanini, L. Impacts on a Sand Aquifer from an Old Septic System: Nitrate and Phosphate. Ground Water 1996, 34, 1105–1114. [Google Scholar] [CrossRef]
- USGS 1998 Basic Groundwater Hydrology. Water-Supply Paper 2220. Available online: https://doi.org/10.3133/wsp2220 (accessed on 14 February 2022).
- Robertson, W.D. Development of Steady-State Phosphate Concentrations in Septic System Plumes. J. Contam. Hydrol. 1995, 19, 289–305. [Google Scholar] [CrossRef]
- Robertson, W.D.; Schiff, S.L.; Ptacek, C.J. Review of Phosphate Mobility and Persistence in 10 Septic System Plumes. Groundwater 1998, 3, 1000–1010. [Google Scholar] [CrossRef]
- Lombardo, P. Phosphorus Geochemistry in Septic Tanks, Soil Absorption Systems, and Groundwater; Lombardo Associates, Inc.: Newton, MA, USA, 2006. [Google Scholar]
- Humphrey, C.P.; O’Driscoll, M.A. Biogeochemistry of Groundwater beneath Onsite Wastewater Systems in a Coastal Watershed. Univer. J. Environ. Res. Technol. 2011, 1, 320–328. [Google Scholar]
- Mechtensimer, S.; Toor, G.S. Fate, Mass Balance, and Transport of Phosphorus in the Septic System Drainfields. Chemosphere 2016, 159, 153–158. [Google Scholar] [CrossRef]
- Robertson, W.D.; Van Stempvoort, D.R.; Schiff, S.L. Review of Phosphorus Attenuation in Groundwater Plumes from 24 Septic Systems. Sci. Total Environ. 2019, 692, 640–652. [Google Scholar] [CrossRef]
- O’Driscoll, M.A.; Humphrey, C.P.; Deal, N.E.; Lindbo, D.L.; Zarate-Bermudez, M.A. Meteorological Influences on Nitrogen Dynamics of a Coastal Onsite Wastewater Treatment System. J. Environ. Qual. 2014, 43, 1873–1885. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, C.; Iverson, G.; O’Driscoll, M. Nitrogen Treatment Efficiency of a Large Onsite Wastewater System in Relation to Water Table Dynamics. CLEAN Soil Air Water 2017, 45, 1700551. [Google Scholar] [CrossRef]
- Geary, P. Effluent Tracing and the Transport of Contaminants from a Domestic Septic System. Water Sci. Technol. 2005, 51, 283–290. [Google Scholar] [CrossRef]
- Humphrey, C.P., Jr.; O’Driscoll, M.; Iverson, G. Comparison of Nitrogen Treatment by Four Onsite Wastewater Systems in Nutrient-Sensitive Watersheds of the North Carolina Coastal Plain. Nitrogen 2021, 2, 268–286. [Google Scholar] [CrossRef]
- Buyanjargal, A.; Kang, J.; Sleep, B.E.; Jeen, S.W. Sequential Treatment of Nitrate and Phosphate in Groundwater Using a Permeable Reactive Barrier System. J. Environ. Manag. 2021, 300, 113699. [Google Scholar] [CrossRef] [PubMed]
- Maamoun, I.; Eljamal, O.; Khalil, A.M.; Sugihara, Y.; Matsunaga, N. Phosphate Removal through Nano-Zero-Valent Iron Permeable Reactive Barrier; Column Experiment and Reactive Solute Transport Modeling. Transp. Porous Media 2018, 125, 395–412. [Google Scholar] [CrossRef]





| Site | Tank Capacity (L) | System Type, Trench Media | Drainfield Trenches Number, Length (m) | System Age Start of Study | Avg. Water Usage (L day−1) |
|---|---|---|---|---|---|
| 1 | 3780 (2) | Conventional, Gravel | 4, 15.2 | 12 | 930 |
| 2 | 3780 | Conventional, Gravel | 3, 15.2; 2, 24.4 | 12, 1 | 1385 |
| 3 | 3780 | Conventional, Gravel | 3, 15.2 | 12 | 590 |
| 4 | 3780 | Conventional, Gravel | 3, 15.2 | 7 | 545 |
| 5 | 3780 | Conventional, Polystyrene | 3, 15.2 | 1 | 798 |
| Site | Vadose Zone Sand/Silt/Clay% | Trench Infiltration Rate Ksat (m day−1) | Hydraulic Gradient | Slug Tests Ksat (m day−1) | Groundwater Vel. (m day−1) |
|---|---|---|---|---|---|
| 1 | 86/4/10 | 2.3 (1.3) | 0.010 (0.002) | 4.1 (2.4) | 0.25 |
| 2 | 67/13/20 | 1.0 (1.1) | 0.020 (0.003) | 3.8 (2.0) | 0.27 |
| 3 | 2.8 (4.3) | 0.019 (.002) | 7.8 (8.5) | 0.59 * | |
| 4 | 0.6 (0.7) | 0.023 (0.002) | 7.2 (5.8) | 0.66 * | |
| 5 | 3.9 (0.9) | 0.007 (0.004) | 1.1 (0.6) | 0.03 * |
| Site | Wastewater PO4−-P (mg L−1) | Flow Rate (L yr−1) | System Age (Y) | Cumulative Flow (L) | Cumulative PO4−-P Loading (kg) | Trench Bottom Area (m2) | PO4−-P Loading to Trenches (kg m−2) |
|---|---|---|---|---|---|---|---|
| 1 | 5.48 | 339,450 | 12 | 4,073,400 | 22.32 | 54 | 0.41 |
| 2 | 6.15 | 505,525 | 1 | 505,525 | 3.11 | 43.7 * | 0.07 |
| 3 | 7.8 | 215,350 | 12 | 2,584,200 | 20.16 | 40.5 | 0.50 |
| 4 | 5.9 | 198,925 | 7 | 1,392,475 | 8.22 | 40.5 | 0.20 |
| 5 | 4.7 | 291,270 | 1 | 291,270 | 1.37 | 40.5 | 0.03 |
| Sampling Location | Samples | pH | ORP | EC (µs cm−1) | Temp (°C) |
|---|---|---|---|---|---|
| S1-Background | 4 | 6.1 (0.3) | 293.5 (7.5) | 203.5 (30.0) | 18.4 (2.4) |
| S1-Drainfield | 24 | 6.4 (0.2) | 90.3 (101.5) | 560.5 (238.9) | 17.3 (3.6) |
| S1-10 m | 20 | 6.3 (0.3) | 143.2 (41.9) | 416.8 (149.5) | 17.3 (3.2) |
| S1-20 m | 8 | 6.3 (0.2) | 99.0 (124.5) | 410.0 (214.9) | 17.6 (3.4) |
| S1-30 m | 11 | 6.0 (0.4) | 95.5 (78.5) | 303.0 (171.6) | 16.4 (2.5) |
| S1-35 m | 15 | 5.2 (1.1) | 135.0 (62.3) | 238.0 (99.5) | 18.0 (5.0) |
| S2-Background | 11 | 5.3 (0.5) | 255.8 (73.6) | 185.8 (63.5) | 18.2 (2.1) |
| S2-Drainfield | 32 | 6.1 (0.3) | 221.4 (27.3) | 470.9 (242.7) | 18.6 (3.8) |
| S2-10 m | 31 | 5.7 (0.5) | 205.5 (54.8) | 304.8 (102.8) | 18.4 (3.6) |
| S2-20 m | 16 | 5.8 (0.3) | 178.3 (31.1) | 289.0 (60.5) | 17.9 (3.0) |
| S2-37 m | 32 | 7.0 (0.2) | 43.2 (64.0) | 401.4 (60.5) | 18.8 (4.8) |
| S3-Drainfield | 18 | 6.6 (0.2) | 136.3 (28.9) | 438.5 (199.1) | 22.2 (3.5) |
| S4-Drainfield | 19 | 5.6 (0.2) | 183.5 (45.4) | 194.3 (93.1) | 17.6 (3.7) |
| S5-Drainfield | 19 | 5.4 (0.5) | 262.3 (48.2) | 269.5 (122.6) | 17.7 (3.4) |
| S3-5 Background | 8 | 5.7 (0.4) | 141.8 (72.7) | 127.0 (31.1) | 19.7 (2.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humphrey, C.P., Jr. Phosphate Treatment by Five Onsite Wastewater Systems in a Nutrient Sensitive Watershed. Earth 2022, 3, 683-698. https://doi.org/10.3390/earth3020039
Humphrey CP Jr. Phosphate Treatment by Five Onsite Wastewater Systems in a Nutrient Sensitive Watershed. Earth. 2022; 3(2):683-698. https://doi.org/10.3390/earth3020039
Chicago/Turabian StyleHumphrey, Charles P., Jr. 2022. "Phosphate Treatment by Five Onsite Wastewater Systems in a Nutrient Sensitive Watershed" Earth 3, no. 2: 683-698. https://doi.org/10.3390/earth3020039
APA StyleHumphrey, C. P., Jr. (2022). Phosphate Treatment by Five Onsite Wastewater Systems in a Nutrient Sensitive Watershed. Earth, 3(2), 683-698. https://doi.org/10.3390/earth3020039






