Perspectives of Polymers in Forensic Analysis
Abstract
:1. Introduction
2. Approaches of Polymer-Based Materials in Forensic Analysis
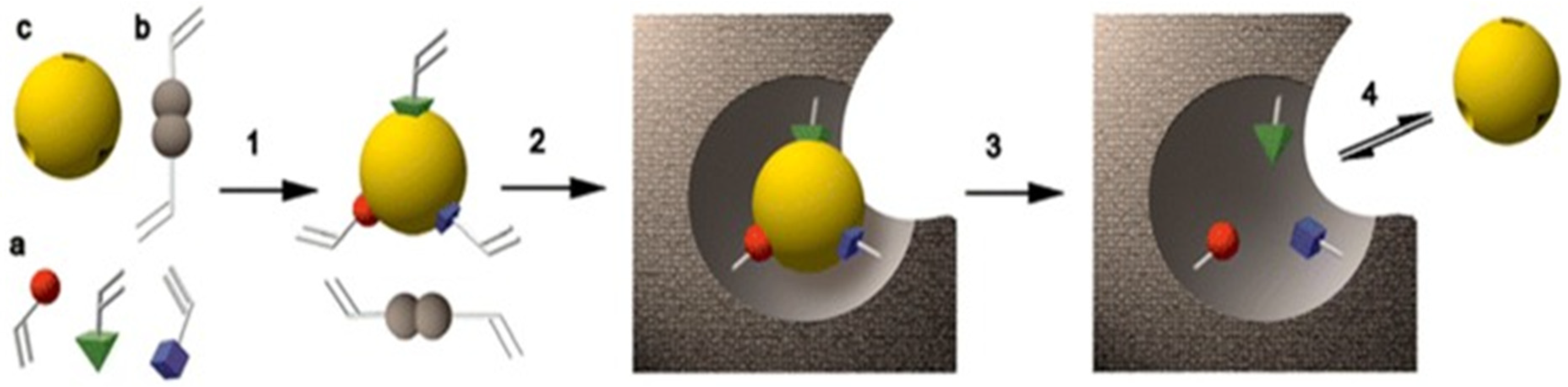
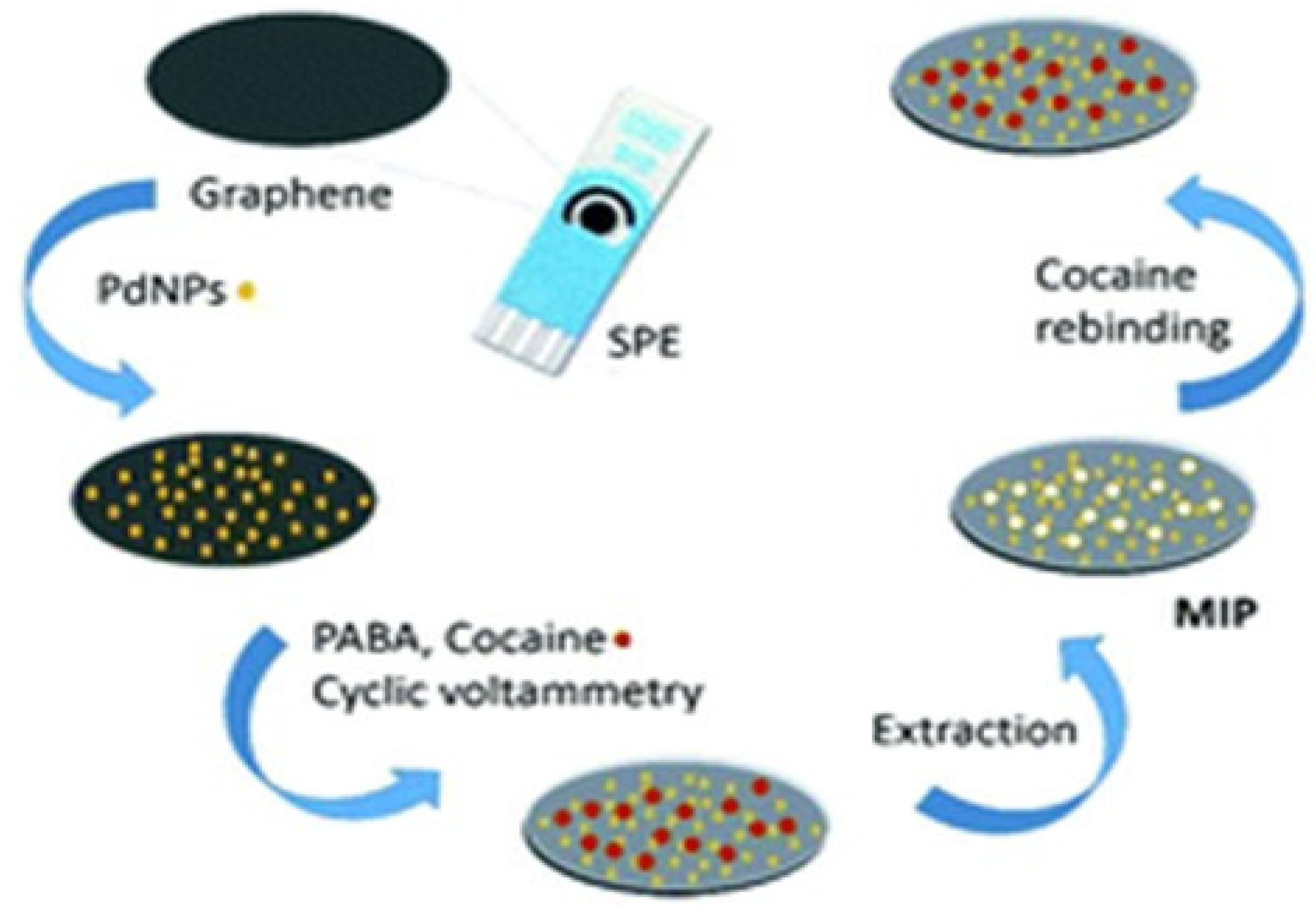
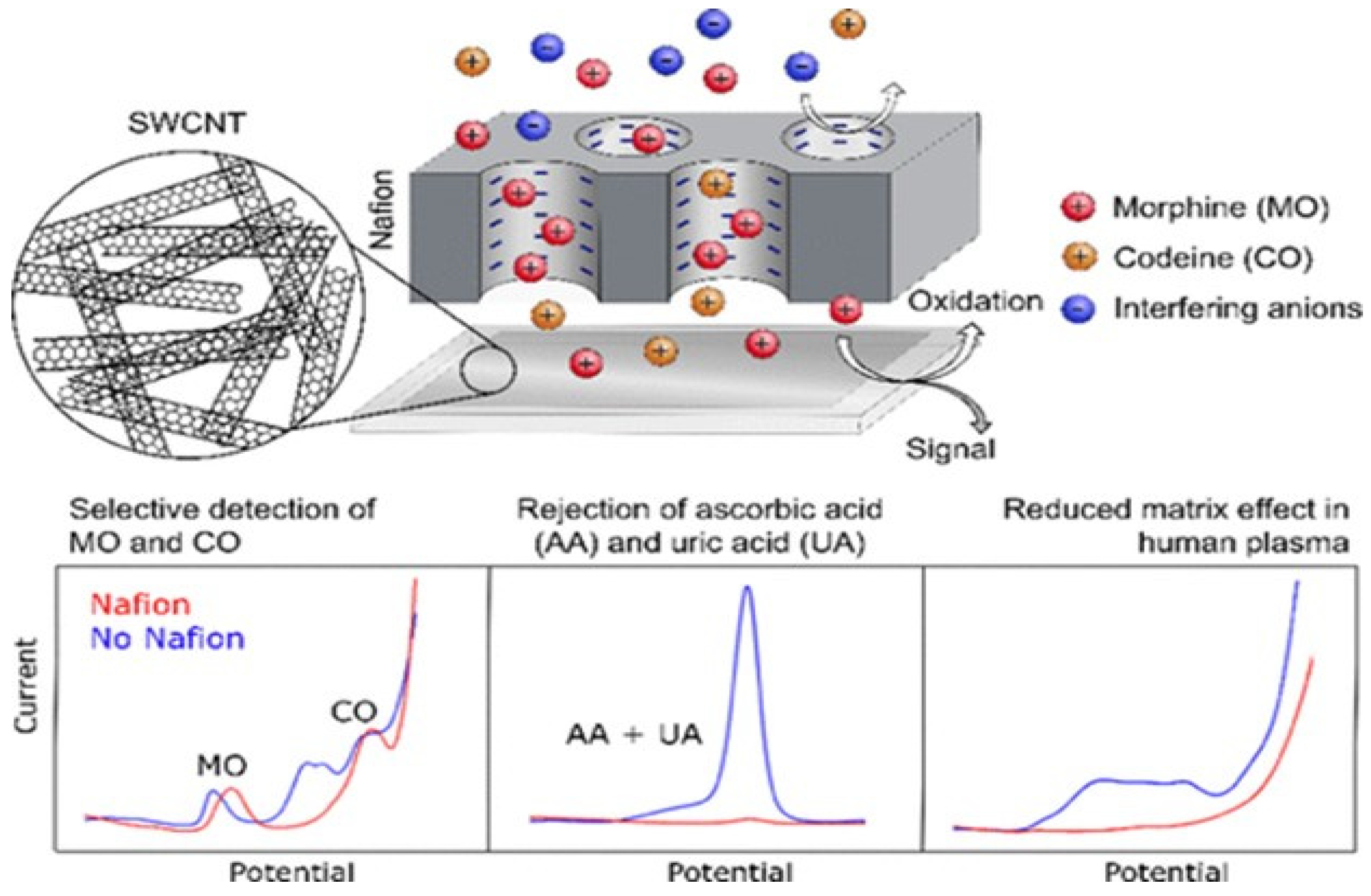
2.1. Detection of Illegal Drugs
2.2. Detection of Doping Compounds
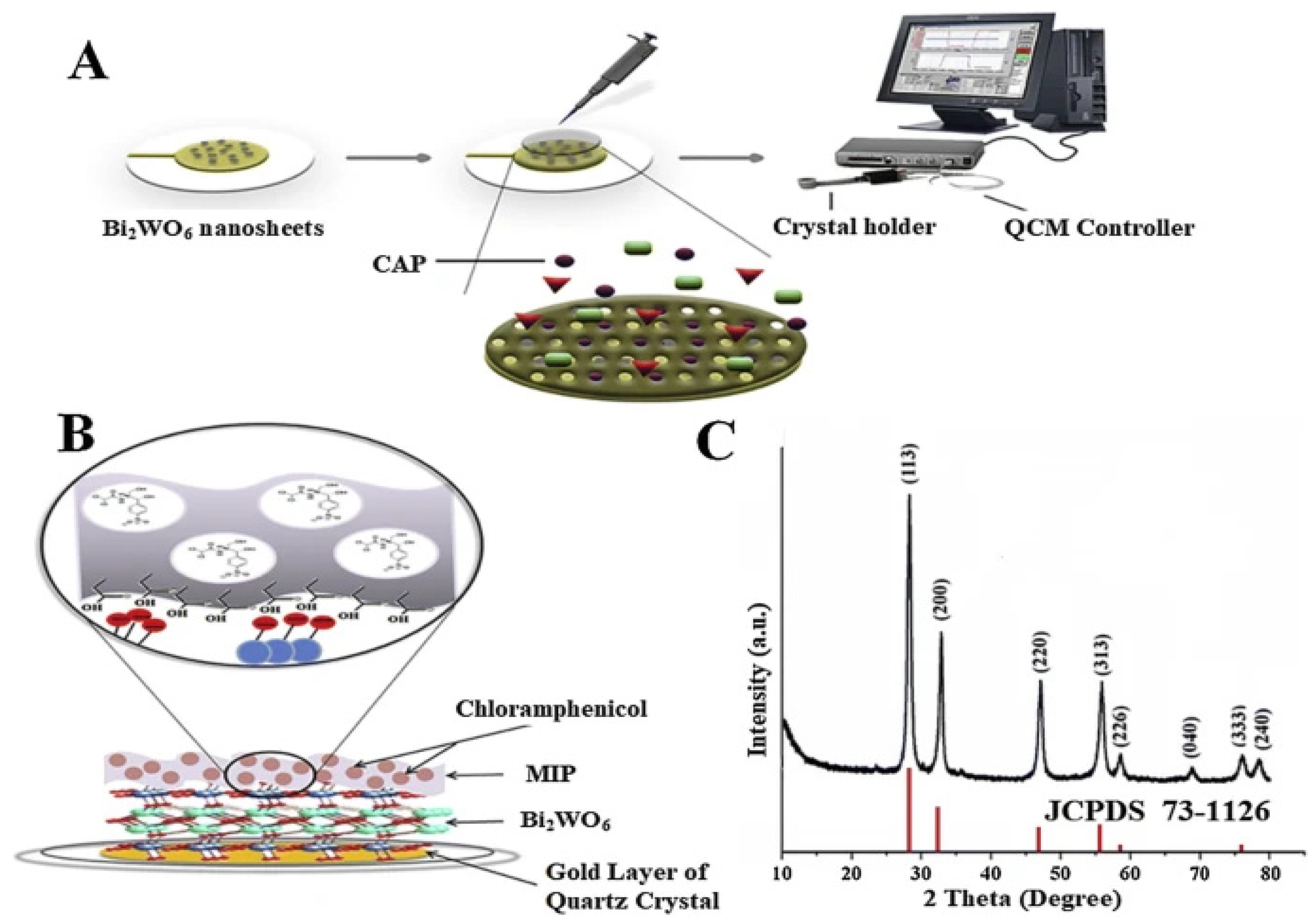
2.3. Detection of Toxins
3. Future Perspectives
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lodha, A.S.; Pandya, A.; Shukla, R.K. Nanotechnology: An applied and robust approach for forensic investigation. Forensic Res. Criminol. Int. J. 2016, 2, 00044. [Google Scholar]
- Diez Pascual, A.M. Carbon-Based Polymer Nanocomposites for High-Performance Applications II. Polymers 2022, 14, 870. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Cruz, D.L.; Redondo, A.L. Advanced Carbon-Based Polymeric Nanocomposites for Forensic Analysis. Polymers 2022, 14, 3598. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhao, X.; Ma, Y.; Zhang, H.; Pan, G. Molecularly Imprinted Nanomaterials with Stimuli Responsiveness for Applications in Biomedicine. Molecules 2023, 28, 918. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, E.C.; Sparrapan, R.; Sanvido, G.B.; Santos, M.G.; Arruda, M.A.; Eberlin, M.N. Quantitation of drugs via molecularly imprinted polymer solid phase extraction and electrospray ionization mass spectrometry: Benzodiazepines in human plasma. Analyst 2011, 136, 3753–3757. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef]
- Anderson, R.A.; Ariffin, M.M.; Cormack, P.A.G.; Miller, E.I. Comparison of molecularly imprinted solid-phase extraction (MISPE) with classical solid-phase extraction (SPE) for the detection of benzodiazepines in post-mortem hair samples. Forensic Sci. Int. 2008, 174, 40–46. [Google Scholar] [CrossRef]
- Panahi, Y.; Motaharian, A.; Hosseini, M.R.M.; Mehrpour, O. High sensitive and selective nano-molecularly imprinted polymer based electrochemical sensor for midazolam drug detection in pharmaceutical formulations and human urine samples. Sens. Actuators B 2018, 273, 1579–1586. [Google Scholar] [CrossRef]
- Rezaei, B.; Boroujeni, M.H.; Ensafi, A.A. A novel electrochemical nanocomposite imprinted sensor for the determination of lorazepam based on modified polypyrrole@sol-gel@gold nanoparticles/pencil graphite electrode. Electrochim. Acta 2014, 123, 332–339. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Zakery, M.; Rezaei, B. An optical sensor with specific binding sites for the detection of thioridazine hydrochloride based on ZnO-QDs coated with molecularly imprinted polymer. Spectrochim. Acta Part A 2019, 206, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xiang, W.; Liu, C.; Shi, H.; Zhou, Y.; Gao, L. Highly sensitive detection for cocaine using graphene oxide-aptamer based sensors in combination with tween 20. Nanosci. Nanotechnol. Lett. 2018, 10, 1707–1712. [Google Scholar] [CrossRef]
- Florea, A.; Cowen, T.; Piletsky, S.; De Wael, K. Electrochemical sensing of cocaine in real samples based on electrodeposited biomimetic affinity ligands. Analyst 2019, 144, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.G.; Ribeiro, J.S.; Santana, M.H.; Richter, E.M.; Muñoz, R.A. 3D-printing for forensic chemistry: Voltammetric determination of cocaine on additively manufactured graphene–polylactic acid electrodes. Anal. Methods 2021, 13, 1788–1794. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Mendonça, D.M.; Silva, W.P.; Silva, M.N.; Nossol, E.; da Silva, R.A.; Richter, E.M.; Muñoz, R.A. 3D printing for electroanalysis: From multiuse electrochemical cells to sensors. Anal. Chim. Acta 2018, 1033, 49–57. [Google Scholar] [CrossRef]
- Hashemi, P.; Bagheri, H.; Afkhami, A.; Ardakani, Y.H.; Madrakian, T. Fabrication of a novel aptasensor based on three-dimensional reduced graphene oxide/polyaniline/gold nanoparticle composite as a novel platform for high sensitive and specific cocaine detection. Anal. Chim. Acta 2017, 996, 10–19. [Google Scholar] [CrossRef]
- Saisahas, K.; Soleh, A.; Somsiri, S.; Senglan, P.; Promsuwan, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Lee, K.; Chang, K.H. Electrochemical Sensor for Methamphetamine Detection Using Laser-Induced Porous Graphene Electrode. Nanomaterials 2022, 12, 73. [Google Scholar] [CrossRef]
- Riahifar, V.; Haghnazari, N.; Keshavarzi, F.; Ahmadi, E. A sensitive voltammetric sensor for methamphetamine determination based on modified glassy carbon electrode using Fe3O4@ poly pyrrole core-shell and graphene oxide. Microchem. J. 2021, 170, 106748. [Google Scholar] [CrossRef]
- Granickowska, K.; PÜtz, M.; Hauser, F.M.; De Saeger, S.; Beloglazova, N.V. Capacitive sensing of N-formylamphetamine based on immobilized molecular imprinted polymers. Biosens. Bioelectron. 2017, 92, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Wester, N.; Mynttinen, E.; Etula, J.; Lilius, T.; Kalso, E.; Kauppinen, E.I.; Laurila, T.; Koskinen, J. Simultaneous detection of morphine and codeine in the presence of ascorbic acid and uric acid and in human plasma at nafion single-walled carbon nanotube thin-film electrode. ACS Omega 2019, 4, 17726–17734. [Google Scholar] [CrossRef] [Green Version]
- Mynttinen, E.; Wester, N.; Lilius, T.; Kalso, E.; Mikladal, B.; Varjos, I.; Sainio, S.; Jiang, H.; Kauppinen, E.I.; Koskinen, J. Electrochemical detection of oxycodone and its main metabolites with Nafion-coated single-walled carbon nanotube electrodes. Anal. Chem. 2020, 92, 8218–8227. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Masoum, S. A hybrid imprinted polymer based on magnetic graphene oxide and carbon dots for ultrasonic assisted dispersive solid-phase microextraction of oxycodone. Microchem. J. 2021, 164, 105988. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, J.; Salgueiro-Fernandez, R.; Cabarcos, P.; Bermejo, A.M.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Cannabinoids assessment in plasma and urine by high performance liquid chromatography–tandem mass spectrometry after molecularly imprinted polymer microsolid-phase extraction. Anal. Bioanal. Chem. 2017, 409, 1207–1220. [Google Scholar] [CrossRef]
- Sánchez-González, J.; Odoardi, S.; Bermejo, A.M.; Bermejo-Barrera, P.; Romolo, F.S.; Moreda-Piñeiro, A.; Strano-Rossi, S. Development of a micro-solid-phase extraction molecularly imprinted polymer technique for synthetic cannabinoids assessment in urine followed by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2018, 1550, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Cela-Perez, M.C.; Bates, F.; Jimenez-Morigosa, C.; Lendoiro, E.; de Castro, A.; Cruz, A.; Lopez-Rivadullab, M.; Lopez-Vilarino, J.M.; Gonzalez-Rodriguez, M.V. Water-compatible imprinted pills for sensitive determination of cannabinoids in urine and oral fluid. J. Chromatogr. A 2016, 1429, 53–64. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Battal, D.; Yalcin, M.S.; Yavuz, H.; Denizli, A. Rapid and sensitive detection of synthetic cannabinoids JWH-018, JWH-073 and their metabolites using molecularly imprinted polymer-coated QCM nanosensor in artificial saliva. Microchem. J. 2020, 153, 104454. [Google Scholar] [CrossRef]
- Alenus, J.; Ethirajan, A.; Horemans, F.; Weustenraed, A.; Csipai, P.; Gruber, J.; Peeters, M.; Cleij, T.J.; Wagner, P. Molecularly imprinted polymers as synthetic receptors for the QCM-D-based detection of l-nicotine in diluted saliva and urine samples. Anal. Bioanal. Chem. 2013, 405, 6479–6487. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, R.; He, J.; Bai, H.; Zhang, G. Sensitive detection of ketamine with an electrochemical sensor based on UV-induced polymerized molecularly imprinted membranes at graphene and MOFs modified electrode. Biosens. Bioelectron. 2019, 143, 111636. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Alkirkit, S.M.O.; Mewis, R.E.; Fulton, D.; Banks, C.E.; Sutcliffe, O.B.; Peeters, M. Engineering molecularly imprinted polymers (MIPs) for the selective extraction and quantification of the novel psychoactive substance (NPS) methoxphenidine and its regioisomers. Analyst 2018, 143, 2002–2007. [Google Scholar] [CrossRef] [Green Version]
- Kellens, E.; Bove, H.; Conradi, M.; D’Olieslaeger, L.; Wagner, P.; Landfester, K.; Ethirajan, A. Improved molecular imprinting based on colloidal particles made from miniemulsion: A case study on testosterone and its structural analogues. Macromolecules 2016, 49, 2559–2567. [Google Scholar] [CrossRef]
- Tu, X.; Muhammad, P.; Liu, J.; Ma, Y.; Wang, S.; Yin, D.; Liu, Z. Molecularly-imprinted polymer-based plasmonic immunosandwich assay for fast and ultrasensitive determination of trace glycoproteins in complex samples. Anal. Chem. 2016, 88, 12363–12370. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhuo, Y.; Chai, Y.Q.; Xiang, Y.; Yuan, R. New type of redox nanoprobe: C60-based nanomaterial and its application in electrochemical immunoassay for doping detection. Anal. Chem. 2015, 87, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H. Doping of transition metal dichalcogenides in molecularly imprinted conductive polymers for the ultrasensitive determination of 17-β estradiol in cell serum. Biosens. Bioelectron. 2020, 150, 111901. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Che, C.; Korolchuk, V.I.; Gan, F.; Pan, C.; Huang, K. Selenomethionine alleviates AFB1-induced damage in primary chicken hepatocytes by inhibiting CYP450 1A5 expression via upregulated SelW expression. J. Agric. Food Chem. 2017, 65, 2495–2502. [Google Scholar] [CrossRef]
- Hu, Z.; Lustig, W.P.; Zhang, J.; Zheng, C.; Wang, H.; Teat, S.J.; Gong, Q.; Rudd, N.D.; Li, J. Efective detection of mycotoxins by a highly luminescent metal-organic framework. J. Am. Chem. Soc. 2015, 137, 16209–16215. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Qian, J.; An, K.; Ren, C.; Lu, X.; Hao, N.; Liu, Q.; Li, H.; Huang, X.; Wang, K. Fabrication of magnetically assembled aptasensing device for label-free determination of aflatoxin B1 based on EIS. Biosens. Bioelectron. 2018, 108, 69–75. [Google Scholar] [CrossRef]
- Bayram, E.; Yılmaz, E.; Uzun, L.; Say, R.; Denizli, A. Multiclonal plastic antibodies for selective aflatoxin extraction from food samples. Food Chem. 2017, 221, 829–837. [Google Scholar] [CrossRef]
- Lu, L.; Seenivasan, R.; Wang, Y.Z.; Yu, J.H.; Gunasekaran, S. An electrochemical immunosensor for rapid and sensitive detection of mycotoxins fumonisin B1 and deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Qiu, X.; Su, K.; Xu, G.; Wang, P. Disposable poly(o-aminophenol)-carbon nanotubes modified screen print electrode-based enzyme sensor for electrochemical detection of marine toxin okadaic acid. Sens. Actuators B Chem. 2016, 235, 170–178. [Google Scholar] [CrossRef]
- Shendy, A.H.; Al-Ghobashy, M.A.; Gad Alla, S.A.; Lotfy, H.M. Development and validation of a modified QuEChERS protocol coupled to LC–MS/MS for simultaneous determination of multi-class antibiotic residues in honey. Food Chem. 2016, 190, 982–989. [Google Scholar] [CrossRef]
- Shaheen, A. Design of heterostructured hybrids comprising ultrathin 2D bismuth tungstate nanosheets reinforced by chloramphenicol imprinted polymers used as biomimetic interfaces for mass-sensitive detection. Colloids Surf. B Biointerfaces 2020, 188, 110775. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhang, L.; Zhao, P.; Lun, X.; Li, W.; Guo, Y.; Hou, X. A joint experimental-computational investigation: Metal organic framework as a vortex assisted dispersive micro-solid-phase extraction sorbent coupled with UPLC-MS/MS for the simultaneous determination of amphenicols and their metabolite in aquaculture water. Microchem. J. 2017, 130, 263–270. [Google Scholar]
- Munawar, A.; Tahir, M.A.; Shaheen, A.; Lieberzeit, P.A.; Khan, W.S.; Bajwa, S.Z. Investigating nanohybrid material based on 3D CNTs@Cu nanoparticle composite and imprinted polymer for highly selective detection of chloramphenicol. J. Hazard. Mater. 2018, 342, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, A.B.; Abdollahi, A. Determination of organothiophosphate insecticides in environmental water samples by a very simple and sensitive spectrofluorimetric method. Bull. Environ. Contam. Toxicol. 2015, 95, 536–541. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Bates, F. Computational design of molecularly imprinted polymer for direct detection of melamine in milk. Sep. Sci. Technol. 2017, 52, 1441–1453. [Google Scholar] [CrossRef] [Green Version]
- Piletska, E.V.; Pink, D.; Karim, K.; Piletsky, S.A. Development of a computationally-designed polymeric adsorbent specific for mycotoxin patulin. Analyst 2017, 142, 4678–4683. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Feng, T.; Xu, J.; Xue, C. Recent advances of molecularly imprinted polymer-based sensors in the detection of food safety hazard factors. Biosens. Bioelectron. 2019, 141, 111447. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef]
- Luppa, P.; Junker, R. Point-of-Care Testing: Principles and Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Smolinska-Kempisty, K.; Guerreiro, A.; Canfarotta, F.; Cáceres, C.; Whitcombe, M.J.; Piletsky, S. A comparison of the performance of molecularly imprinted polymer nanoparticles for small molecule targets and antibodies in the ELISA format. Sci. Rep. 2016, 6, 37638. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Pascual, A.M. Perspectives of Polymers in Forensic Analysis. Macromol 2023, 3, 108-119. https://doi.org/10.3390/macromol3020008
Díez-Pascual AM. Perspectives of Polymers in Forensic Analysis. Macromol. 2023; 3(2):108-119. https://doi.org/10.3390/macromol3020008
Chicago/Turabian StyleDíez-Pascual, Ana M. 2023. "Perspectives of Polymers in Forensic Analysis" Macromol 3, no. 2: 108-119. https://doi.org/10.3390/macromol3020008
APA StyleDíez-Pascual, A. M. (2023). Perspectives of Polymers in Forensic Analysis. Macromol, 3(2), 108-119. https://doi.org/10.3390/macromol3020008





