Active Agents Incorporated in Polymeric Substrates to Enhance Antibacterial and Antioxidant Properties in Food Packaging Applications
Abstract
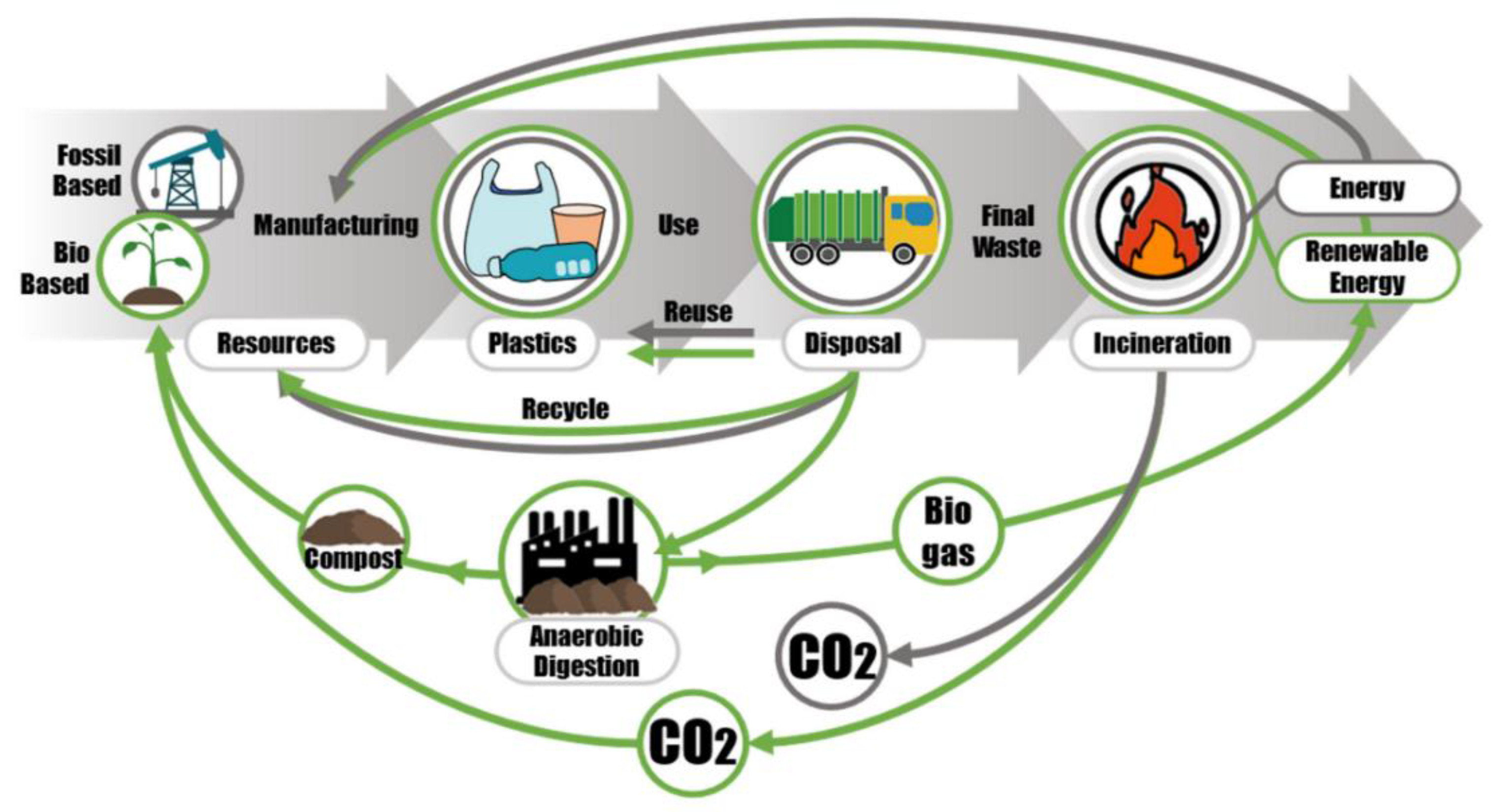
:1. Introduction
2. Bulk Preparation Technologies
2.1. Compression Molding
2.2. Injection Molding
2.3. Extrusion Molding
2.4. Blow Molding
2.5. Thermoforming
2.6. Electrospinning
3. Active Agents incorporated in Bulk Preparation Technologies
3.1. Inorganic Active Agents
3.1.1. Metals and Metal Oxides
3.1.2. Nanoclays
3.1.3. Other Inorganic Compounds
3.2. Organic Active Agents
4. Detailed Overview of Extrusion Molding’s Industrial Use for Active Food Packaging
4.1. Active Agents Incorporated into Synthetic Polymers
4.2. Active Agents Incorporated into Biopolymers
5. Challenges and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food Packaging Market Size, Growth and Industry Trends 2028. Available online: https://www.fortunebusinessinsights.com/industry-reports/food-packaging-market-101941 (accessed on 2 November 2022).
- Hakovirta, M.; Denuwara, N. How COVID-19 Redefines the Concept of Sustainability. Sustainability 2020, 12, 3727. [Google Scholar] [CrossRef]
- Barone, A.S.; Matheus, J.R.V.; Souza, T.S.P.; Moreira, R.F.A.; Fai, A.E.C. Green-based Active Packaging: Opportunities beyond COVID-19, Food Applications, and Perspectives in Circular Economy—A Brief Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4881–4905. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Recent Breakthroughs of Antibacterial and Antiviral Protective Polymeric Materials during COVID-19 Pandemic and after Pandemic: Coating, Packaging, and Textile Applications. Curr. Opin. Colloid Interface Sci. 2021, 55, 101480. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- de Jong, A.R.; Boumans, H.; Slaghek, T.; van Veen, J.; Rijk, R.; van Zandvoort, M. Active and Intelligent Packaging for Food: Is It the Future? Food Addit. Contam. 2005, 22, 975–979. [Google Scholar] [CrossRef]
- EUR-Lex-32009R0450-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R0450 (accessed on 2 November 2022).
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial Edible Films in Food Packaging: Current Scenario and Recent Nanotechnological Advancements-a Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Licciardello, F.; Muratore, G.; Mercea, P.; Tosa, V.; Nerin, C. Diffusional Behaviour of Essential Oil Components in Active Packaging Polypropylene Films by Multiple Headspace Solid Phase Microextraction-Gas Chromatography. Packag. Technol. Sci. 2013, 26, 173–185. [Google Scholar] [CrossRef]
- Conte, A.; Longano, D.; Costa, C.; Ditaranto, N.; Ancona, A.; Cioffi, N.; Scrocco, C.; Sabbatini, L.; Contò, F.; del Nobile, M.A. A Novel Preservation Technique Applied to Fiordilatte Cheese. Innov. Food Sci. Emerg. Technol. 2013, 19, 158–165. [Google Scholar] [CrossRef]
- Brebu, M. Environmental Degradation of Plastic Composites with Natural Fillers—A Review. Polymers 2020, 12, 166. [Google Scholar] [CrossRef] [Green Version]
- Muniyasamy, S.; John, M.J. Biodegradability of biobased polymeric materials in natural environments. In Handbook of Composites from Renewable Materials; Wiley: New York, NY, USA, 2017; Volume 1–8, pp. 625–653. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial Agents and Packaging Systems in Antimicrobial Active Food Packaging: An Overview of Approaches and Interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Yam, K.L. The Wiley Encyclopedia of Packaging Technology, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Afr. 2022, 1–28. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable Polymers for Food Packaging: A Review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Subramanian, M.N. Plastics and Polymers Collection Basics of Polymers Fabrication and Processing Technology; Momentum Press: London, UK, 2015. [Google Scholar]
- Schmidtchen, L.; Roleda, M.Y.; Majschak, J.-P.; Mayser, M. Processing Technologies for Solid and Flexible Packaging Materials from Macroalgae. Algal. Res. 2022, 61, 102300. [Google Scholar] [CrossRef]
- González, K.; Iturriaga, L.; González, A.; Eceiza, A.; Gabilondo, N. Improving Mechanical and Barrier Properties of Thermoplastic Starch and Polysaccharide Nanocrystals Nanocomposites. Eur. Polym. J. 2020, 123, 109415. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- di Bartolo, A.; Infurna, G.; Dintcheva, N.T. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef]
- Tatara, R.A. Compression molding. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–320. [Google Scholar] [CrossRef]
- Farris, S. Main manufacturing processes for food packaging materials. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Kobayashi, M. Study of Monitoring Method and Melt Flow Behavior in Compression Molding Process Using Thermoplastic Sheets Reinforced with Discontinuous Long-Fibers. J. Compos. Sci. 2021, 5, 50. [Google Scholar] [CrossRef]
- Asim, M.; Jawaid, M.; Saba, N.; Ramengmawii; Nasir, M.; Sultan, M.T.H. Processing of hybrid polymer composites—A review. In Hybrid Polymer Composite Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. [Google Scholar] [CrossRef]
- Xie, F.; Flanagan, B.M.; Li, M.; Sangwan, P.; Truss, R.W.; Halley, P.J.; Strounina, E.v.; Whittaker, A.K.; Gidley, M.J.; Dean, K.M.; et al. Characteristics of Starch-Based Films Plasticised by Glycerol and by the Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate: A Comparative Study. Carbohydr. Polym. 2014, 111, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Simaafrookhteh, S.; Khorshidian, M.; Momenifar, M. Fabrication of Multi-Filler Thermoset-Based Composite Bipolar Plates for PEMFCs Applications: Molding Defects and Properties Characterizations. Int J. Hydrogen Energy 2020, 45, 14119–14132. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; de la Rosa-Ramírez, H.; López-Martínez, J.; Arrieta, M.P. Improvement of PBAT Processability and Mechanical Performance by Blending with Pine Resin Derivatives for Injection Moulding Rigid Packaging with Enhanced Hydrophobicity. Polymers 2020, 12, 2891. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.T.; Nuradibah, M.A.; Chin, K.M.; Hani, N. Current application and challenges on packaging industry based on natural polymer blending. In Natural Polymers; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–184. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Tengku Yasim-Anuar, T.A.; Sapuan, S.M.; Ilyas, R.A.; Hakimi, M.I.; Syed Najmuddin, S.U.F.; Jenol, M.A. Nanocellulose reinforced polypropylene and polyethylene composite for packaging application. In Bio-Based Packaging; Wiley: New York, NY, USA, 2021; pp. 133–150. [Google Scholar] [CrossRef]
- Shrivastava, A. Introduction to Plastics Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Chokshi, R.; Zia, H. Hot-Melt Extrusion Technique: A Review. Iran. J. Pharm. Res. 2004, 3, 3–16. [Google Scholar]
- Okubo, H.; Kaneyasu, H.; Kimura, T.; Phanthong, P.; Yao, S. Effects of a Twin-Screw Extruder Equipped with a Molten Resin Reservoir on the Mechanical Properties and Microstructure of Recycled Waste Plastic Polyethylene Pellet Moldings. Polymers 2021, 13, 1058. [Google Scholar] [CrossRef] [PubMed]
- Belcher, S.L. Blow molding. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2017; pp. 265–289. [Google Scholar] [CrossRef]
- Kazmer, D. Design of plastic parts. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–615. [Google Scholar] [CrossRef]
- Ashter, S.A. Matching material characteristics to commercial thermoforming. In Thermoforming of Single and Multilayer Laminates; Elsevier: Amsterdam, The Netherlands, 2014; pp. 193–209. [Google Scholar] [CrossRef]
- Throne, J. Thermoforming. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2011; pp. 333–358. [Google Scholar] [CrossRef]
- Banús, N.; Boada, I.; Xiberta, P.; Toldrà, P.; Bustins, N. Deep Learning for the Quality Control of Thermoforming Food Packages. Sci. Rep. 2021, 11, 21887. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Guo, H.; Liu, L.; Xu, W.; Duan, G. Structural Design toward Functional Materials by Electrospinning: A Review. e-Polymers 2020, 20, 682–712. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Ding, Y.; Agarwal, S.; Greiner, A.; Duan, G. A Review of Smart Electrospun Fibers toward Textiles. Compos. Commun. 2020, 22, 100506. [Google Scholar] [CrossRef]
- Phan, D.-N.; Dorjjugder, N.; Khan, M.Q.; Saito, Y.; Taguchi, G.; Lee, H.; Mukai, Y.; Kim, I.-S. Synthesis and Attachment of Silver and Copper Nanoparticles on Cellulose Nanofibers and Comparative Antibacterial Study. Cellulose 2019, 26, 6629–6640. [Google Scholar] [CrossRef]
- Amna, T.; Yang, J.; Ryu, K.-S.; Hwang, I.H. Electrospun Antimicrobial Hybrid Mats: Innovative Packaging Material for Meat and Meat-Products. J. Food Sci. Technol. 2015, 52, 4600–4606. [Google Scholar] [CrossRef]
- Vega-Lugo, A.-C.; Lim, L.-T. Controlled Release of Allyl Isothiocyanate Using Soy Protein and Poly(Lactic Acid) Electrospun Fibers. Food Res. Int. 2009, 42, 933–940. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Isaac, B.; Taylor, R.M.; Reifsnider, K. Mechanical and Dielectric Properties of Aligned Electrospun Fibers. Fibers 2021, 9, 4. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of Nanofibers: Potentials and Perspectives for Active Food Packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A Review on Techniques Utilized for Design of Controlled Release Food Active Packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 2601–2621. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.-S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled Release of Antioxidants from Active Food Packaging: A Review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Pušnikčrešnar, K.; Pušnikčrešnar, P.; Aulova, A.; Bikiaris, D.N.; Lambropoulou, D.; Kuzmič, K.; Zemljič, L.F.; Stoleru, E. Incorporation of Metal-Based Nanoadditives into the PLA Matrix: Effect of Surface Properties on Antibacterial Activity and Mechanical Performance of PLA Nanoadditive Films. Molecules 2021, 26, 4161. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, W.; Wang, B.; Kang, X.; Liu, P.; Cui, B.; Abd El-Aty, A.M. Preparation and Evaluation of Starch-Based Extrusion-Blown Nanocomposite Films Incorporated with Nano-ZnO and Nano-SiO2. Int. J. Biol. Macromol. 2021, 183, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of Nanocomposite Packaging Containing Ag and ZnO on Shelf Life of Fresh Orange Juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Črešnar, K.P.; Zemljič, L.F.; Papadopoulos, L.; Terzopoulou, Z.; Zamboulis, A.; Klonos, P.A.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Effects of Ag, ZnO and TiO2 Nanoparticles at Low Contents on the Crystallization, Semicrystalline Morphology, Interfacial Phenomena and Segmental Dynamics of PLA. Mater. Today Commun. 2021, 27, 102192. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Ghanbarzadeh, B.; Divband, B. Polyvinyl Alcohol/Gelatin Nanocomposite Containing ZnO, TiO2 or ZnO/TiO2 Nanoparticles Doped on 4A Zeolite: Microbial and Sensory Qualities of Packaged White Shrimp during Refrigeration. Int. J. Food Microbiol. 2020, 312, 108375. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Hong, S.I.; Ha, C.S. Tensile, Water Vapor Barrier and Antimicrobial Properties of PLA/Nanoclay Composite Films. LWT-Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Vidotti, S.E.; Chinellato, A.C.; Hu, G.-H.; Pessan, L.A. Effect of an Organo-Modified Montmorillonite on the Barrier Properties of PET Nanocomposites Using a Polyester Ionomer as a Compatibilizing Agent. Mater. Res. 2017, 20, 826–834. [Google Scholar] [CrossRef] [Green Version]
- Majdzadeh-Ardakani, K.; Zekriardehani, S.; Coleman, M.R.; Jabarin, S.A. A Novel Approach to Improve the Barrier Properties of PET/Clay. Int. J. Polym. Sci. 2017, 2017, 7625906. [Google Scholar] [CrossRef]
- Ceballos, R.L.; von Bilderling, C.; Guz, L.; Bernal, C.; Famá, L. Effect of Greenly Synthetized Silver Nanoparticles on the Properties of Active Starch Films Obtained by Extrusion and Compression Molding. Carbohydr. Polym. 2021, 261, 117871. [Google Scholar] [CrossRef]
- Simbine, E.O.; Rodrigues, L.D.C.; Lapa-Guimaraes, J.; Kamimura, E.S.; Corassin, C.H.; Oliveira, C.A.F.D. Application of Silver Nanoparticles in Food Packages: A Review. Food Sci. Technol. 2019, 39, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Wu, Y.; Li, Y. Development of Tea Extracts and Chitosan Composite Films for Active Packaging Materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- Chenwei, C.; Zhipeng, T.; Yarui, M.; Weiqiang, Q.; Fuxin, Y.; Jun, M.; Jing, X. Physicochemical, Microstructural, Antioxidant and Antimicrobial Properties of Active Packaging Films Based on Poly(Vinyl Alcohol)/Clay Nanocomposite Incorporated with Tea Polyphenols. Prog. Org. Coat. 2018, 123, 176–184. [Google Scholar] [CrossRef]
- Zia, J.; Paul, U.C.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Low-Density Polyethylene/Curcumin Melt Extruded Composites with Enhanced Water Vapor Barrier and Antioxidant Properties for Active Food Packaging. Polymer (Guildf.) 2019, 175, 137–145. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular Interactions, Characterization and Antimicrobial Activity of Curcumin–Chitosan Blend Films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Xin Seow, Y.; Rou Yeo, C.; Ling Chung, H.; Yuk, H.-G.; Yuk, H. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Fras Zemljič, L.; Plohl, O.; Vesel, A.; Luxbacher, T.; Potrč, S. Physicochemical Characterization of Packaging Foils Coated by Chitosan and Polyphenols Colloidal Formulations. Int. J. Mol. Sci. 2020, 21, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potrč, S.; Sterniša, M.; Možina, S.S.; Hrnčič, M.K.; Zemljič, L.F. Bioactive Characterization of Packaging Foils Coated by Chitosan and Polyphenol Colloidal Formulations. Int J. Mol. Sci. 2020, 21, 2610. [Google Scholar] [CrossRef] [Green Version]
- Solihat, N.N.; Sari, F.P.; Falah, F.; Ismayati, M.; Lubis, M.A.R.; Fatriasari, W.; Santoso, E.B.; Syafii, W. Lignin as an Active Biomaterial: A Review. J. Sylva Lestari 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Črešnar, K.P.; Klonos, P.A.; Zamboulis, A.; Terzopoulou, Z.; Xanthopoulou, E.; Papadopoulos, L.; Kyritsis, A.; Kuzmič, K.; Zemljič, L.F.; Bikiaris, D.N. Structure-Properties Relationships in Renewable Composites Based on Polylactide Filled with Tannin and Kraft Lignin-Crystallization and Molecular Mobility. Acta 2021, 703, 178998. [Google Scholar] [CrossRef]
- Črešnar, K.P.; Zamboulis, A.; Bikiaris, D.N.; Aulova, A.; Zemljič, L. Kraft Lignin/Tannin as a Potential Accelerator of Antioxidant and Antibacterial Properties in an Active Thermoplastic Polyester-Based Multifunctional Material. Polymers 2022, 14, 1532. [Google Scholar] [CrossRef]
- Ahn, B.J.; Gaikwad, K.K.; Lee, Y.S. Characterization and Properties of LDPE Film with Gallic-Acid-Based Oxygen Scavenging System Useful as a Functional Packaging Material. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- di Giuseppe, F.; Coffigniez, F.; Aouf, C.; Guillard, V.; Torrieri, E. Activated Gallic Acid as Radical and Oxygen Scavenger in Biodegradable Packaging Film. Food Packag. Shelf Life 2022, 31, 100811. [Google Scholar] [CrossRef]
- Sung, S.Y.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.C.; Vikhraman, M. Antimicrobial Agents for Food Packaging Applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver Nanoparticles in Polymeric Matrices for Fresh Food Packaging. J. King Saud Univ.-Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Ahari, H.; Lahijani, L.K. Migration of Silver and Copper Nanoparticles from Food Coating. Coatings 2021, 11, 380. [Google Scholar] [CrossRef]
- Llorens, A.; Lloret, E.; Picouet, P.; Fernandez, A. Study of the Antifungal Potential of Novel Cellulose/Copper Composites as Absorbent Materials for Fruit Juices. Int. J. Food Microbiol. 2012, 158, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D.N.; Triantafyllidis, K.S. HDPE/Cu-Nanofiber Nanocomposites with Enhanced Antibacterial and Oxygen Barrier Properties Appropriate for Food Packaging Applications. Mater. Lett. 2013, 93, 1–4. [Google Scholar] [CrossRef]
- de Azeredo, H.M.C. Antimicrobial Nanostructures in Food Packaging. Trends Food Sci. Technol. 2013, 30, 56–69. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V. Nanocomposites Based on Biosafe Nano ZnO and Different Polymeric Matrixes for Antibacterial, Optical, Thermal and Mechanical Applications. Eur. Polym. J. 2016, 84, 377–403. [Google Scholar] [CrossRef]
- Esmailzadeh, H.; Sangpour, P.; Shahraz, F.; Hejazi, J.; Khaksar, R. Effect of Nanocomposite Packaging Containing ZnO on Growth of Bacillus Subtilis and Enterobacter Aerogenes. Mater. Sci. Eng. C 2016, 58, 1058–1063. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of Nanocomposite Packaging Containing Ag and ZnO on Inactivation of Lactobacillus Plantarum in Orange Juice. Food Control. 2011, 22, 408–413. [Google Scholar] [CrossRef]
- Esmailzadeh, H.; Sangpour, P.; Shahraz, F.; Eskandari, A.; Hejazi, J.; Khaksar, R.; Hejazi jhejazi, J. CuO/LDPE Nanocomposite for Active Food Packaging Application: A Comparative Study of Its Antibacterial Activities with ZnO/LDPE Nanocomposite. Polym. Bull. 2021, 78, 1671–1682. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Zhang, L.; Xu, Q.; Che, Z.; Li, W.; Bai, Y.; Li, K. Effect of TiO 2 Nanoparticles on the Antibacterial and Physical Properties of Polyethylene-Based Film. Prog Org. Coat. 2012, 73, 219–224. [Google Scholar] [CrossRef]
- Tarani, E.; Pušnikčrešnar, K.; Pušnikčrešnar, P.; Fras Zemljič, L.; Chrissafis, K.; Papageorgiou, G.Z.; Lambropoulou, D.; Zamboulis, A.; Bikiaris, D.N.; Terzopoulou, Z. Cold Crystallization Kinetics and Thermal Degradation of PLA Composites with Metal Oxide Nanofillers. Appl. Sci. 2021, 11, 3004. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Purohit, S.D.; Roy, S.; Ghosh, T.; Rhim, J.W.; Han, S.S. Antiviral Biodegradable Food Packaging and Edible Coating Materials in the COVID-19 Era: A Mini-Review. Coatings 2022, 12, 577. [Google Scholar] [CrossRef]
- Randazzo, W.; Fabra, M.J.; Falcó, I.; López-Rubio, A.; Sánchez, G. Polymers and Biopolymers with Antiviral Activity: Potential Applications for Improving Food Safety. Compr. Rev. Food Sci. Food Saf. 2018, 17, 754–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarzadeh, A.; Kafshdooz, L.; Razban, Z.; Dastranj Tbrizi, A.; Rasoulpour, S.; Khalilov, R.; Kavetskyy, T.; Saghfi, S.; Nasibova, A.N.; Kaamyabi, S.; et al. An Overview Application of Silver Nanoparticles in Inhibition of Herpes Simplex Virus. Nanomed. Biotechnol. 2018, 46, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, A.; Hashemzadeh, M.S. Inhibition of Herpes Simplex Virus Type 1 by Copper Oxide Nanoparticles. J. Virol. Methods 2020, 275, 113688. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Hakim, L.; Akhila, K.; Ramakanth, D.; Gaikwad, K.K. Nano Clays and Its Composites for Food Packaging Applications. Int. Nano Lett. 2022, 1–23. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Antioxidant Polyethylene Films Based on a Resveratrol Containing Clay of Interest in Food Packaging Applications. Food Packag. Shelf Life 2015, 6, 30–41. [Google Scholar] [CrossRef]
- Khan, I.; Bhat, A.H.; Sharma, D.K.; Usmani, M.A.; Khan, F. Overview of nanofluids to ionanofluids: Applications and challenges. In Nanomaterials for Healthcare, Energy and Environment; Advanced Structured Materials Book Series; Springer: Berlin/Heidelberg, Germany, 2019; Volume 118, pp. 199–227. [Google Scholar] [CrossRef]
- Hundáková, M.; Tokarský, J.; Valášková, M.; Slobodian, P.; Pazdziora, E.; Kimmer, D. Structure and Antibacterial Properties of Polyethylene/Organo-Vermiculite Composites. Solid State Sci. 2015, 48, 197–204. [Google Scholar] [CrossRef]
- Tas, C.E.; Hendessi, S.; Baysal, M.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Halloysite Nanotubes/Polyethylene Nanocomposites for Active Food Packaging Materials with Ethylene Scavenging and Gas Barrier Properties. Food Bioprocess Technol. 2017, 10, 789–798. [Google Scholar] [CrossRef]
- Tornuk, F.; Sagdic, O.; Hancer, M.; Yetim, H. Development of LLDPE Based Active Nanocomposite Films with Nanoclays Impregnated with Volatile Compounds. Food Res. Int. 2018, 107, 337–345. [Google Scholar] [CrossRef]
- Hadj-Hamou, A.S.; Metref, F.; Yahiaoui, F. Thermal Stability and Decomposition Kinetic Studies of Antimicrobial PCL/Nanoclay Packaging Films. Polym. Bull. 2017, 74, 3833–3853. [Google Scholar] [CrossRef]
- NanoBioMatters Industries Sociedad Limited. Oxygen Scavenging Additive_Nanotechnology Product NPD (Nantotechnology Product Database). Available online: https://product.statnano.com/product/6748/o2block-barrier (accessed on 8 December 2022).
- NanoBioMatters Industries Sociedad Limited. Nanotechnology Products_NPD (Nantotechnology Product Database). 2016. Available online: https://product.statnano.com/product/6746/bactiblock (accessed on 8 December 2022).
- García-Quiles, L.; Cuello, Á.F.; Castell, P. Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite. Polymers 2019, 11, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alejandro Marzano-Barreda, L.; Yamashita, F.; Paula Bilck, A. Effect of Biodegradable Active Packaging with Zeolites on Fresh Broccoli Florets. J. Food Sci. Technol. 2021, 58, 197–204. [Google Scholar] [CrossRef]
- Soysal, Ç.; Bozkurt, H.; Dirican, E.; Güçlü, M.; Bozhüyük, E.D.; Uslu, A.E.; Kaya, S. Effect of Antimicrobial Packaging on Physicochemical and Microbial Quality of Chicken Drumsticks. Food Control. 2015, 54, 294–299. [Google Scholar] [CrossRef]
- Kenyó, C.; Renner, K.; Móczó, J.; Fekete, E.; Kröhnke, C.; Pukánszky, B. Hips/Zeolite Hybrid Composites as Active Packaging Materials: Structure and Functional Properties. Eur. Polym. J. 2018, 103, 88–94. [Google Scholar] [CrossRef]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef]
- Sängerlaub, S.; Seibel, K.; Miesbauer, O.; Pant, A.; Kiese, S.; Rodler, N.; Schmid, M.; Müller, K. Functional Properties of Foamed and/or Stretched Polypropylene-Films Containing Sodium Chloride Particles for Humidity Regulation. Polym. Test. 2018, 65, 339–351. [Google Scholar] [CrossRef]
- Pant, A.F.; Sängerlaub, S.; Müller, K. Materials Gallic Acid as an Oxygen Scavenger in Bio-Based Multilayer Packaging Films. Materials 2017, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the Shelf-Life of Foal Meat with Two Antioxidant Active Packaging Systems. LWT-Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Al-Malaika, S. Oxidative Degradation and Stabilisation of Polymers. International Materials Reviews 2003, 48, 165–185. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Antioxidant Packaging with Encapsulated Green Tea for Fresh Minced Meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Dopico-García, M.S.; Castro-López, M.M.; López-Vilariño, J.M.; González-Rodríguez, M.v.; Valentão, P.; Andrade, P.B.; García-Garabal, S.; Abad, M.J. Natural Extracts as Potential Source of Antioxidants to Stabilize Polyolefins. J. Appl. Polym. Sci. 2011, 119, 3553–3559. [Google Scholar] [CrossRef]
- Granda-Restrepo, D.; Peralta, E.; Troncoso-Rojas, R.; Soto-Valdez, H. Release of Antioxidants from Co-Extruded Active Packaging Developed for Whole Milk Powder. Int. Dairy J. 2009, 19, 481–488. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, D.S.; Yam, K.L. Food Additives &Contaminants: Part A Chemistry, Analysis, Control, Exposure & Risk Assessment Release Property and Antioxidant Effectiveness of Tocopherol-Incorporated LDPE/PP. Blend Films Release Property and Antioxidant Effectiveness of Tocopherol-Incorporated LDPE/PP. Blend Films. Food Addit. Contam. 2012, 29, 461–468. [Google Scholar] [CrossRef]
- Manzanarez-López, F.; Soto-Valdez, H.; Auras, R.; Peralta, E. Release of α-Tocopherol from Poly(Lactic Acid) Films, and Its Effect on the Oxidative Stability of Soybean Oil. J. Food Eng. 2011, 104, 508–517. [Google Scholar] [CrossRef]
- Lopes, A.C.; Barcia, M.K.; Veiga, T.B.; Yamashita, F.; Grossmann, M.V.E.; Olivato, J.B. Eco-Friendly Materials Produced by Blown-Film Extrusion as Potential Active Food Packaging. Polym. Adv. Technol. 2021, 32, 779–788. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendón, R.; Rodríguez Bernaldo de Quirós, A.; Ares, A.; Castro-López, M.; Abad, M.J.; Maroto, J.; Paseiro-Losada, P. Development of Antioxidant Active Films Containing Tocopherols to Extend the Shelf Life of Fish. Food Control. 2013, 31, 236–243. [Google Scholar] [CrossRef]
- Hu, S.; Wang, H.; Han, W.; Ma, Y.; Shao, Z.; Li, L. Development of Double-Layer Active Films Containing Pomegranate Peel Extract for the Application of Pork Packaging. J. Food Process. Eng. 2017, 40, e12388. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic Effect of Cellulose and Lignin Nanostructures in PLA Based Systems for Food Antibacterial Packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A. Development of Novel Active Polypropylene Based Packaging Films Containing Different Concentrations of Sorbic Acid. Food Packag. Shelf Life 2018, 18, 87–94. [Google Scholar] [CrossRef]
- Colak, B.Y.; Peynichou, P.; Galland, S.; Oulahal, N.; Prochazka, F.; Degraeve, P. Antimicrobial Activity of Nisin and Natamycin Incorporated Sodium Caseinate Extrusion-Blown Films: A Comparative Study with Heat-Pressed/Solution Cast Films. J. Food Sci. 2016, 81, E1141–E1150. [Google Scholar] [CrossRef] [PubMed]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and Degradability Properties of Polyvinyl Alcohol/Gelatin Nanocomposite Films Filled Water Hyacinth Cellulose Nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Hayat, N.A.B.M.; Nor, M.S.M. Development and Characterization of Food Packaging Bioplastic Film from Cocoa Pod Husk Cellulose Incorporated with Sugarcane Bagasse Fibre. J. Bioresour. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Reza Bakhsheshi-Rad, H.; Nur, H.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Berto, F. Antioxidants Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Herniou--Julien, C.; Gutiérrez, T.J. Reactive Extrusion-Processed Shape-Memory Food Packaging Films Made from Native and Carboxymethylated Plantain Flour/Polystyrene Blends. Starch-Stärke 2021, 73, 2100053. [Google Scholar] [CrossRef]
- Deng, J.; Ding, Q.M.; Li, W.; Wang, J.H.; Liu, D.M.; Zeng, X.X.; Liu, X.Y.; Ma, L.; Deng, Y.; Su, W.; et al. Preparation of Nano-Silver-Containing Polyethylene Composite Film and Ag Ion Migration into Food-Simulants. J. Nanosci. Nanotechnol. 2020, 20, 1613–1621. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and Characterization of Polypropylene/Sodium Propionate (PP/SP) Composite Films for Bread Packaging Application. Packag. Technol. Sci. 2018, 31, 221–231. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Fages, J. Extrusion Assisted by Supercritical CO2: A Review on Its Application to Biopolymers. J. Supercrit. Fluids 2017, 120, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Darie-Niță, R.N.; Râpă, M.; Sivertsvik, M.; Rosnes, J.T.; Popa, E.E.; Dumitriu, R.P.; Marincaș, O.; Matei, E.; Predescu, C.; Vasile, C. PLA-Based Materials Containing Bio-Plasticizers and Chitosan Modified with Rosehip Seed Oil for Ecological Packaging. Polymers 2021, 13, 1610. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Barajas, D.L.; Arévalo-Prada, J.A.; Fenollar, O.; Rueda-Ordóñez, Y.J.; Torres-Giner, S. Torrefaction of Coffee Husk Flour for the Development of Injection-Molded Green Composite Pieces of Polylactide with High Sustainability. Appl. Sci. 2020, 10, 6468. [Google Scholar] [CrossRef]
- Pal, A.K.; Wu, F.; Misra, M.; Mohanty, A.K. Reactive Extrusion of Sustainable PHBV/PBAT-Based Nanocomposite Films with Organically Modified Nanoclay for Packaging Applications: Compression Moulding vs. Cast Film Extrusion. Compos. B Eng. 2020, 198, 108141. [Google Scholar] [CrossRef]
- Mistretta, M.C.; Botta, L.; Arrigo, R.; Leto, F.; Malucelli, G.; la Mantia, F.P. Bionanocomposite Blown Films: Insights on the Rheological and Mechanical Behavior. Polymers 2021, 13, 1167. [Google Scholar] [CrossRef]
- Althues, H.; Henle, J.; Kaskel, S. Functional Inorganic Nanofillers for Transparent Polymers. Chem. Soc. Rev. 2007, 36, 1454–1465. [Google Scholar] [CrossRef]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology Development in Food Packaging: A Review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]













| Active Agents | Advantages | References |
|---|---|---|
| ZnO | Low-cost, high ultraviolet absorption capability, and strong antibacterial activity on a wide range of bacteria | [51,52,53] |
| TiO2 | Enhances ethylene-scavenging activity | [54,55,56] |
| Nanoclays | Improved mechanical, gas barrier, optical properties at low filler content | [57,58,59] |
| Nanosilver | Antimicrobial activity | [60,61] |
| Catechin | Antioxidant and antimicrobial activities | [62,63] |
| Curcumin | Excellent antiviral, anticancer, antimicrobial, anti-inflammatory, and antioxidant activities. | [64,65] |
| Essential oils | Antioxidant, anticancer, and antimicrobial, cost-efficient | [66,67,68,69] |
| Lignin | Excellent antioxidant and antimicrobial activity attributed to the large number of phenolic groups; mechanical properties and fire-resistance | [70,71,72] |
| Tannin | Antioxidant and antimicrobial activities | [71,72] |
| Gallic acid | Oxygen-scavenging ability | [73,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanley, J.; John, A.; Pušnik Črešnar, K.; Fras Zemljič, L.; Lambropoulou, D.A.; Bikiaris, D.N. Active Agents Incorporated in Polymeric Substrates to Enhance Antibacterial and Antioxidant Properties in Food Packaging Applications. Macromol 2023, 3, 1-27. https://doi.org/10.3390/macromol3010001
Stanley J, John A, Pušnik Črešnar K, Fras Zemljič L, Lambropoulou DA, Bikiaris DN. Active Agents Incorporated in Polymeric Substrates to Enhance Antibacterial and Antioxidant Properties in Food Packaging Applications. Macromol. 2023; 3(1):1-27. https://doi.org/10.3390/macromol3010001
Chicago/Turabian StyleStanley, Johan, Athira John, Klementina Pušnik Črešnar, Lidija Fras Zemljič, Dimitra A. Lambropoulou, and Dimitrios N. Bikiaris. 2023. "Active Agents Incorporated in Polymeric Substrates to Enhance Antibacterial and Antioxidant Properties in Food Packaging Applications" Macromol 3, no. 1: 1-27. https://doi.org/10.3390/macromol3010001
APA StyleStanley, J., John, A., Pušnik Črešnar, K., Fras Zemljič, L., Lambropoulou, D. A., & Bikiaris, D. N. (2023). Active Agents Incorporated in Polymeric Substrates to Enhance Antibacterial and Antioxidant Properties in Food Packaging Applications. Macromol, 3(1), 1-27. https://doi.org/10.3390/macromol3010001









