Simple Summary
Steel production emits manganese and other heavy metals into the environment, where these byproducts can lead to health problems in humans and other animals. Birds have historically been used to detect harmful chemicals in the air, and in this paper, we show that this role continues into the present day. Feathers of a small terrestrial songbird, the Tufted Titmouse, were collected from live birds captured both near (0.3 km) and far (4.0 km) from steel production facilities in western Pennsylvania, USA. Tail feathers from birds living near the facilities had significantly higher amounts of manganese embedded in their structure compared to the feathers of birds living farther away. This shows that manganese is present in the environment, is localized at industrial sites, is encountered by birds, and is sequestered in their tissues. Animals could suffer ill health if exposures are high enough. Feather data cannot be used to assess the magnitude of this health risk since metals laid down within feathers are no longer circulating in the birds’ bodies. However, our data do suggest that the environmental monitoring of manganese should continue, as inadvertent exposure is still occurring.
Abstract
Human industry and land use has led to the anthropogenic release of manganese (Mn) into the air and soil near manufacturing centers. Overexposure to Mn can cause considerable health problems in birds. We studied whether the concentration of Mn in bird feathers correlates with the distance to point sources of Mn air emissions. Feathers were collected from Tufted Titmice (Baeolophus bicolor) at two sites in western Pennsylvania, USA. One site was in proximity (0.3 km) to a steel plant with documented Mn releases, and the other site was in a different town about 4.0 km away from other steel plants with documented Mn releases. Using the microwave plasma–atomic emission spectrometer (MP–AES), we found that tail feathers collected from nearest to a steel plant had a significantly higher concentration of Mn compared to the samples from the site further from the emission source. A body mass index was calculated for each set of birds; however, the indices did not vary significantly. This is the first published study of Mn sequestration in Tufted Titmouse feathers. This study develops our general understanding of the potential use of bird feathers as non-invasive bioindicators of environmental metal exposure.
1. Introduction
There is a long history of using birds as indicators to detect potential environmental hazards [1]. Rachel Carson’s Silent Spring used the impact of the pesticide DDT on birds to increase the level of public attention to environmental chemicals [2]. More directly, birds were used in underground mines to detect the presence of poisonous gasses [3]. Birds have also been used to monitor the release of industrial byproducts such as manganese (Mn) [4], a heavy metal that is naturally found in soil and water. Human activity can increase Mn concentrations in water and air considerably over ambient levels. Contaminated water comes from mining activities, industrial waste, or landfill leachate [5,6]. Manganese soil pollution is increased in areas near mines and factories [4,5]. Manganese is also released into the air by industrial processes, including raw steel production, ferroalloy production, production of dry-cell batteries, and plant fertilizer production [7]. The mean concentration of Mn in ambient air near industrial sources can range from 0.22 to 0.30 μg/m3, which is four to six times greater than the U.S. Environmental Protection Agency’s airborne Mn reference concentration of 0.05 μm/m3 for occupational exposure [8]. Concentrations exceeding the reference value may cause harm. Manganese air emissions in the form of the total suspended particulate have been shown to spread from 7.5 to 16 km from a point source [9,10].
Manganese is an essential mineral for all living organisms, including birds. It is an important component of many enzymes that are needed in metabolism, immune function, tissue growth, and reproduction. Bone, cartilage, and tissues that are dense with mitochondria or melanocytes contain the highest levels of Mn [11]. Levels in blood are kept within narrow homeostatic limits, and concentrations above these limits cause toxic effects. Excess Mn may block acetylcholine at the neuromuscular junction [12]. It will also oxidize dopamine, leading to a reduction in neurotransmitter effectiveness [8,13]. Birds require a smaller amount of Mn to reach the same level of Mn toxicity as humans [4,6]. A sub-lethal exposure of Mn to birds causes a variety of negative impacts, including reproductive dysfunction, immune deficiency, and cellular damage [14]. Mn accumulates in avian brains, and this can cause detrimental behaviors and a reduced growth rate among nestlings [12,15]. In field studies, it is difficult to assess such serious and systemic impacts on physiology and behavior without sacrificing the animals. Therefore, a simple measure of physical condition called the body mass index (BMI) is frequently employed to provide information on how environmental conditions might affect animal populations. BMI or related body mass metrics have been shown to vary in birds in response to immune challenges, food availability, the risk of predation, an urbanized habitat, and exposure to heavy metals [16,17,18,19,20]. With regard to Mn, laboratory research on rodents has shown that excess Mn leads to a lower body mass over time [21,22].
Manganese is subject to bioaccumulation in living organisms, particularly in plants and invertebrates that form the basis of the food chain [4,6,23]. In higher vertebrates, excess Mn will be stored in various tissues for extended periods of time, though once the source of excess is removed, Mn levels will slowly diminish to homeostatic baselines. For example, the half-life of Mn in specific rat brain regions ranged from 52 to 74 days [24].
Analyzing the Mn accumulation in feathers is a useful way to determine exposure, as shown by numerous studies [23,25,26]. Feathers are not permanent structures, which has implications for collection procedures. Birds molt and regrow their feathers at predictable times of year. When a bird molts, it begins by shedding an old feather and then growing a pin feather in its place [27]. The pin feather has a constant blood supply flowing through it. This blood supply allows contaminants (e.g., Mn) to be brought into the feathers. The contaminants will stay in the feather permanently, since there is no longer a blood supply to the feather once it has fully grown [28,29]. However, many contaminants, including Mn, appear to collect on a feather’s outer surface due to external exposure, independent of ingestion and bioaccumulation [30]. Regardless of the exposure route, measuring environmental contamination using feathers is non-invasive and a popular choice for use as a biological indicator [4,23,26,31].
We measured Mn from the feathers of the Tufted Titmouse (Baeolophus bicolor). The aim of this study was to determine whether Mn could be measured in feathers in this species, and if so, what concentrations would be found, especially as they would relate to anthropogenic point sources located nearby. We hypothesized that Mn emissions from these sources would settle into the environment downwind and expose the birds enough to cause the metal to accumulate in or on their feathers. We predicted that there would be an inverse relationship between the Mn measured from the feathers of the Tufted Titmouse and their distance to a steel plant documented to release Mn into the air.
We also measured the body mass of the birds and hypothesized that challenges to health or physical stress would be manifested in their overall mass corrected for body size. We predicted that birds living closer to, or downwind from, a metal plant would show reduced mass. Excess Mn in animal diets has that effect, but a reduced mass might also be caused by the indirect effects of environmental contamination, such as poor nutrition and infection [19].
2. Materials and Methods
2.1. Study Species
The Tufted Titmouse is a sexually monomorphic songbird found year-round in the deciduous forests of eastern North America, including Pennsylvania. Titmouse body masses range from 18 to 26 g [32], which makes this bird species smaller than most others used in studies of Mn. The Titmouse has a diet primarily consisting of seeds and arthropods, and their preference for seeds makes them easy to trap for research. Tufted Titmice nest in spring and their young are fully grown and independent by mid-summer. The birds go through several molts in their lifetimes, including a pre-juvenile molt, a pre-formative molt, and a definitive pre-basic molt that completely replaces all their feathers [32]. The pre-formative molt occurs in a bird’s first year. In subsequent years, birds molt all feathers during the definitive pre-basic molt. Both the pre-formative and pre-basic molts take place over the summer and are nearly complete by late September [32].
2.2. Site Descriptions
Feathers were sampled from Tufted Titmice at two study sites in fall 2019. The first site was Bridgeville, PA, USA. Bridgeville is a town of 16,000 people living at a density of 480 per km2. It is 12 km SW from the city center of Pittsburgh, whose metropolitan area contains 1.7 million people. The second site was at Saint Vincent College, located in Latrobe, Pennsylvania. Latrobe is a suburban area about 53 km SE from Pittsburgh. Latrobe’s population is 28,000, and is spread across 193 km2 at a density of 147 per km2. Bridgeville and Latrobe are both in the Köppen–Geiger humid continental climate zone with hot summers and no dry season [33]. Temperatures at the nearest airport range from a mean daily low temperature of −5.9 °C in winter (January) to a mean daily high temperature of 28.3 °C in summer (July) [34]. Much of the forest area was cleared in the nineteenth and twentieth centuries to support a thriving economy based in manufacturing and coal mining. An economic downturn in the late twentieth century has caused some industries to close and vegetation to return [35]. Existing forest patches now contain a mixed oak and maple canopy that is characteristic of the Eastern Temperate Forest ecoregion of North America [36]. Previously disturbed sites, including where we captured Tufted Titmice for this study, are dense with invasive shrub species, including several varieties of bush honeysuckle (Lonicera spp.).
We captured eight Tufted Titmice in Bridgeville (Table S1). These individuals resided on private property 0.3 km from factory buildings at Universal Stainless and Alloy Products, Inc. (Bridgeville, PA, USA). which produces specialty steels and is the largest source of airborne Mn emissions within a radius of 30 km. The major emitters of Mn near our study sites were identified using the “MyEnvironment” finder tool of the U.S. Environmental Protection Agency [37]. In 2019, the Universal Stainless plant released 150 kg of Mn via its smokestack [38]. This annual release was below the average of 214 kg, calculated using the 35 years for which data are available online. Combining the years, the plant has released 7480 kg of Mn or Mn compounds into the air since 1987. The Tufted Titmice in Bridgeville were captured in a narrow patch of disturbed woods about 6 ha in area and located between a residential neighborhood and factory buildings. Both a high voltage aerial powerline and a creek (Chartiers Creek) about 14 m wide pass through the woodland. The steel plant was located SW of the capture site. Prevailing winds at this location are from the W and SW [39], so air emissions from Universal Stainless and Alloy Products frequently impact this site.
Another eight Tufted Titmice were collected at Saint Vincent College in Latrobe, Pennsylvania (Table S1), approximately 60 km east from Universal Stainless. The capture location on campus was in a 14 ha patch of woods fragmented by gravel roads, natural gas wells, and corn and hay fields. Birds captured near Latrobe had a more limited exogenous exposure to Mn. The nearest 2 emitters of Mn to the Latrobe population were Latrobe Specialty Steel Company and Lehigh Specialty Melting Company (Latrobe, PA, USA) both located 4 km away [40,41]. Latrobe Specialty Steel Company makes stainless steel and alloy products, including tools used in other manufacturing plants. Lehigh Specialty Melting makes steel ingots that are sold to other manufacturers. A third metal manufacturing facility, Allegheny Ludlum, was 5 km away [42]. It produces stainless steel and silicon electrical steels. Combined, the three manufacturing plants in Latrobe emitted 796 kg of Mn into the air in 2019, and 33,551 kg in the last 35 years, more than 4 times the amount released by Universal Stainless in Bridgeville [38]. However, prevailing winds in Latrobe are SW [43], and our study site was upwind (SW) of all 3 metal plants. With the wind direction and extra distance between these plants and the sampling site, we assume that the birds had less direct exposure to Mn emissions here than at Bridgeville. The prevailing wind direction and distance from a point source have been shown to significantly affect the degree of exposure to airborne Mn emissions [10].
2.3. Feather Sampling and Morphometrics
Tufted Titmice were caught using treadle traps. Treadle traps are baited walk-in traps with a pressure sensitive trigger, or treadle. When a bird steps on the treadle, the trap closes and prevents the bird from leaving.
Traps were opened and baited with black oil sunflower seeds three days before trapping began. This allowed birds to become familiar with the trap. Trapping only occurred at times without precipitation and when the temperature was above −4 °C. On the day of trapping, traps were checked every 20 min. When a Tufted Titmouse was caught, two feathers were plucked: a feather from the outer tail (r6) and a single contour feather from the breast. Both kinds of feather have been used in previous studies, so we collected both [4,31]. Neither of the sampled feathers is crucial to survival and they grow back after being plucked.
The 16 birds in this study were sampled between 18 September and 9 November 2019 following the typical period of pre-formative or pre-basic molt (Table S1). All feathers appeared to be of full length, so it was assumed that the collected feathers were recently grown. After the feathers were plucked, the bird’s body mass and linear measurements were taken. This enabled the calculation of a body mass index (BMI) in order to compare the physical condition of the birds in Bridgeville to those in Latrobe. To prevent interobserver bias in measurement, only one of us (R.S.) collected these data. Body mass was measured by placing a bird in a cloth bag and weighing it with a spring scale to the nearest 0.3 g (Pesola 100 g capacity, Switzerland). Linear measurements were collected using calipers to measure the length of the head and bill running from the back of the head to the tip of the bill. Since linear measurements are difficult to measure precisely, each bird was measured three times in succession to the nearest mm and the values were averaged. We applied a U.S. Fish and Wildlife Service leg band to enable identification and avoid resampling. The bird was then released into the wild.
2.4. Feather Preprocessing
Feathers were stored in paper envelopes until analysis. In the lab, feathers were washed in deionized water and then acetone [26]. Feather washing included scrubbing the feathers in order to remove external contaminants such as dust or preen oil. It was recently found that chemically and mechanically washing feathers does not remove all surface contaminants, and as a result, the age of the feather positively correlates to the amount of trace elements found, including Mn [30]. As noted earlier, feathers used in this study were assumed to be only a few months old. Feathers were dried on lint-free paper at room temperature. After the feathers dried, they were weighed using an electronic balance with 0.1 mg precision (Fisher Scientific XD-100A, Hanover Park, IL, USA). Feathers were cut into pieces approximately 1 mm each using surgical scissors. The entirety of each feather was then placed into its own individual 15 mL capped polystyrene test tube [26]. Next, 2.0 mL of high purity concentrated nitric acid and 1.0 mL of 30% hydrogen peroxide were added into the test tube. Tubes were placed into a 90 °C water bath. Test tube caps were loosely applied so that gases did not build up inside. The test tubes remained in the hot water bath for 60 min.
2.5. Microwave Plasma-Atomic Emission Spectrometry
In order to analyze the samples, the sample was split into two replicates and each was diluted with doubly distilled water to a final volume of 15.0 mL [26]. Sample replicates were analyzed with the microwave plasma–atomic emission spectrometer (MP–AES; Agilent, Santa Clara, CA, USA) at a wavelength of 403.076 nm. The MP–AES causes atoms of specific elements to emit light at wavelengths characteristic of each element. In order to calculate the concentration of Mn at the wavelength of 403.076 nm, a series of standards were made, ranging from 1.00 μg/L to 100 μg/L. The intra-assay error between replicates was 3.5%
2.6. Statistical Analyses
We used parametric statistics in the form of unpaired, two-tailed t-tests. The Jarque–Bera goodness-of-fit test showed that the data used in these tests were not significantly different from normal distributions (JB value range: 0.28–4.58, p-value range: 0.10–0.87). Likewise, one-tailed, two-sample F-tests showed that the groups being compared by the t-tests did not have variances that were significantly different from one another (F value range: 0.29–0.89, df = 7, p-value range: 0.06–0.40).
The first t-test was used to compare the tail feathers collected from the birds at the Bridgeville traps to the feathers collected from the birds near Latrobe. This allowed us to see the relationship between the distance from the known point source emitter of Mn (independent variable) and the concentration of Mn found in or on the feathers (dependent variable). The concentration of Mn, as determined by MP–AES, was corrected for the weight of the feather sample used [26]. Because the variances in the tail feather Mn at the two sites were considerably different, if not significantly so (F = 0.89, df = 7, p = 0.06), we employed a Welch t-test that assumes unequal variances and reported the results from that test in addition to the standard t-test. We used standard two-tailed t-tests assuming equal variance in order to compare the body mass, combined head and bill length, weights of plucked feathers, and the BMI of the two groups of birds. The BMI value was calculated by the following formula:
BMI = mass/(head length + bill length)
The BMI was compared to the tail feather Mn using a Pearson correlation test. All averages are reported ± SD.
3. Results
A total of sixteen birds were caught (Table S1). None of the datasets were significantly different from a normal distribution so we proceeded with parametric statistics. Tufted Titmice in Latrobe averaged 21.3 ± 1.2 g in mass, while the Bridgeville group averaged 20.3 ± 1.2 g. This 5% difference was not statistically significant (t14 = 1.65, p = 0.12). The Latrobe birds averaged slightly smaller in their combined head and bill length by 1%, but this again was not a significant difference (t14 = 0.93, p = 0.37). Group differences in BMI values were not statistically significant (t14 = 1.59, p = 0.26). The average BMI for the Latrobe group was 0.60 ± 0.03 g/mm. The average BMI for the Bridgeville group was 0.65 ± 0.03 g/mm.
The Latrobe tail feathers had slightly larger weight values than those of the Bridgeville group (Figure 1), but like the difference in body mass, this difference in feather weight was not statistically significant (t14 = 0.96, p = 0.70). The average feather weight for the Latrobe group was 10.5 ± 1.0 mg. The average weight for the Bridgeville group was 10.0 ± 1.2 mg.
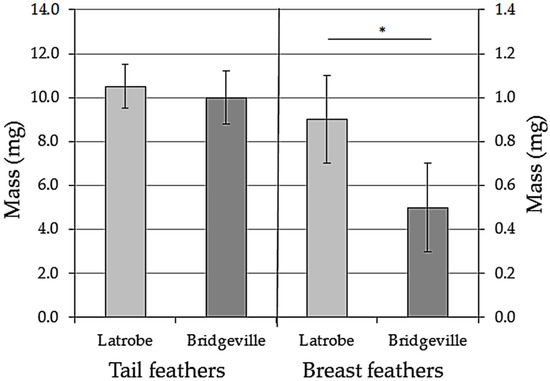
Figure 1.
Average (±SD) dry mass of feathers collected from Tufted Titmice captured at Latrobe, Pennsylvania, 4 km and upwind from a steel plant, and Bridgeville, Pennsylvania, 0.3 km and downwind from a different steel plant. A single outer tail feather and a single breast feather were collected from each bird. There were eight birds per group. The * symbol indicates that the mass of the breast feathers was significantly different between birds living in Latrobe and Bridgeville.
The breast feathers collected from both the Latrobe and Bridgeville groups were weighed after being washed in acetone. The weight values for the Latrobe feathers (0.9 ± 0.2 mg) were significantly higher than those of the Bridgeville sample (0.5 ± 0.2 mg; t14 = 3.11, p = 0.014; Figure 1).
The Bridgeville tail feathers had a greater concentration of Mn than those of the Latrobe group (Figure 2), and the difference was statistically significant using both a t-test assuming equal variance (t14 = 4.48, p = 0.0003) and the Welch’s t-test, which assumes unequal variance (t11 = 4.48, p = 0.0009). The average concentration of Mn for the Latrobe tail feathers was 3.97 ± 1.92 μg/L (range = 1.93–7.94). The average concentration of Mn for the Bridgeville tail feathers was 2.6 times higher, averaging 10.40 ± 3.58 μg/L (range = 4.39–15.35). The range of values within the two groups overlapped, and this is due to an extended tail in the Latrobe distribution that was created by two outliers of 5.69 μg/L and 7.94 μg/L. These sample values were not large enough to violate the assumption of normality (JB = 4.58, p = 0.10). BMI values were not correlated with tail feather Mn concentrations (r16 = −0.18, p = 0.51).
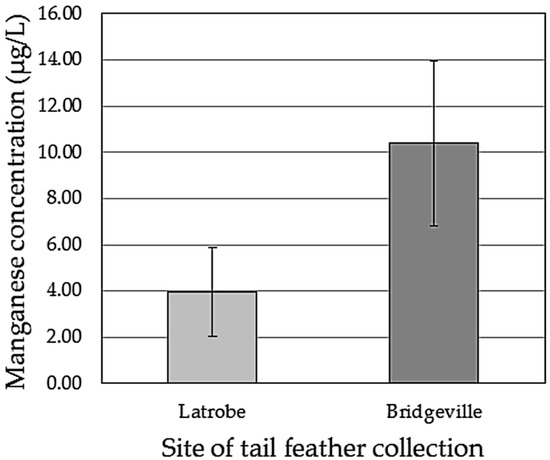
Figure 2.
Average (±SD) concentration of manganese found in outer tail feathers collected from Tufted Titmice captured at Latrobe, Pennsylvania, 4 km and upwind from a steel plant, and Bridgeville, Pennsylvania, 0.3 km and downwind from a different steel plant. Eight birds per group. Concentrations were calculated from the amount of manganese present in 15.0 mL sample tubes after acid digestion. Manganese was found in significantly higher amounts in the Bridgeville population.
Given that the Tufted Titmouse tail feathers in our study averaged 0.01 g of dry weight, and each feather was digested and then diluted into a 15.0 mL sample, every 1.0 μg/L in concentration equals approximately 1.5 μg/g of dry feather weight. Thus, the content of Mn in the Latrobe feathers ranged from 2.9 to 11.9 μg/g, and the content of Mn in the Bridgeville feathers ranged from 6.6 to 23.0 μg/g.
The quantity of Mn in breast feathers was below the limit of detectability (<1.00 μg/L).
4. Discussion
The average body mass index (BMI) for the Latrobe group was smaller than that of the Bridgeville group, but that difference was not statistically significant, and the BMI values were not correlated to the tail feather Mn concentration. It is important to remember that the physical size and health of the bird may be affected by several different factors. Dominance status within a social group impacts body mass and the foraging site. In Tufted Titmice, older males are more dominant than other age–sex combinations, and they carry less weight due to having better access to food [44]. Season, molt, and geographical variations also affect mass [28]. Since we did not assess age or make assumptions about the sex or dominance status of birds, there may be variations in the BMI and other study parameters related to those factors that we could not detect. Likewise, only 16 birds were sampled. This is a weakness of the study, and the finding of non-significant relationships may be due to low statistical power rather than a true lack of pattern. We were unable to detect Mn in the single breast feathers plucked from the Tufted Titmice. This is probably due to the smaller mass of breast feathers in comparison to tail feathers. It is therefore advisable to collect more than one breast feather from each bird, especially when using birds as small as Titmice.
Despite the low sample size, our results demonstrate that the tail feathers of Tufted Titmice accumulate Mn. No prior published study has attempted to measure Mn exposure in Tufted Titmice. Further, the steel plant-adjacent Bridgeville tail feathers had a statistically higher concentration of Mn than the Latrobe tail feathers, collected from birds living at a distance and upwind from the nearest Mn emitter. It is uncertain whether this increase is due solely to the air emissions of Universal Stainless or if the exposure was due to other pathways. The ambient Mn concentrations in both locations’ soil and air were not tested, so the route by which Mn found its way to the bird feathers is unclear.
Prior research on the Mn content of bird feathers has often centered on birds that consume ocean-based prey. The route of Mn contamination in these species appears to be via atmospheric deposition into the water, where it then bioaccumulates in the aquatic prey of the birds measured [4]. In this situation, there is no point source for the Mn, and the individual levels of Mn in each bird do not depend on their distance and direction from a known emitter. Inter-individual differences in Mn concentration would be based on differences in diet, rather than foraging location.
Meanwhile, there have been a few other studies of Mn in land-based bird species that do not consume ocean-based prey. The route of Mn exposure in these species is usually more direct. In our study and others, the Mn could have been ingested when birds consumed prey living in contaminated soil [1,6,25,26]. Or, it could have been introduced onto feather surfaces by birds coming into contact with contaminated soil [45]. Ingestion was proposed in a study of Cattle Egrets (Bubulcus ibis) in Pakistan, in which there were no reported differences in feather Mn between two areas that saw extensive industrial activity. However, no control group of birds living in an area with no known Mn pollution was used [6]. The Mn levels at one of the industrial sites in Pakistan were 2.7 times higher than those found in this study at the Latrobe study site and about equal (Pakistan: 16 μg/g) to those found in the feathers from Bridgeville (15.6 μg/g). The birds in Pakistan could have also been exposed to Mn via breathing contaminated dust, diesel and leaded gasoline fumes. Another study determined the concentrations of trace elements in the primary flight feathers of Rock Dove (Columba livia) and Carrion Crow (Corvus corone) in Israel. The study demonstrated that the lowest concentrations of most elements were found in dove feathers from the control population (captive population with known dietary habits), with increasing concentrations found in samples collected from free-living birds in areas of industrialization [26]. The average levels of Mn detected in Tufted Titmice at Latrobe were lower than those found in all the groups of free-living doves in Israel, even at rural sites [26]. The Mn levels in the Latrobe birds were also slightly lower than the estimated marginal mean value of 5.28 ug/g, which was calculated for feathers collected from 26 bird species at a site in Spain used by researchers as a “reference area”, with background levels of trace elements [45]. Data from this reference area were compared to two other sites in Spain with a long history of mining and metallurgy, where concentrations of Mn were higher [45]. We did not collect feathers from a reference or control site, so even though we consider the Titmice living near Latrobe to have only background levels of Mn in their feathers, we cannot know whether the proximity of the factories there still contributes to the levels detected.
Perhaps the best comparison between this study’s findings and a prior publication is that of Muhawenimana [1] in Poland, where researchers found that Mn in feathers increased linearly as the distance to a coal mine decreased. They measured levels in Snow Bunting (Plectrophenax nivalis) nestlings closest to the mine at around 2.9 μg/g of dry feather weight, and at 0.7 μg/g of dry feather weight at another location 7 km away. These values are much lower than those measured in the present study, but here, adult feathers were measured rather than those of nestlings. Varying diets and species differences might contribute to these differences. Muhawenimana [1] suggested that the metal was likely ingested with food, but the inhaled route could not be ruled out. The findings of Borgheri et al. [30] show that Mn may be found on feather surfaces, even after washing, so this reduces clarity regarding how Mn was introduced to the birds in the present study. In either case, the differences among individuals could be due to differences in the foraging location.
It is interesting that the range in the Mn values of the tail feathers from the two groups of birds overlapped by a small degree, with two Latrobe birds having values higher than the two lowest values found in the Bridgeville birds. It is possible that the two Latrobe birds could have frequented areas downwind of the metal-producing plants during or after feather development, and likewise, the birds in Bridgeville with lower levels may have lived farther away or perhaps upwind from the Universal Stainless plant during or after feather development. No attempt was made to age the Tufted Titmice in the present study, so it could be that some birds were the young of the year. Young disperse 75 km from their natal territory [46]. Birds older than the hatch-year are territorial and are unlikely to have spent much time beyond territory boundaries. The average winter territory size is 7.9 ha, and the linear dimension of a territory has not been reported to exceed 914 m [32]. There are slight feather molt patterns that can be used to distinguish Tufted Titmice less than and greater than one year in age. Any future study of the trace metal environmental contaminates in feathers should employ aging techniques.
Biomonitoring through feather analysis is a non-destructive and non-invasive way to monitor environmental contamination, and it has several advantages over other techniques. First, a single or small number of feathers may be taken without causing harm to the birds [47]. Second, the contaminant will stay in the feather indefinitely, so the concentration found represents a defined period in which the animal was exposed. Third, the variations found between birds are likely to be due to differences in their environment rather than to metabolic patterns related to the time of day or the time since exposure. The concentration of Mn in blood samples is subject to a greater level of variation due to these variables. Finally, contaminate concentrations will be higher in feather material than in blood or soft tissue since contaminates are sequestered there throughout feather development. This makes it easier to measure trace elements without extraordinary laboratory analysis.
Our study is particularly significant to environmental interests in our region of the United States, where there is a long history of coal mining and steel manufacturing. These industries operate on much smaller scales today than earlier in the twentieth century, and emissions are more restricted and enforced. Therefore, it is possible that the Mn found in the Titmouse feathers measured in this study is not only from contemporary Mn emissions, but is also from legacy contamination, caused by the accumulation of the element in the soil. Additional, non-biological samples from the environment should be collected when conducting studies like ours, as that may help determine the route of Mn exposure.
As new vegetation and animals colonize former industrial sites, the human population may not be aware of past contamination, but human and non-human organisms continue to be exposed. We can make use of non-invasive biomarkers to identify locations that still have the potential to generate environmental harm.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/birds4010012/s1, Table S1: Manganese concentrations in feathers collected from Tufted Titmouse caught at varying distances from a metal processing plant.
Author Contributions
Conceptualization, R.S.; methodology, R.S. and J.S.K.; validation, R.S.; formal analysis, R.S.; investigation, R.S.; resources, J.S.K.; writing—original draft preparation, R.S.; writing—review and editing, J.S.K.; visualization, J.S.K.; supervision, J.S.K.; project administration, R.S.; funding acquisition, R.S. and J.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Saint Vincent College A.J. Palumbo Endowment Grant awarded to R.S., and by the Biological Sciences Department at Saint Vincent College.
Institutional Review Board Statement
Birds were banded and feathers collected under U.S. Fish and Wildlife Service bird-banding permit #23382 issued to J.S.K., and the work was approved by the Saint Vincent College Animal Care and Use Committee under protocol #19-017.
Data Availability Statement
This study’s complete dataset is contained within Table S1.
Acknowledgments
We thank the landowner in Bridgeville for access to our bird trapping site. We thank I. Taylor of the Chemistry Department at Saint Vincent College for assisting with the metal analysis using the MP-AES.
Conflicts of Interest
J.K. has no conflict of interest in publishing this study. R.S. has no financial conflict of interest. She resided near Universal Stainless in Bridgeport, and public reports of air emissions coming from the plant prompted her to conduct this study. No blame is assigned here to either Universal Stainless or the three facilities in Latrobe for producing the Mn that was found in the bird feathers, and the authors make no claim that the birds were harmed by the Mn. In fact, the authors did not use the word “pollution” to describe Mn released into the environment by the steel plants, as that word might show negative connotation and bias.
References
- Muhawenimana, F. Essential and Non-Essential Elements in Feathers of Snow Bunting Nestlings of Longyearbyen and Adventdalen—Svalbard 2016. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2016. [Google Scholar]
- Carson, R. Silent Spring; Penguin: London, UK, 2002. [Google Scholar]
- Pollock, C. The canary in the coal mine. J. Avian Med. Surg. 2016, 30, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Comparison of arsenic, cadmium, chromium, lead, manganese, mercury and selenium in feathers in Bald Eagle (Haliaeetus leucocephalus), and comparison with Common Eider (Somateria mollissima), Glaucous-Winged Gull (Larus glaucescens), Pigeon Guillemot (Cepphus columba), and Tufted Puffin (Fratercula cirrhata) from the Aleutian Chain of Alaska. Environ. Monit. Assess. 2009, 152, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.M.; Jerrett, M. A study of the relationships between Parkinson’s Disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ. Res. 2007, 104, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Fasola, M.; Muhammad, A.; Malik, S.A.; Bostan, N.; Bokhari, H.; Kamran, M.A.; Shafqat, M.N.; Alamdar, A.; Khan, M.; et al. Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: A case study from severely contaminated areas. Chemosphere 2015, 119, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Hagelstein, K. Globally sustainable manganese metal production and use. J. Environ. Manag. 2009, 90, 3736–3740. [Google Scholar] [CrossRef] [PubMed]
- Fitsanakis, V.A.; Garcia, S.J.; Aschner, M. Manganese dynamics, distribution, and neurotoxicity. In The Role of Glia in Neurotoxicity; Aschner, M., Costa, L.G., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 395–416. [Google Scholar]
- Colledge, M.A.; Julian, J.R.; Gocheva, V.V.; Beseler, C.L.; Roles, H.A.; Lobdell, D.T.; Bowler, R.M. Characterization of air manganese exposure estimates for residents in two Ohio towns. J. Air Waste Manag. Assoc. 2015, 65, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Haynes, E.N.; Heckel, P.; Ryan, P.; Roda, S.; Leung, Y.-K.; Sebastian, K.; Succop, P. Environmental manganese exposure in residents living near a ferromanganese refinery in Southeast Ohio: A pilot study. NeuroToxicology 2010, 31, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kalisinska, E.; Budis, H. Manganese, Mn: An Ecotoxicological Assessment of the Northern Hemisphere. In Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments; Kalisinska, E., Ed.; Springer: Cham, Switzerland, 2019; pp. 213–246. [Google Scholar] [CrossRef]
- Hui, C.A. Concentrations of chromium, manganese, and lead in air and in avian eggs. Environ. Pollut. 2002, 120, 201–206. [Google Scholar] [CrossRef]
- Röllin, H.B.; Nogueira, C.M.C.A. Manganese: Environmental pollution and health effects. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J.O., Ed.; Elsevier Science: San Diego, CA, USA, 2011; pp. 617–629. ISBN 978-0-444-52272-6. [Google Scholar]
- Jankowski, J.; Ognik, K.; Stępniowska, A.; Zduńczyk, Z.; Kozłowski, K. The effect of the source and dose of manganese on the performance, digestibility and distribution of selected minerals, redox, and immune status of turkeys. Poult. Sci. 2019, 98, 1379–1389. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Growth and behavioral effects of early postnatal chromium and manganese exposure in herring gull (Larus argentatus) chicks. Pharmacol. Biochem. Behav. 1995, 50, 607–612. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Body-Mass-Dependent Trade-off between Immune Response and Uropygial Gland Size in House Sparrows Passer domesticus. J. Avian Biol. 2015, 46, 40–45. [Google Scholar] [CrossRef]
- Vafidis, J.O.; Vaughan, I.P.; Jones, T.H.; Facey, R.J.; Parry, R.; Thomas, R.J. Habitat Use and Body Mass Regulation among Warblers in the Sahel Region during the Non-Breeding Season. PLoS ONE 2014, 9, e113665. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Senar, J.C. Resident but not transient Eurasian Siskins reduce body mass in response to increasing predation risk: A natural experiment. J. Ornithol. 2015, 156, 451–456. [Google Scholar] [CrossRef]
- Liker, A.; Papp, Z.; Bókony, V.; Lendvai, Á.Z. Lean birds in the city: Body size and condition of house sparrows along the urbanization gradient. J. Anim. Ecol. 2008, 77, 789–795. [Google Scholar] [CrossRef]
- Theuerkauf, J.; Haneda, T.; Okahisa, Y.; Sato, N.J.; Rouys, S.; Bloc, H.; Ueda, K.; Watanabe, I.; Kuehn, R.; Gula, R. Elevated concentrations of naturally occurring heavy metals inversely correlate with reproductive output and body mass of the Kagu Rhynochetos jubatus. IBIS 2017, 159, 580–587. [Google Scholar] [CrossRef]
- Bouabid, S.; Delaville, C.; De Deurwaerdère, P.; Lakhdar Ghazal, N.; Benazzouz, A. Manganese-Induced Atypical Parkinsonism Is Associated with Altered Basal Ganglia Activity and Changes in Tissue Levels of Monoamines in the Rat. PLoS ONE 2014, 9, e98952. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, A.J.; Fakunle, P.B.; Fatoba, O.; Olayemi, O.T. Some effects of manganese dichloride administration on the body weight, Purkinje cell population, brain, and cerebellar weights of adult Wistar rats. J. Neurosci. Behav. Health 2011, 3, 87–90. [Google Scholar]
- Rutkowska, M.; Plotka-Wasylka, J.; Lubinska-Szczygel, M.; Rozanska, A.; Mozejko-Ciesielska, J.; Namiesnik, J. Birds’ feathers—Suitable samples for determination of environmental pollutants. TrAC Trends Anal. Chem. 2018, 109, 97–115. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Veerle, J.; Dauwe, T.; Pinxten, R.; Bervoets, L.; Blust, R.; Eens, M. The importance of exogenous contamination on heavy metal levels in bird feathers. A field experiment with free-living Great Tits, Parus major. J. Environ. Monit. 2004, 6, 356–360. [Google Scholar] [CrossRef]
- Adout, A.; Hawlena, D.; Maman, R.; Paz-Tal, O.; Karpas, Z. Determination of trace elements in pigeon and raven feathers by ICPMS. Int. J. Mass Spectrom. 2007, 267, 109–116. [Google Scholar] [CrossRef]
- Terres, J.K. The Audubon Society Encyclopedia of North American Birds; Knopf: New York, NY, USA, 1980; pp. 616–617. [Google Scholar]
- Monteiro, L.R.; Furness, R.W. Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut. 1995, 80, 851–870. [Google Scholar] [CrossRef]
- Pedro, S.; Xavier, J.C.; Tavares, S.; Trathan, P.N.; Ratcliffe, N.; Paiva, V.H.; Medeiros, R.; Pereira, E.; Pardal, M.A. Feathers as a tool to assess mercury contamination in Gentoo Penguins: Variations at the individual level. PLoS ONE 2015, 10, e0137622. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, F.; Migani, F.; Andreotti, A.; Baccetti, N.; Bianchi, N.; Birke, M.; Dinelli, E. Metals and trace elements in feathers: A geochemical approach to avoid misinterpretation of analytical responses. Sci. Total Environ. 2016, 544, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, T.; Bervoets, L.; Blust, R.; Eens, M. Tissue levels of lead in experimentally exposed Zebra Finches (Taeniopygia guttata) with particular attention on the use of feathers as biomonitors. Arch. Environ. Contam. Toxicol. 2001, 42, 88–92. [Google Scholar] [CrossRef]
- Ritchison, G.; Grubb, T.C., Jr.; Pravosudov, V.V. Tufted Titmouse (Baeolophus bicolor), Version 2.0. In The Birds of North America; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2015. [Google Scholar]
- Beck, H.; Zimmermann, N.; McVicar, T.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. NOAA Online Weather Data. Available online: https://www.weather.gov/wrh/Climate?wfo=pbz (accessed on 16 March 2023).
- Ghosh, S.; Byahut, S.; Masilela, C. Metropolitan Regional Scale Smart City Approaches in a Shrinking City in the American Rust Belt—Case of Pittsburgh, Pennsylvania. In Smart Metropolitan Regional Development; Advances in 21st Century Human Settlements; Vinod Kumar, T., Ed.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Ecoregions of North America. Available online: https://www.epa.gov/eco-research/ecoregions-north-america (accessed on 16 March 2023).
- U.S. Environmental Protection Agency. My Environment. Available online: https://geopub.epa.gov/myem/envmap/find.html (accessed on 30 December 2021).
- U.S. Environmental Protection Agency. Enforcement and Compliance History Online. Detailed Facility Reports for Universal Stainless and Alloy Products, Inc. Available online: https://echo.epa.gov/detailed-facility-report?fid=110000328743 (accessed on 8 March 2023).
- Windfinder.com GmbH & Co. KG, Windfinder app. Wind and Weather Statistics for Allegheny County Airport. Available online: https://www.windfinder.com/windstatistics/allegheny_county_airport (accessed on 8 March 2023).
- U.S. Environmental Protection Agency. Enforcement and Compliance History Online. Detailed Facility Report for Latrobe Specialty Steel Co. Available online: https://echo.epa.gov/detailed-facility-report?fid=110000330188 (accessed on 8 March 2023).
- U.S. Environmental Protection Agency. Enforcement and Compliance History Online. Detailed Facility Report for Lehigh Specialty Melting Co. Available online: https://echo.epa.gov/detailed-facility-report?fid=110000330204 (accessed on 8 March 2023).
- U.S. Environmental Protection Agency. Enforcement and Compliance History Online. Detailed Facility Report for Allegheny Ludlum Latrobe Plant. Available online: https://echo.epa.gov/detailed-facility-report?fid=110056954381 (accessed on 8 March 2023).
- Windfinder.com GmbH & Co. KG, Windfinder app. Wind and Weather Statistics for Arnold Palmer Regional Airport. Available online: https://www.windfinder.com/windstatistics/latrobe_arnold-palmer-airport (accessed on 7 March 2023).
- Pravosudov, V.V.; Grubb, T.C., Jr.; Doherty, P.F., Jr.; Bronson, C.L.; Pravosudova, E.V.; Dolby, A.S. Social dominance and energy reserves in wintering woodland birds. Condor 1999, 101, 880–884. [Google Scholar] [CrossRef]
- Durkalec, M.; Martínez-Haro, M.; Nawrocka, A.; Pareja-Carrera, J.; Smits, J.E.G.; Mateo, R. Factors influencing lead, mercury and other trace element exposure in birds from metal mining areas. Environ. Res. 2022, 212, 113575. [Google Scholar] [CrossRef]
- Tittler, R.; Villard, M.-A.; Fahrig, L. How far do songbirds disperse? Ecography 2009, 32, 1051–1061. [Google Scholar] [CrossRef]
- Fossi, M.C. Nondestructive biomarkers in ecotoxicology. Environ. Health Perspect. 1994, 102, 49–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).