Biological Carbon Sequestration: From Deep History to the Present Day
Abstract
1. Introduction
2. Mechanisms of Carbon Sequestration
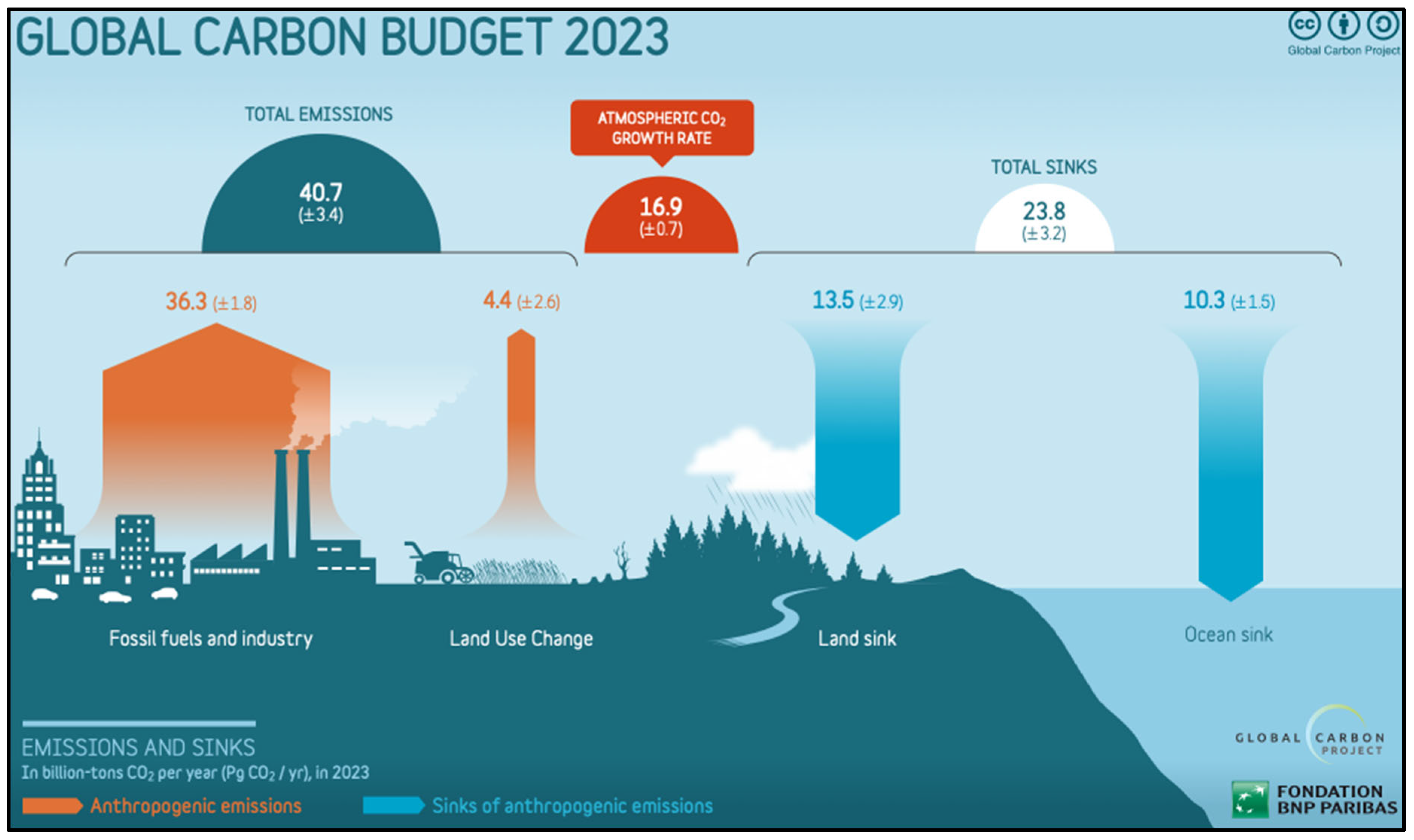
3. Biological Carbon Sequestration
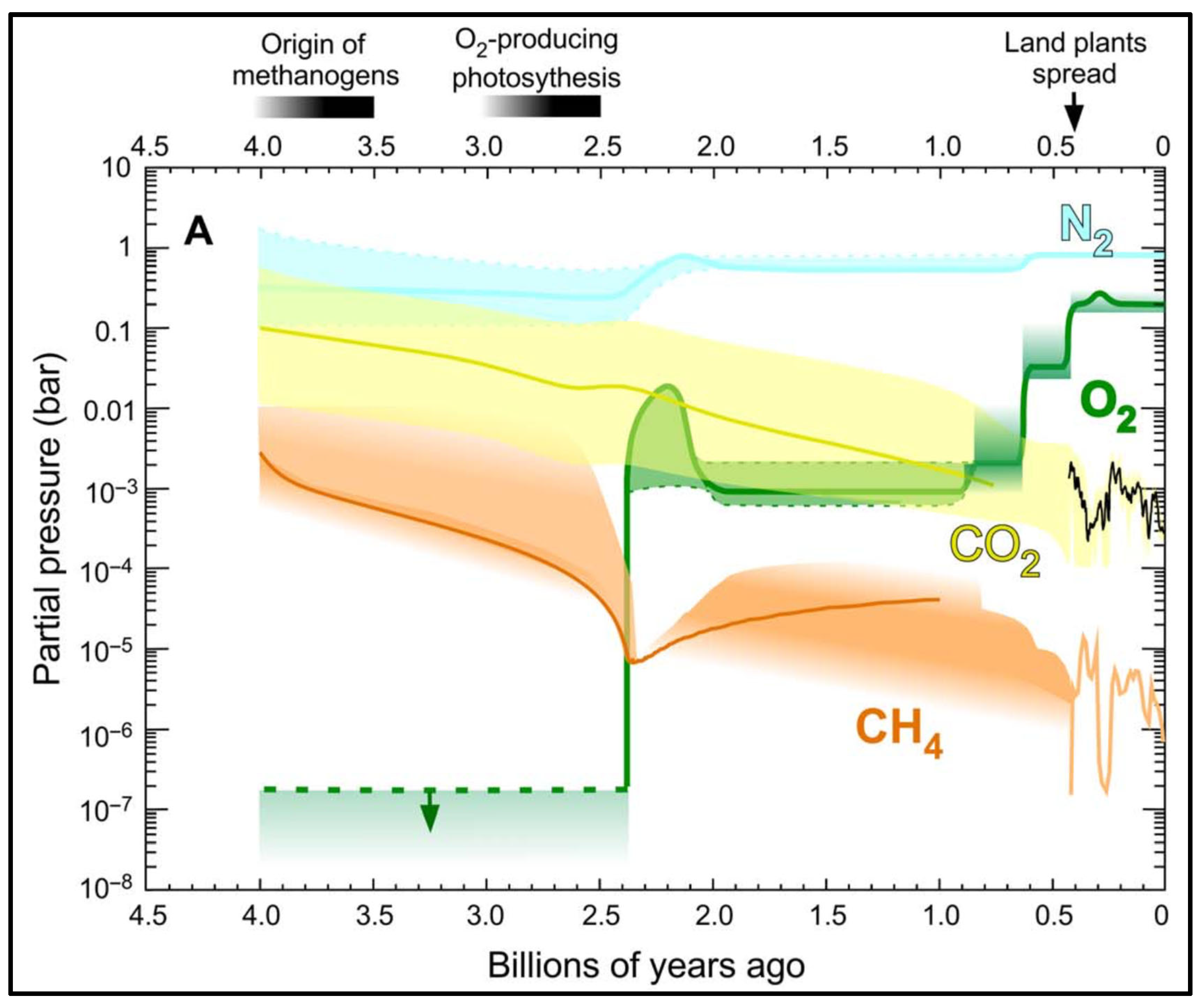
4. Photosynthesis and Carbon Sequestration in the Early Earth
5. Photosynthesis and Carbon Sequestration by Land Plants from 500 Million Years Ago
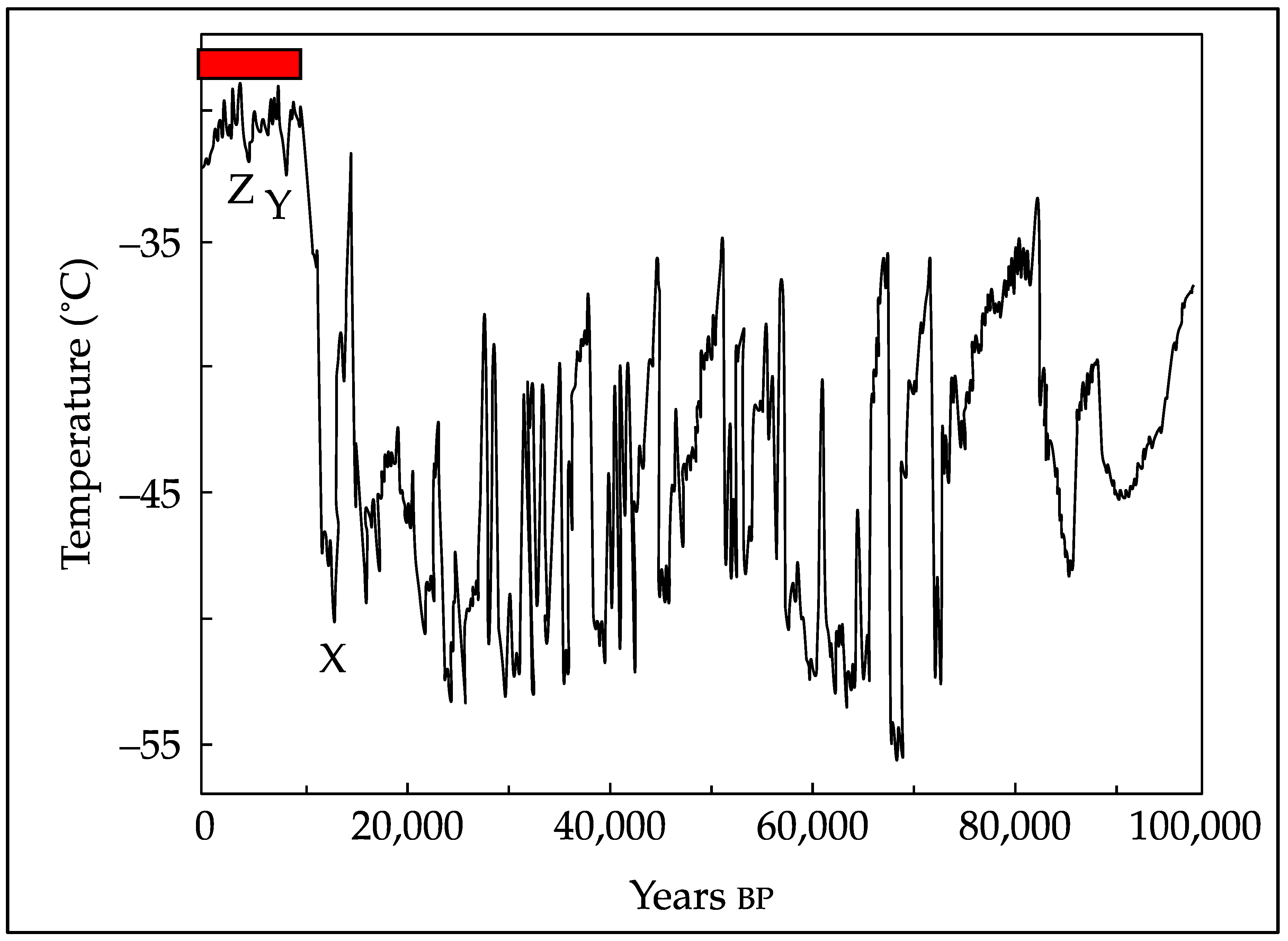
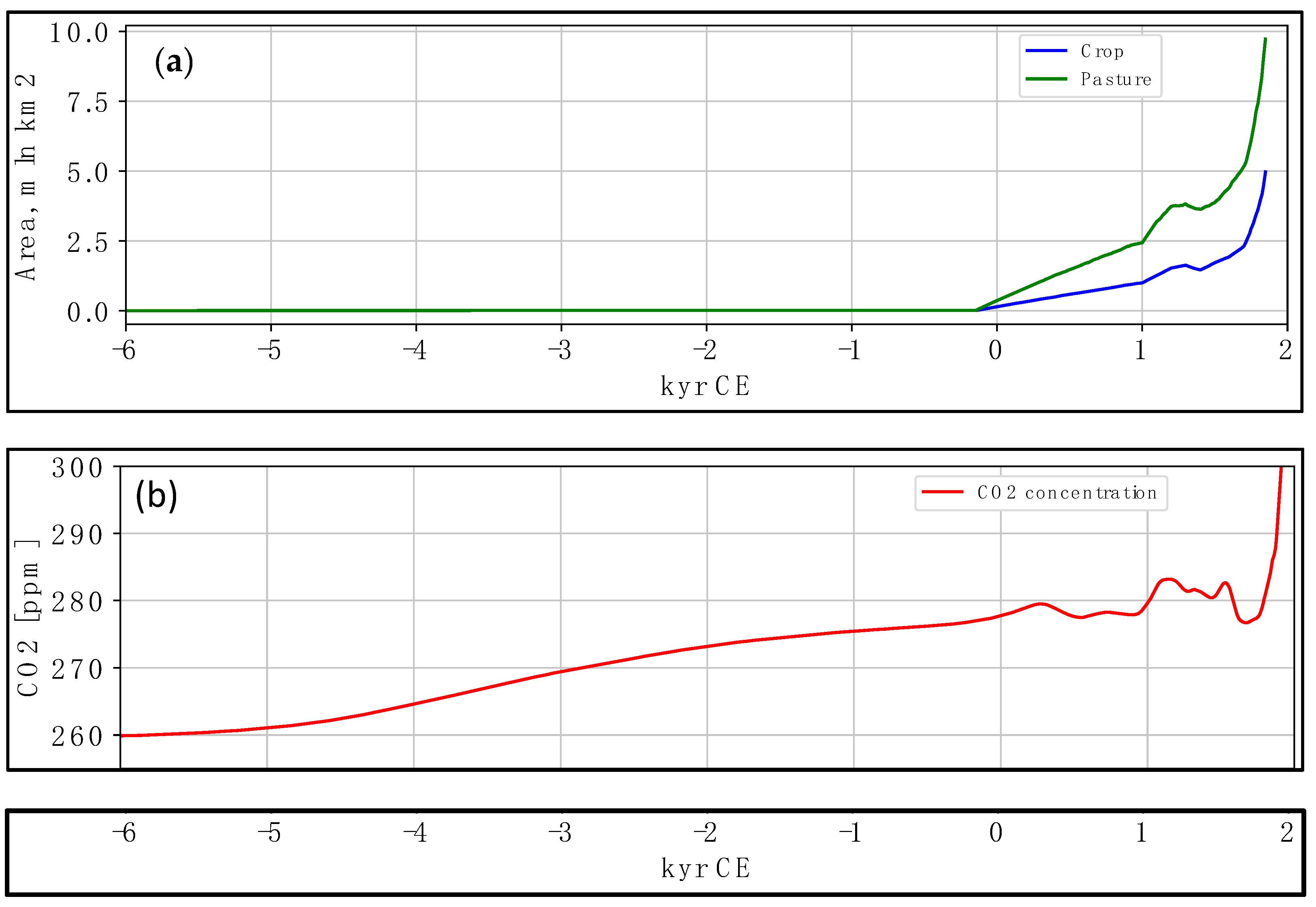
6. Carbon Dynamics during the Holocene Epoch until 1950 CE
7. The Post-1950 Inflection Point or ‘Anthropocene Event’
8. Conclusions
- Over the past ~4.5 billion years, the global carbon cycle has fluctuated considerably as geological and biological factors modulated the balance between carbon sequestration from and emission into the atmosphere.
- About 300 million years ago, atmospheric CO2 levels declined considerably following carbon sequestration due to coal formation and global cooling. This was followed by an erratic climate, especially over the past million years, when CO2 levels averaged ~220 ppm.
- The Holocene epoch, beginning ~11,700 years ago, ushered in a period of unusual climatic stability that enabled humans to develop agro-urban cultures and advanced technologies.
- This resulted in a gradual decline in carbon sequestration as cropland replaced natural vegetation and increased CO2 emissions due to fossil fuel combustion. By ~1950, an inflection point was reached, with anthropogenic CO2 emissions rising exponentially while carbon sequestration remained static or declined.
- In 2023, plants sequestered 6.6 gigatonnes of CO2 eq, but this was greatly exceeded by anthropogenic emissions of 59 gigatonnes of CO2 eq. These emissions are a major factor in ongoing climatic changes that could undermine the Holocene climatic stability that underpins food production for billions of people.
- In order to mitigate this situation, it will be necessary to restore the global carbon cycle by drastically reducing emissions and increasing carbon sequestration. As part of this strategy, the IPCC has set a carbon sequestration target of 10 gigatonnes/yr plus measures to reduce emissions via decarbonisation.
- The most effective carbon sequestration agents include coastal wetlands, tropical forests, and tree crops; hence, their removal should be avoided, and high-yield perennial tropical food crops encouraged.
Funding
Acknowledgments
Conflicts of Interest
References
- Schlesinger, W.H.; Bernhardt, E.S. The global carbon cycle. In Biogeochemistry, An Analysis of Global Change, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 419–444. [Google Scholar]
- Rackley, S.; Sewel, A.; Clery, D.; Dowson, G.; Styring, P.; Andrews, G.; McCord, S.; Knops, P.; de Richter, R.; Ming, T.; et al. (Eds.) The global carbon cycle. In Negative Emissions Technologies for Climate Change Mitigation; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-12-819663-2. [Google Scholar]
- The Royal Society. The carbon cycle: Better understanding carbon-climate feedbacks and reducing future risks. In Climate Change Briefing 7; The Royal Society: London, UK, 2021; Available online: https://royalsociety.org/-/media/policy/projects/climate-change-science-solutions/climate-science-solutions-carbon-cycle.pdf (accessed on 26 May 2024).
- Xu, L.; Saatchi, S.S.; Yang, Y.; Yu, Y.; Pongratz, J.; Bloom, A.A.; Bowman, K.; Worden, J.; Liu, J.; Yin, Y.; et al. Changes in global terrestrial live biomass over the 21st century. Sci. Adv. 2021, 7, eabe9829. [Google Scholar]
- Ocean Chemistry. The Oceans—The Largest CO2-Reservoir. World Ocean Review. 2010. Available online: https://worldoceanreview.com/en/wor-1/ocean-chemistry/co2-reservoir/ (accessed on 26 May 2024).
- IPCC. Annex B—Glossary of Terms in Climate Change 2001: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001; Volume 2. [Google Scholar]
- IPCC. Annex VII: Glossary. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Matthews, J.B.R., Ed.; Cambridge University Press: Cambridge, UK, 2001; pp. 2215–2256. [Google Scholar]
- Don, A.; Seidel, F.; Leifeld, J.; Kätterer, T.; Martin, M.; Pellerin, S.; Emde, D.; Seitz, D.; Chenu, C. Carbon sequestration in soils and climate change mitigation—Definitions and pitfalls. Glob. Change Biol. 2024, 30, e16983. [Google Scholar] [CrossRef] [PubMed]
- Scotese, C.R.; Song, H.; Mills, B.J.W.; van der Meer, D. Phanerozoic Paleotemperatures: The Earth’s Changing Climate during the Last 540 million years. Earth-Sci. Rev. 2021, 215, 103503. [Google Scholar] [CrossRef]
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- NASA. The Relentless Rise of Carbon Dioxide. 2024. Available online: https://climate.nasa.gov/climate_resources/24/graphic-the-relentless-rise-of-carbon-dioxide/#:~:text=If%20fossil%2Dfuel%20burning%20continues,of%20years%20into%20the%20future (accessed on 26 May 2024).
- NOAA. Doesn’t Carbon Dioxide in the Atmosphere Come from Natural Sources? 2020. Available online: https://www.climate.gov/news-features/climate-qa/doesnt-carbon-dioxide-atmosphere-come-natural-sources#:~:text=Yes%2C%20there%20are%20natural%20sources,even%20belches%20from%20ruminant%20animals (accessed on 26 May 2024).
- Yue, X.L.; Gao, Q.X. Contributions of natural systems and human activity to greenhouse gas emissions. Adv. Clim. Change Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Ito, A. Disequilibrium of terrestrial ecosystem CO2 budget caused by disturbance-induced emissions and non-CO2 carbon export flows: A global model assessment. Earth Syst. Dynam. 2019, 10, 685–709. [Google Scholar] [CrossRef]
- Jones, M.W.; Peters, G.P.; Gasser, T.; Andrew, R.M.; Schwingshackl, C.; Gütschow, J.; Houghton, R.A.; Friedlingstein, P.; Pongratz, J.; Le Quéré, C. National Contributions to Climate Change. 2023. Available online: https://ourworldindata.org/grapher/methane-emissions (accessed on 26 May 2024).
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Luijkx, I.T.; Peters, G.P.; et al. Global Carbon Budget 2023. Earth System Science Data 2023. Available online: https://globalcarbonbudget.org/carbonbudget2023/ (accessed on 26 May 2024).
- Neeraj; Yadav, S. Carbon storage by mineral carbonation and industrial applications of CO2. Mater. Sci. Energy Appl. 2020, 3, 494–500. [Google Scholar]
- Liu, X.; Wang, X.; Licht, G.; Licht, S. Transformation of the greenhouse gas carbon dioxide to graphene. J. CO2 Util. 2018, 36, 288–294. [Google Scholar] [CrossRef]
- Goren, A.Y.; Erdemir, D.; Dincer, I. Comprehensive review and assessment of carbon capturing methods and technologies: An environmental research. Environ. Res. 2024, 240, 117503. [Google Scholar] [CrossRef]
- Zhao, K.; Cunqi, J.; Zihao, L.; Xiaodong, D.; Yubei, W.; Jingjing, L.; Zechen, Y.; Jun, Y. Recent Advances and Future Perspectives in Carbon Capture, Transportation, Utilization, and Storage (CCTUS) Technologies: A Comprehensive Review. Fuel 2023, 351, 128913. [Google Scholar] [CrossRef]
- Ozkan, M.; Quiros, K.A.M.; Watkins, J.M.; Nelson, T.M.; Singh, N.D.; Chowdhury, M.; Namboodiri, T.; Talluri, K.M.; Yuan, E. Curbing pollutant CO2 by using two-dimensional MXenes and MBenes. Chem 2024, 10, 443–483. [Google Scholar] [CrossRef]
- Zhu, Q.; Qu, H.; Avci, G.; Hafizi, R.; Zhao, C.; Day, G.M.; Jelfs, K.E.; Little, M.A.; Cooper, A.I. Computationally guided synthesis of a hierarchical [4[2+3]+6] porous organic ‘cage of cages’. Nat. Synth 2024. [Google Scholar] [CrossRef]
- Robertson, B.; Mousavian, M. The Carbon Capture Crux, Lessons Learned; Institute for Energy Economics and Financial Analysis: Lakewood, OH, USA, 2020; Available online: https://ieefa.org/resources/carbon-capture-remains-risky-investment-achieving-decarbonisation?gad_source=1&gclid=EAIaIQobChMIoorZ7djuhAMVLZdQBh0Pwgn_EAAYAyAAEgIhi_D_BwE (accessed on 26 May 2024).
- Murphy, D.J.; Cardona, T. Photosynthetic Life. Origin, Evolution and Future; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Pongratz, J.; Manning, A.C.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; Jackson, R.B.; et al. Global Carbon Budget. Earth Syst. Sci. Data 2018, 10, 405–448. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Baslam, M.; Sanz-Saez, A. Photosynthesis in a changing global climate: A matter of scale. Front. Plant Sci. 2023, 14, 1158816. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Luo, X.; De Kauwe, M.G.; Medlyn, B.E.; Prentice, I.C.; Stocker, B.D.; Smith, N.G.; Terrer, C.; Wang, H.; Zhang, Y.; et al. A constraint on historic growth in global photosynthesis due to rising CO2. Nat. Clim. Change 2023, 13, 1376–1381. [Google Scholar] [CrossRef]
- Ruehr, S.; Keenan, T.F.; Williams, C.; Zhou, Y.; Lu, X.; Bastos, A.; Canadell, J.G.; Prentice, I.; Sitch, S.; César, T. Publisher Correction: Evidence and attribution of the enhanced land carbon sink. Nat. Rev. Earth Environ. 2023, 4, 864. [Google Scholar] [CrossRef]
- Beringer, T.; Müller, C.; Chatterton, J.; Kulak, M.; Schaphoff, S.; Jans, Y. CO2 fertilization effect may balance climate change impacts on oil palm cultivation. Environ. Res. Lett. 2023, 18, 054019. [Google Scholar] [CrossRef]
- Winkler, A.J.; Myneni, R.B.; Alexandrov, G.A.; Brovkin, V. Earth system models underestimate carbon fixation by plants in the high latitudes. Nat. Commun. 2019, 10, 885. [Google Scholar] [CrossRef]
- Gutiérrez-Salazar, P.; Medrano-Vizcaíno, P. The effects of climate change on decomposition processes in Andean Paramo ecosystem-synthesis, a systematic review. Appl. Ecol. Environ. Res. 2019, 17, 4957–4970. [Google Scholar] [CrossRef]
- Stuble, K.L.; Ma, S.; Liang, J.; Luo, Y.; Classen, A.T.; Souza, L. Long-term impacts of warming drive decomposition and accelerate the turnover of labile, not recalcitrant, carbon. Ecosphere 2019, 10, e02715. [Google Scholar] [CrossRef]
- Dawson-Glass, E.; Hewins, C.R.; Burke, D.J.; Souza, L.; Stuble, K.L. Warming-induced functional shifts in the decomposer community interact with plant community compositional shifts to impact litter decomposition. Funct. Ecol. 2023, 37, 2583–2597. [Google Scholar] [CrossRef]
- Harris, N.L.; Brown, S.; Hagen, S.C.; Saatchi, S.S.; Petrova, S.; Salas, W.; Hansen, M.C.; Potapov, P.V.; Lotsch, A. Baseline map of carbon emissions from deforestation in tropical regions. Science 2012, 336, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R.A. Carbon emissions and the drivers of deforestation and forest degradation in the tropics. Curr. Opin. Environ. Sustain. 2012, 4, 597–603. [Google Scholar] [CrossRef]
- Smit, H.H.; Meijaard, E.; van der Laan, C.; Mantel, S.; Budiman, A.; Verweij, P. Breaking the link between environmental degradation and oil palm expansion: A method for enabling sustainable oil palm expansion. PLoS ONE 2013, 8, e68610. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3765141/ (accessed on 26 May 2024). [CrossRef] [PubMed]
- Baccini, A.; Walker, W.; Carvalho, L.; Farina, M.; Sulla-Menashe, D.; Houghton, R.A. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 2017, 358, 230–234. [Google Scholar] [CrossRef]
- Pearson, T.R.H.; Brown, S.; Murray, L.T.; Sidman, G. Greenhouse gas emissions from tropical forest degradation: An underestimated source. Carbon Balance Manag. 2017, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Pendrill, F.; Gardner, T.A.; Meyfroidt, P.; Persson, U.M.; Adams, J.; Azevedo, T.; Bastos Lima, M.G.; Baumann, M.; Curtis, P.G.; De Sy, V.; et al. Disentangling the numbers behind agriculture-driven tropical deforestation. Science 2022, 377, eabm9267. [Google Scholar] [CrossRef]
- Pugh, T.A.M.; Lindeskog, M.; Smith, B.; Poulter, B.; Arneth, A.; Haverd, V.; Calle, L. Role of forest regrowth in global carbon sink dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 4382–4387. [Google Scholar] [CrossRef]
- Harris, N.; Gibbs, D. Forests Absorb Twice as Much Carbon as They Emit Each Year; World Resources Institute: Washington, DC, USA, 2021; Available online: https://www.wri.org/insights/forests-absorb-twice-much-carbon-they-emit-each-year (accessed on 26 May 2024).
- FAO. Global Forest Resources Assessment 2015, 2nd ed.; FAO: Rome, Italy, 2015; Available online: https://www.fao.org/3/i4808e/i4808e.pdf (accessed on 26 May 2024).
- FAO. The State of the World’s Forests 2018—Forest Pathways to Sustainable Development; FAO: Rome, Italy, 2018; Available online: http://www.fao.org/3/I9535EN/i9535en.pdf (accessed on 26 May 2024).
- Tyukavina, A.; Baccini, A.; Hansen, M.C.; Potapov, P.V.; Stehman, S.V.; Houghton, R.A.; Goetz, S.J. Aboveground carbon loss in natural and managed tropical forests from 2000 to 2012. Environ. Res. Lett. 2015, 10, 074002. [Google Scholar] [CrossRef]
- FAO. Land Use in Agriculture by the Numbers; FAO: Rome, Italy, 2020; Available online: https://www.fao.org/sustainability/news/detail/en/c/1274219/ (accessed on 26 May 2024).
- Bernal, B.; Murray, L.T.; Pearson, T.R.H. Global carbon dioxide removal rates from forest landscape restoration activities. Carbon Balance Manag. 2018, 13, 22. [Google Scholar] [CrossRef]
- Geden, O.; Gidden, M.; Lamb, W.F.; Minx, J.C.; Nemet, G.F.; Powis, C.; Smith, S.M. The State of Carbon Dioxide Removal, 1st ed.; University of Oxford: Oxford, UK, 2023. [Google Scholar]
- Lepot, K. Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth-Sci. Rev. 2020, 209, 103296. [Google Scholar] [CrossRef]
- Hanson, A.D.; Millar, A.H.; Nikoloski, Z.; Way, D.A. Focus on respiration. Plant Physiol. 2023, 191, 2067–2069. [Google Scholar] [CrossRef]
- Walker, J.C.G. Carbon dioxide on the early earth. Orig. Life Evol. Biosph. 1985, 16, 117–127. [Google Scholar] [CrossRef]
- Herwartz, D.; Pack, A.; Nagel, T.J. A CO2 greenhouse efficiently warmed the early Earth and decreased seawater 18O/16O before the onset of plate tectonics. Proc. Natl. Acad. Sci. USA 2021, 118, e2023617118. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Tahata, M.; Kitajima, K.; Ueno, Y.; Komiya, T.; Yamamoto, S.; Igisu, M.; Terabayashi, M.; Sawaki, Y.; Takai, K.; et al. Depth variation of carbon and oxygen isotopes of calcites in Archean altered upper oceanic crust: Implications for the CO2 flux from ocean to oceanic crust in the Archean. Earth Planet Sci. Lett. 2012, 321, 64–73. [Google Scholar] [CrossRef]
- Franks, P.J.; Royer, D.L.; Beerling, D.J.; Van de Water, P.K.; Cantrill, D.J.; Barbour, M.M.; Berry, J.A. New constraints on atmospheric CO2 concentration for the Phanerozoic. Geophys. Res. Lett. 2014, 41, 4685–4694. [Google Scholar] [CrossRef]
- Kiehl, J. Data from Earth’s Past Holds a Warning for Our Future under Climate Change; Yale University: New Haven, CT, USA, 2019; Available online: https://yaleclimateconnections.org/2019/06/data-from-earths-past-holds-a-warning-for-our-future-under-climate-change/ (accessed on 26 May 2024).
- Volkova, I.B.; Bogdanova, M.V. Petrology and genesis of Karelian shungite—High rank coal. Int. J. Coal Geol. 1986, 6, 369–379. [Google Scholar] [CrossRef]
- Morris, J.L.; Puttick, M.N.; Clark, J.W.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Yang, Z.; Schneider, H.; Donoghue, P.C.J. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 2274–2283. [Google Scholar] [CrossRef]
- Dahl, T.W.; Arens, S.K.M. The impacts of land plant evolution on Earth’s climate and oxygenation state—An interdisciplinary review. Chem. Ecol. 2020, 547, 119665. [Google Scholar] [CrossRef]
- Gensel, P.G.; Glasspool, I.; Gastaldo, R.A.; Libertin, M.; Kvaček, J. Back to the beginnings: The silurian-devonian as a time of major innovation in plants and their communities. In Nature through Time. Springer Textbooks in Earth Sciences, Geography and Environment; Martinetto, E., Tschopp, E., Gastaldo, R.A., Eds.; Springer: Champaign, IL, USA, 2020. [Google Scholar]
- Davies, N.S.; McMahon, W.J.; Barry, C.M. Earth’s earliest forest: Fossilized trees and vegetation-induced sedimentary structures from the Middle Devonian (Eifelian) Hangman Sandstone Formation, Somerset and Devon, SW England. J. Geol. Soc. 2024, 181, jgs2-23-204. [Google Scholar] [CrossRef]
- Barham, L.; Duller, G.A.T.; Candy, I.; Scott, C.; Cartwright, C.R.; Peterson, J.R.; Kabukcu, C.; Chapot, M.S.; Melia, F.; Rots, V.; et al. Evidence for the earliest structural use of wood at least 476,000 years ago. Nature 2023, 622, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.; Blanchette, R.; Kenrick, P.; Mills, B. Climate, decay, and the death of the coal forests. Curr. Biol. 2016, 26, R563–R567. [Google Scholar] [CrossRef] [PubMed]
- Feulner, G. Formation of most of our coal brought Earth close to global glaciation. Proc. Natl. Acad. Sci. USA 2017, 114, 11333–11337. [Google Scholar] [CrossRef] [PubMed]
- Holdinghausen, H. Geology and Geography: Subterranean Forests; Heinrich Böll Stiftung: Brussels, Belgium, 2015. [Google Scholar]
- Statista. Coal—Worldwide. 2024. Available online: https://www.statista.com/outlook/io/mining-quarrying/coal/worldwide (accessed on 26 May 2024).
- Libes, S.M. The origin of petroleum in the marine environment. In Introduction to Marine Biogeochemistry, 2nd ed.; John Willey and Sons: Hoboken, NJ, USA, 2009; ISBN 0-471-50946-9. [Google Scholar]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Horsfield, B.; Schulz, H.M.; Bernard, S.; Mahlstedt, N.; Han, Y.; Kuske, S. Oil and gas shales. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate. Handbook of Hydrocarbon and Lipid Microbiology; Wilkes, H., Ed.; Springer: Champaign, IL, USA, 2018. [Google Scholar]
- BP. Statistical Review of World Energy, 70th ed. 2021. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-oil.pdf (accessed on 26 May 2024).
- Kiehl, J.T.; Shields, C.A.; Snyder, M.A.; Zachos, J.C.; Rothstein, M. Greenhouse-and orbital-forced climate extremes during the early Eocene. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170085. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Pagani, M.; Liu, Z.; Bohaty, S.M.; DeConto, R. A 40-million-year history of atmospheric CO2. Philos. Trans R. Soc. A 2013, 371, 20130096. [Google Scholar] [CrossRef] [PubMed]
- Mounier, A.; Mirazón Lahr, M. Deciphering African late middle Pleistocene hominin diversity and the origin of our species. Nat. Commun. 2019, 10, 3406. [Google Scholar] [CrossRef]
- Lemoine, R.T.; Buitenwerf, R.; Svenning, J.C. Megafauna extinctions in the late-Quaternary are linked to human range expansion, not climate change. Anthropocene 2023, 44, 100403. [Google Scholar] [CrossRef]
- Sim, T.G.; Swindles, G.T.; Morris, P.J.; Baird, A.J.; Gallego-Sala, A.V.; Wang, Y.; Blaauw, M.; Camill, P.; Garneau, M.; Hardiman, M.; et al. Regional variability in peatland burning at mid-to high-latitudes during the Holocene. Quat. Sci. Rev. 2023, 305, 108020. [Google Scholar] [CrossRef]
- Zalasiewicz, J.; Williams, M. Birth and Death of the Holocene, The Goldilocks Planet: The 4 Billion Year Story of Earth’s Climate; Oxford Academic: Oxford, UK, 2012. [Google Scholar]
- Murphy, D.J. People, Plants and Genes. The Story of Crops and Humanity; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Marcott, S.A.; Shakun, J.D. Holocene climate change and its context for the future. Past. Glob. Changes 2015, 23, 28. [Google Scholar] [CrossRef]
- Elias, S.A. The Quaternary. In Reference Module in Earth Sciences and Environmental Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Grootes, P.M.; Stuiver, M. Oxygen 18/16 variability in Greenland snow and ice with 10−3- to 10−5-year time resolution. J. Geophys. Res. 1997, 102, 455–470. [Google Scholar] [CrossRef]
- Weiss, H. Beyond the Younger Dryas—Collapse as adaptation to abrupt climate change. In Confronting Natural Disaster, Engaging the Past to Understand the Future; Bawden, G., Reycraft, R., Eds.; University of New Mexico Press: Albuquerque, NM, USA, 2000; pp. 75–98. [Google Scholar]
- DeMenocal, P. Cultural responses to climate change during the late Holocene. Science 2001, 292, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.; Ferrio, J.; Voltas, J.; Aguilera, M.; Buxo, R. Agronomic conditions and crop evolution in ancient Near East agriculture. Nat. Commun. 2014, 5, 3953. [Google Scholar] [CrossRef] [PubMed]
- Schug, G.W.; Buikstra, J.E.; DeWitte, S.N.; Baker, B.J.; Berger, E.; Buzon, M.R.; Davies-Barrett, A.M.; Goldstein, L.; Grauer, A.L.; Gregoricka, L.A.; et al. Climate change, human health, and resilience in the Holocene. Proc. Natl. Acad. Sci. USA 2023, 120, e2209472120. [Google Scholar] [CrossRef] [PubMed]
- Brovkin, V.; Lorenz, S.; Raddatz, T.; Ilyina, T.; Stemmler, I.; Toohey, M.; Claussen, M. What was the source of the atmospheric CO2 increase during the Holocene? Biogeosciences 2019, 16, 2543–2555. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Stoermer, E.F. The Anthropocene. Glob. Change Newsl. 2000, 41, 17–18. [Google Scholar]
- Steffen, W.; Grinewald, J.; Crutzen, P.; McNeill, J. The Anthropocene: Conceptual and historical perspectives. Philos. Trans R. Soc. Ser. A 2011, 369, 842–867. [Google Scholar] [CrossRef] [PubMed]
- Malm, A.; Hornborg, A. The geology of mankind? A critique of the Anthropocene narrative. Anthr. Rev. 2014, 1, 62–69. [Google Scholar] [CrossRef]
- Waters, C.N.; Turner, S.D. Defining the onset of the Anthropocene. Science 2022, 378, 706–708. [Google Scholar] [CrossRef]
- Waters, C.N.; Williams, M.; Zalasiewicz, J.; Turner, S.D.; Barnosky, A.D.; Head, M.J.; Wing, S.L.; Wagreich, M.; Steffen, W.; Summerhayes, C.P.; et al. Epochs, events and episodes: Marking the geological impact of humans. Earth-Sci. Rev. 2022, 234, 104171. [Google Scholar] [CrossRef]
- Waters, C.N.; Head, M.J.; Zalasiewicz, J.; McCarthy, F.M.G.; Wing, S.L.; Haff, P.K.; Williams, M.; Barnosky, A.D.; Fiałkiewicz-Kozieł, B.; Leinfelder, R.; et al. Response to Merritts et al. (2023): The Anthropocene is complex. Defining it is not. Earth-Sci Rev. 2023, 238, 104335. [Google Scholar] [CrossRef]
- Merritts, D.; Edwards, L.E.; Ellis, E.; Walker, M.; Finney, S.; Gibbard, P.; Gill, J.L.; Maslin, M.; Bauer, A.; Edgeworth, M. The Anthropocene is complex. Defining it is not. Earth-Sci. Rev. 2023, 238, 104340. [Google Scholar] [CrossRef]
- Edgeworth, M.; Gibbard, P.; Walker, M.; Merritts, D.; Finney, S.; Maslin, M. The stratigraphic basis of the Anthropocene Event. Quat. Sci. Adv. 2023, 11, 100088. [Google Scholar] [CrossRef]
- Walker, M.J.C.; Bauer, A.M.; Edgeworth, M.; Ellis, E.C.; Finney, S.C.; Gibbard, P.L.; Maslin, M. The Anthropocene is best understood as an ongoing, intensifying, diachronous event. Boreas 2024, 53, 1–3. [Google Scholar] [CrossRef]
- Ruddiman, W.F.; Kutzbach, J.E.; Vavrus, S.J. Can natural or anthropogenic explanations of late-Holocene CO2 and CH4 increases be falsified? Holocene 2011, 21, 865–887. [Google Scholar] [CrossRef]
- Nunes, L.J.R. The Rising Threat of Atmospheric CO2: A Review on the Causes, Impacts, and Mitigation Strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Ynalvez, R.A.; Dinamarca, J.; Moroney, J.V. Algal photosynthesis. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar]
- NOAA. Climate Change: Atmospheric Carbon Dioxide, 2023. 2023. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide#:~:text=In%20May%202023%2C%20carbon%20dioxide,people%20are%20burning%20for%20energy (accessed on 26 May 2024).
- Lüthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J.M.; Siegenthaler, U.; Raynaud, D.; Jouzel, J.; Fischer, H.; Kawamura, K.; et al. High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 2008, 453, 379–382. [Google Scholar] [CrossRef]
- Arrhenius, S. On the Influence of Carbonic Acid in the Air Upon the Temperature of the Ground. Philos. Mag. 1896, 41, 237–276. [Google Scholar] [CrossRef]
- CenCO2PIP Consortium. Cenozoic CO2 Proxy Integration Project (CenCO2PIP) Consortium. Toward a Cenozoic history of atmospheric CO2. Science 2023, 382, eadi5177. [Google Scholar] [CrossRef]
- IPCC. IPCC Sixth Assessment Report. Climate Change 2021: The Physical Science Basis. 2021. Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 26 May 2024).
- Copernicus Climate Change Service. Copernicus: March 2024 is the Tenth Month in a Row to be the Hottest on Record. 2024. Available online: https://climate.copernicus.eu/copernicus-march-2024-tenth-month-row-be-hottest-record (accessed on 26 May 2024).
- Bevan, A.; Colledge, S.; Fuller, D.; Fyfe, R.; Shennan, S.; Stevens, C. Holocene fluctuations in human population demonstrate repeated links to food production and climate. Proc. Nat. Acad. Sci. USA 2017, 114, E10524–E10531. [Google Scholar] [CrossRef] [PubMed]
- OGL. United Kingdom Food Security Report 2021. 2023. Available online: https://www.gov.uk/government/statistics/united-kingdom-food-security-report-2021 (accessed on 26 May 2024).
- Bapela, T.; Shimelis, H.; Tsilo, T.J.; Mathew, I. Genetic Improvement of Wheat for Drought Tolerance: Progress, Challenges and Opportunities. Plants 2022, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Jiang, C.; Tang, C.; Nie, X.; Du, L.; Liu, Y.; Cheng, P.; Wu, Y.; Liu, H.; Kang, Z.; et al. Wheat adaptation to environmental stresses under climate change: Molecular basis and genetic improvement. Mol. Plant 2023, 16, 1564–1589. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.K.; Khanday, D.M.; Kumar, P.; Magotra, I.; Choudhary, S.M.; Kosser, R.; Kalunke, R.; Giordano, M.; Corrado, G.; Rouphael, Y.; et al. Enhancing Crop Resilience to Drought Stress through CRISPR-Cas9 Genome Editing. Plants 2023, 12, 2306. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.H.; Siddique, K.H.M.; Zhuang, W.; et al. Developing drought-smart, ready-to-grow future crops. Plant Genome 2023, 16, e20279. [Google Scholar] [CrossRef]
- The World Counts. Globally, We Consume around 350 Million Tons of Meat a Year. 2024. Available online: https://www.theworldcounts.com/challenges/consumption/foods-and-beverages/world-consumption-of-meat (accessed on 26 May 2024).
- Krug, A.S.; BM Drummond, E.; Van Tassel, D.L.; Warschefsky, E.J. The next era of crop domestication starts now. Proc. Natl. Acad. Sci. USA 2023, 120, e2205769120. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, D.J. Biological Carbon Sequestration: From Deep History to the Present Day. Earth 2024, 5, 195-213. https://doi.org/10.3390/earth5020010
Murphy DJ. Biological Carbon Sequestration: From Deep History to the Present Day. Earth. 2024; 5(2):195-213. https://doi.org/10.3390/earth5020010
Chicago/Turabian StyleMurphy, Denis J. 2024. "Biological Carbon Sequestration: From Deep History to the Present Day" Earth 5, no. 2: 195-213. https://doi.org/10.3390/earth5020010
APA StyleMurphy, D. J. (2024). Biological Carbon Sequestration: From Deep History to the Present Day. Earth, 5(2), 195-213. https://doi.org/10.3390/earth5020010




