Biodegradation of Polymers: Stages, Measurement, Standards and Prospects
Abstract
1. Introduction and Definitions
2. Biodegradable Polymers and Stages of Biodegradation
2.1. Mechanisms of Degradation
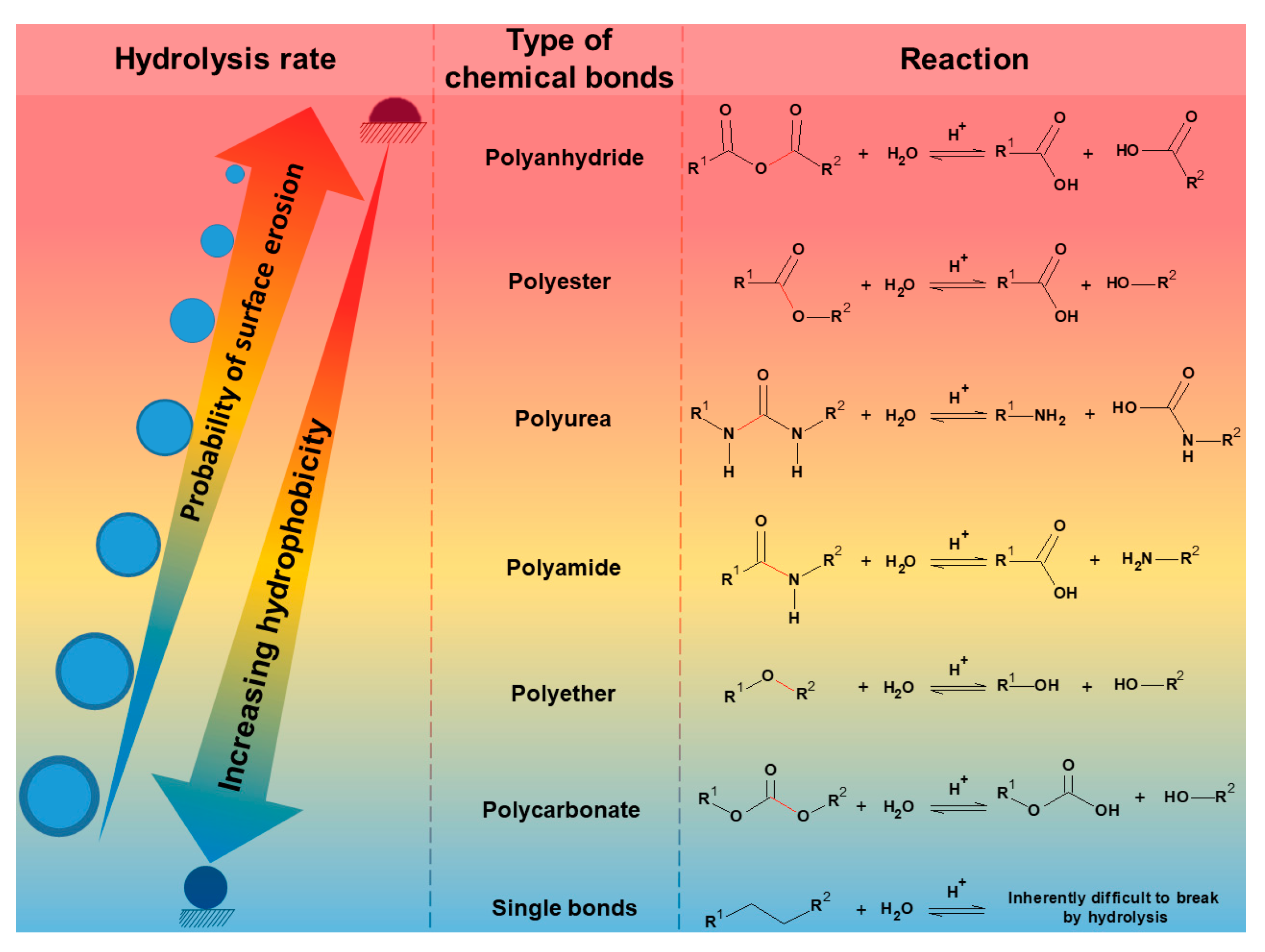
2.1.1. Hydrolysis
2.1.2. Thermolysis
2.1.3. Oxidation and Thermo-Oxidative Fission
2.2. Abiotic and Biotic Degradation
2.3. Stages of Biodegradation
2.3.1. (Bio)Deterioration
2.3.2. (Bio)Fragmentation
2.3.3. Assimilation
2.3.4. Mineralization
2.4. Greenwashing Concept
3. Standardized Norms to Evaluate Biodegradation
3.1. Biodegradation Test in Soil and Landfilling
3.2. Biodegradation Test in Compost
3.3. Biodegradation Test in Aquatic Systems
4. Methods and Analytical Tools for Evaluating Polymers’ Biodegradation Process
- Loss of mass over time.
- Alterations in surface morphology, e.g., increased roughness, formation of pits or cracks.
- Changes in surface energy or wettability.
- Modifications in color or appearance.
- Changes in mechanical properties, e.g., decreased tensile strength or elongation at break.
- Changes in thermal properties, e.g., Tg or melting point (Tm).
- Alterations in chemical structure, e.g., molecular weight or functional groups.
- Release of degradation products, e.g., monomers or oligomers.
- Release of gases, e.g., carbon dioxide or methane.
4.1. Macro and Microscopic Changes
4.2. Gas Evolution Methods
4.3. Methods Based on Mechanical Properties
4.4. Methods Based on Thermal Properties
4.5. Spectroscopic Techniques
5. Prospects
- Public education campaigns: Develop a targeted public education campaign focused on promoting awareness of biodegradable polymers, their benefits, and their proper disposal. This could include media outreach, social media campaigns, and public events.
- Collaborations with industries: Partner with industries that use polymers to promote the use of biodegradable alternatives and educate consumers on their benefits.
- Improved labeling: Develop standardized labeling for biodegradable polymers that is easy for consumers to understand and includes information on proper disposal.
- School curriculum: Incorporate the science of biodegradable polymers into school curriculum, starting at a young age. This can help to create a more informed and environmentally conscious future generation.
6. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in Applications and Prospects of Bioplastics and Biopolymers: A Review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. Nat. Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste- A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Schumann, B.; Schmid, M. Packaging Concepts for Fresh and Processed Meat—Recent Progresses. Innov. Food Sci. Emerg. Technol. 2018, 47, 88–100. [Google Scholar] [CrossRef]
- Porta, R.; Sabbah, M.; Di Pierro, P. Biopolymers as Food Packaging Materials. Int. J. Mol. Sci. 2020, 21, 4942. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”). In Blackwell Scientific Publications; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Hoboken, NJ, USA, 2019; ISBN 0-9678550-9-8. [Google Scholar]
- ASTM D883−17; Standard Terminology Relating to Plastics. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–16.
- Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ. Sci. 2021, 33, 101538. [Google Scholar]
- Abhilash, M.; Thomas, D. Biopolymers for Biocomposites and Chemical Sensor Applications. Biopolym. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 405–435. [Google Scholar]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Canevarolo, S.V., Jr. Ciência dos Polímeros; Artliber: São Paulo, Brazil, 2006; p. 277. [Google Scholar]
- Lyu, S.; Untereker, D. Degradability of Polymers for Implantable Biomedical Devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef]
- Silva, R.R.A.; de Freitas, P.A.V.; Teixeira, S.C.; de Oliveira, T.V.; Marques, C.S.; Stringheta, P.C.; dos Santos Pires, A.C.; Ferreira, S.O.; Soares, N.F.F. Plasticizer Effect and Ionic Cross-Linking: The Impact of Incorporating Divalent Salts in Methylcellulose Films for Colorimetric Detection of Volatile Ammonia. Food Biophys. 2021, 17, 59–74. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V.; Stringheta, P.C.; dos Santos, A.C.P.; Soares, N.F.F. Ionic Strength of Methylcellulose-Based Films: An Alternative for Modulating Mechanical Performance and Hydrophobicity for Potential Food Packaging Application. Polysaccharides 2022, 3, 426–440. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Hodgson, J.L.; Coote, M.L. Revising the mechanism of polymer autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ahn, J.-H.; Hur, H.-G. Biodegradation of polyethylene: A brief review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer biodegradation: Mechanisms and estimation techniques—A review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Oliveira, J.; Belchior, A.; da Silva, V.D.; Rotter, A.; Petrovski, Ž.; Almeida, P.L.; Lourenço, N.D.; Gaudêncio, S.P. Marine Environmental Plastic Pollution: Mitigation by Microorganism Degradation and Recycling Valorization. Front. Mar. Sci. 2020, 7, 567126. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.; Ugwu, C.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Kaabel, S.; Therien, J.P.; Dschênes, C.; Dustin, D.D.; Friščić, T.; Auclair, K. Enzymatic depolymerization of highly crystalline polyethylene terephthalate enabled in moist-solid reaction mixtures. Proc. Natl. Acad. Sci. USA 2021, 118, e2026452118. [Google Scholar] [CrossRef]
- Kawai, F. Biodegradation of Polymers (Bioassimilation, Biomineralization, Biodisintegration, Compost), Overview. Encycl. Polym. Nanomater. 2015, 1, 155–160. [Google Scholar]
- Gu, J.D.; Ford, T.E.; Mitton, D.B.; Mitchell, R. Microbial Corrosion of Metals. In The Uhlig Corrosion Handbook, 2nd ed.; Revie, W., Ed.; Wiley: New York, NY, USA, 2000; pp. 915–927. [Google Scholar]
- Greene, J.P. Degradation and Biodegradation Standards for Biodegradable Food Packaging Materials. In Reference Module in Food Science, Handbook of Biodegradable Materials; Elsevier: Zürich, Switzerland, 2019. [Google Scholar]
- Yadav, N.; Hakkarainen, M. Degradable or Not? Cellulose Acetate is a Model for the Complicated Interplay between Structure, Environment, and Degradation. Chemosphere 2021, 265, 128731. [Google Scholar] [CrossRef] [PubMed]
- FAO; ITPS; GSBI; SCBD. State of Knowledge of Soil Biodiversity—Status, Challenges and Potentialities, Summary for Policymakers; FAO: Rome, Italy, 2022; ISBN 978-92-5-133583-3. [Google Scholar]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Premi, A.; Giri, A.S. Synthesis of Polyvinyl Alcohol/Modified Starch-Based Biodegradable Nanocomposite Films Reinforced with Starch Nanocrystals for Packaging Applications. Polym. Compos. 2021, 29, 405–416. [Google Scholar] [CrossRef]
- Chin, K.; Sam, S.T.; Ong, H.L.; Wong, Y.S.; Tan, W.K. Biodegradation Improvement of Bioinspired Crosslinked and Noncrosslinked Polyvinyl Alcohol Nanocomposites with Cellulose Nanocrystals Extracted from Rice Straw through Natural Soil Burial Exposure. Polym. Compos. 2022, 43, 6955–6965. [Google Scholar] [CrossRef]
- D5988-18; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil. ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 17556:19; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. ISO: Geneva, Switzerland, 2019.
- Anunciado, M.B.; Hayes, D.G.; Astner, A.F.; Wadsworth, L.C.; Cowan-Banker, C.D.; Gonzalez, J.E.L.Y.; DeBruyn, J.M. Effect of Environmental Weathering on Biodegradation of Biodegradable Plastic Mulch Films under Ambient Soil and Composting Conditions. J. Polym. Environ. 2021, 29, 2916–2931. [Google Scholar] [CrossRef]
- Caligiuri, V.; Tedeschi, G.; Palei, M.; Miscuglio, M.; Martin-Garcia, B.; Guzman-Puyol, S.; Hedayati, M.K.; Kristensen, A.; Athanassiou, A.; Cingolani, R.; et al. Biodegradable and Insoluble Cellulose Photonic Crystals and Metasurfaces. ACS Nano. 2020, 14, 9502–9511. [Google Scholar] [CrossRef]
- Torres-Martínez, L.M.; Kharissova, O.V.; Kharisov, B.I. Handbook of Eco Materials; Springer International Publishing: Cham, Switzerland, 2019; Volume 1, ISBN 9783319682556. [Google Scholar]
- ISO 14855-1:2012; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 1: General Method. ISO: Geneva, Switzerland, 2012.
- ISO 14855-2:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory. ISO: Geneva, Switzerland, 2018.
- ASTM D5338-15; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials under Controlled Composting Conditions, Incorporating Thermophilic Temperatures. ASTM International: West Conshohocken, PA, USA, 2015.
- Rudnik, E.; Briassoulis, D. Degradation Behaviour of Poly(Lactic Acid) Films and Fibres in Soil under Mediterranean Field Conditions and Laboratory Simulations Testing. Ind. Crops Prod. 2011, 33, 648–658. [Google Scholar] [CrossRef]
- ISO 16929:2021; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Defined Composting Conditions in a Pi-lot-Scale Test. ISO: Geneva, Switzerland, 2021.
- ASTM D6400-21; Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM 17088:2021; Plastics–Organic Recycling–Specifications for Compostable Plastics. ISO: Geneva, Switzerland, 2021.
- Pires, J.R.A.; Souza, V.G.L.; Fuciños, P.; Pastrana, L.; Fernando, A.L. Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers 2022, 14, 1359. [Google Scholar] [CrossRef]
- Krause, C.J.; von Nordheim, H.; Brager, S. Marine Nature Conservation in Europe 2006. In Proceedings of the Symposium, Stralsund, Germany, 8–12 May 2006; BfN-Skripten 193. [Google Scholar]
- UNEP (United Nations Environment Programme). From Pollution to Solution: A Global Assessment of Marine Litter and Plastic Pollution; UNEP (United Nations Environment Programme): Nairobi, Kenya, 2021; p. 237. ISBN 9789280738810. [Google Scholar]
- Beltrán-Sanahuja, A.; Casado-Coy, N.; Simó-Cabrera, L.; Sanz-Lázaro, C. Monitoring Polymer Degradation under Different Conditions in the Marine Environment. Environ. Pollut. 2020, 259, 113836. [Google Scholar] [CrossRef]
- ASTM D6691-17; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D7991-15; Standard Test Method for Determining Aerobic Biodegradation of Plastics Buried in Sandy Marine Sediment under Controlled Laboratory Conditions. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D7473/D7473M-21; Standard Test Method for Weight Attrition of Non-Floating Plastic Materials by Open System Aquarium Incubations. ASTM International: West Conshohocken, PA, USA, 2021.
- ISO 19679:2016; Plastics—Determination of Aerobic Biodegradation of Non-Floating Plastic Materials in a Seawater/Sediment Interface—Method by Analysis of Evolved Carbon Dioxide. ISO: Geneva, Switzerland, 2016.
- ISO 18830:2016; Plastics—Determination of Aerobic Biodegradation of Non-Floating Plastic Materials in a Seawater/Sandy Sediment Interface—Method by Measuring the Oxygen Demand in Closed Respirometer. ISO: Geneva, Switzerland, 2016.
- ISO 22766:2020; Plastics—Determination of the Degree of Disintegration of Plastic Materials in Marine Habitats under Real Field Conditions. ISO: Geneva, Switzerland, 2020.
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Râpă, M.; Darie-Niţă, R.N.; Mitelut, A.C.; Popa, E.E.; Popescu, P.A.; Draghici, M.C.; Popa, M.E. Study of the soil burial degradation of some PLA/CS biocomposites. Compos. Part B 2018, 142, 251–262. [Google Scholar] [CrossRef]
- Ferreira, W.H.; Andrade, C.T. Physical and Biodegradation Properties of Graphene Derivatives/Thermoplastic Starch Composites. Polysaccharides 2021, 2, 582–593. [Google Scholar] [CrossRef]
- Calabro, P.S.; Folino, A.; Fazzino, F.; Komilis, D. Preliminary evaluation of the anaerobic biodegradability of three biobased materials used for the production of disposable plastics. J. Hazard. Mater. 2020, 390, 121653. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, F.P.; Rizzarelli, P. Comparative investigation on the soil burial degradation behaviour of polymer films for agriculture before and after photo-oxidation. Polymers 2020, 12, 753. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef]
- Munir, E.; Harefa, R.S.M.; Priyani, N.; Suryanto, D. Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012145. [Google Scholar] [CrossRef]
- Mbarki, K.; Fersi, M.; Louati, I.; Elleuch, B.; Sayari, A. Biodegradation study of PDLA/cellulose microfibres biocomposites by Pseudomonas aeruginosa. Environ. Technol. 2021, 42, 731–742. [Google Scholar] [CrossRef]
- Mroczkowska, M.; Germaine, K.; Culliton, D.; Kakouli Duarte, T.; Neves, A.C. Assessment of biodegradation and eco-toxic properties of novel starch and gelatine blend bioplastics. Recycling 2021, 6, 81. [Google Scholar] [CrossRef]
- Ammala, A.; Hill, A.J.; Meakin, P.; Pas, S.J.; Turney, T.W. Degradation studies of polyolefins incorporating transparent nanoparticulate zinc oxide UV stabilizers. J. Nanopart. Res. 2002, 4, 167–174. [Google Scholar] [CrossRef]
- Andersson, S.R.; Hakkarainen, M.; Albertsson, A.-C. Tuning the polylactide hydrolysis rate by plasticizer architecture and hydrophilicity without introducing new migrants. Biomacromolecules 2010, 11, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, G.; Cucci, C.; Garcia, O.; Piantanida, G.; Elnaggar, A.; Cassar, M.; Strlič, M. Environmentally induced colour change during natural degradation of selected polymers. Polym. Degrad. Stab. 2014, 107, 198–209. [Google Scholar] [CrossRef]
- Gajendiran, A.; Krishnamoorthy, S.; Abraham, J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech. 2016, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Patil, A.A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–68. [Google Scholar] [CrossRef]
- Teodonio, L.; Missori, M.; Pawcenis, D.; Łojewska, J.; Valle, F. Nanoscale analysis of degradation processes of cellulose fibers. Micron 2016, 91, 75–81. [Google Scholar] [CrossRef]
- Schmidt, J.; Wei, R.; Oeser, T.; Dedavid e Silva, L.A.; Breite, D.; Schulze, A.; Zimmermann, W. Degradation of polyester polyurethane by bacterial polyester hydrolases. Polymers 2017, 9, 65. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepanska, B.; Sionkowska, M.M.; Mazur, O.; Swiątczak, J.; Brzezinska, M.S. The role of microorganisms in biodegradation of chitosan/tannic acid materials. Int. J. Biol. Macromol. 2021, 184, 584–592. [Google Scholar] [CrossRef]
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A sustainable bioplastic obtained from rice straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Rogovina, S.; Aleksanyan, K.; Prut, E.; Gorenberg, A. Biodegradable blends of cellulose with synthetic polymers and some other polysaccharides. Eur. Polym. J. 2013, 49, 194–202. [Google Scholar] [CrossRef]
- Yamashita, Y.; Endo, T. Biodegradation behavior of acid-containing cellulose acetate film in soil. J. Appl. Polym. Sci. 2005, 98, 466–473. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, G.; Ma, S.; Cai, Z.; Zhang, L. Crystallization behavior, mechanical properties, and environmental biodegradability of poly(β-hydroxybutyrate)/cellulose acetate butyrate blends. J. Appl. Polym. Sci. 2003, 89, 2116–2122. [Google Scholar] [CrossRef]
- Kumar, A.G.; Hinduja, M.; Sujitha, K.; Rajan, N.N.; Marine, G.D. Biodegradation of polystyrene by deep-sea Bacillus paralicheniformis G1 and genome analysis. Sci. Total Environ. 2021, 774, 145002. [Google Scholar] [CrossRef] [PubMed]
- Quintana, R.; Persenaire, O.; Lemmouchi, Y.; Sampson, J.; Martin, S.; Bonnaud, L.; Dubois, P. Enhancement of cellulose acetate degradation under accelerated weathering by plasticization with eco-friendly plasticizers. Polym. Degrad. Stab. 2013, 98, 1556–1562. [Google Scholar] [CrossRef]
- Da Luz, J.M.R.; Paes, S.A.; Bazzolli, D.M.S.; Tótola, M.R.; Demuner, A.J.; Kasuya, M.C.M. Abiotic and Biotic Degradation of Oxo- Biodegradable Plastic Bags by Pleurotus ostreatus. PLoS ONE 2014, 9, e107438. [Google Scholar] [CrossRef]
- Rose, R.-S.; Richardson, K.H.; Latvanen, E.J.; Hanson, C.A.; Resmini, M.; Sanders, I.A. Microbial degradation of plastic in aqueous solutions demonstrated by CO2 evolution and quantification. Int. J. Mol. Sci. 2020, 21, 1176. [Google Scholar] [CrossRef] [PubMed]
- Kalita, N.K.; Sarmah, A.; Bhasney, S.M.; Kalamdhad, A.; Katiyar, V. Demonstrating an ideal compostable plastic using biodegradability kinetics of poly(lactic acid) (PLA) based green biocomposite films under aerobic composting conditions. Environ. Chall. 2021, 3, 100030. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Insights on the aerobic biodegradation of polymers by analysis of evolved carbon dioxide in simulated composting conditions. Polym. Degrad. Stab. 2017, 137, 251–271. [Google Scholar] [CrossRef]
- Reuschenbach, P.; Pagga, U.; Strotmann, U. A critical comparison of respirometric biodegradation tests based on OECD 301 and related test methods. Water Res. 2003, 37, 1571–1582. [Google Scholar] [CrossRef]
- Zhang, W.; Heaven, S.; Banks, C.J. Degradation of some EN13432 compliant plastics in simulated mesophilic anaerobic digestion of food waste. Polym. Degrad. Stab. 2018, 147, 76–88. [Google Scholar] [CrossRef]
- Saharudin, M.S.; Atif, R.; Shyha, I.; Inam, F. The degradation of mechanical properties in polymer nano-composites exposed to liquid media—A review. RSC Adv. 2016, 6, 1076–1089. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Bonilla, J.; Paiano, R.B.; Lourenço, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.A. Biodegradability in aquatic system of thin materials based on chitosan, PBAT and HDPE polymers: Respirometric and physical-chemical analysis. Int. J. Biol. Macromol. 2020, 164, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Sangale, M.K.; Shahnawaz, M.; Ade, A.B. Potential of fungi isolated from the dumping sites mangrove rhizosphere soil to degrade polythene. Sci. Rep. 2019, 9, 5390. [Google Scholar] [CrossRef] [PubMed]
- Luthra, P.; Vimal, K.K.; Goel, V.; Singh, R.; Kapur, G.S. Biodegradation studies of polypropylene/natural fiber composites. SN Appl. Sci. 2020, 2, 512. [Google Scholar] [CrossRef]
- De Freitas, R.R.M.; Botaro, V.R. Biodegradation behavior of cellulose acetate with ds 2.5 in simulated soil. Int. Sch. Sci. Res. Innov. 2018, 12, 347–351. [Google Scholar]
- Ruggero, F.; Carreti, E.; Gori, R.; Lotti, T.; Lubello, C. Monitoring of degradation of starch-based biopolymer film under different composting conditions, using TGA, FTIR and SEM analysis. Chemosphere 2020, 246, 125770. [Google Scholar] [CrossRef]
- Liu, L.; Gong, D.; Bratasz, L.; Zhu, Z.; Wang, C. Degradation markers and plasticizer loss of cellulose acetate films during ageing. Polym. Degrad. Stab. 2019, 168, 108952. [Google Scholar] [CrossRef]
- Schlemmer, D.; Sales, M.J.A.; Resck, I.S. Preparation, characterization and degradation of PS/TPS blends using glycerol and buriti oil as plastiscizers. Polímeros Ciência E Tecnol. 2010, 20, 6–13. [Google Scholar] [CrossRef]
- Brandon, A.M.; Gao, S.-H.; Tian, R.; Ning, D.; Yang, S.-S.; Zhou, J.; Wu, W.-M.; Criddle, C.S. Biodegradation of polyethylene and plastic mixtures in mealworms (larvae of Tenebrio molitor) and effects on the gut microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 25–29. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.G.; Dennis, J.S.; von Blottnitz, H.; Harrison, S.T.L. Environmental analysis of plastic production processes: Comparing petroleum-based polypropylene and polyethylene with biologicallybased poly-β-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007, 130, 57–66. [Google Scholar] [CrossRef]
- American Society of Testing Materials (ASTM). Available online: https://sn.astm.org/features/standards-circular-economy.html (accessed on 12 January 2023).
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 692–3706. [Google Scholar] [CrossRef]
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020, 5, 501–516. [Google Scholar] [CrossRef]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Biopolymer production and end of life comparisons using life cycle assessment. Resour. Conserv. Recycl. 2017, 122, 295–306. [Google Scholar] [CrossRef]
- Basel Convention. Technical Guidelines for the Identification and Environmentally Sound Management of Plastic Wastes and for Their Disposal. Matters Related to Work Program 1–94. Available online: http://www.basel.int/Implementation/Plasticwaste/Technicalguidelines/Overview/tabid/7992/Default.aspx (accessed on 12 January 2023).
- Gundupalli, S.P.; Hait, S.; Thakur, A. A review on automated sorting of source-separated municipal solid waste for recycling. Waste Manag. 2017, 60, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Bucknall, D.G. Plastics as a materials system in a circular economy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190268. [Google Scholar] [CrossRef]
- Briassoulis, D.; Hiskakis, M.; Babou, E. Technical specifications for mechanical recycling of agricultural plastic waste. Waste Manag. 2013, 33, 1516–1530. [Google Scholar] [CrossRef]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of bioplastics: Routes and benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Walker, S.; Rothman, R. Life cycle assessment of bio-based and fossil-based plastic: A review. J. Clean. Prod. 2020, 261, 121158. [Google Scholar] [CrossRef]
- Miller, S.A. Five misperceptions surrounding the environmental impacts of single-use plastic. Environ. Sci. Technol. 2020, 54, 14143–14151. [Google Scholar] [CrossRef]
- Chanda, M.; Roy, S.K. Plastics Technology Handbook; Taylor & Francis: Abingdon, UK, 2006. [Google Scholar]
- Mülhaupt, R. Green polymer chemistry and bio-based plastics: Dreams and reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Künkel, A.; Becker, J.; Borger, L.; Hamprecht, J.; Koltzenburg, S.; Loos, R.; Schick, M.B.; Schlegel, K.; Sinkel, C.; Skupin, G.; et al. Polymers, Biodegradable. In Ullmann’s Encyclopedia of Industrial Chemistry; Verlag Chemie: Hoboken, NJ, USA, 2023; pp. 1–29. [Google Scholar]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Chen, C.; Dai, L.; Ma, L.; Guo, R. Enzymatic degradation of plant biomass and synthetic polymers. Nat. Rev. Chem. 2020, 4, 114–126. [Google Scholar] [CrossRef]
- Hann, S.; Sherrington, C.; Jamieson, O.; Hickman, M.; Kershaw, P.; Bapasola, A.; Cole, G. Investigating Options for Reducing Releases in the Aquatic Environment of Microplastics Emitted by (but Not Intentionally Added in) Products. Report for European Commission, DG Environment (European Union, 2020). Available online: https://environment.ec.europa.eu/topics/circular-economy_en (accessed on 12 January 2023).
- Themelis, N.J.; Ulloa, P.A. Methane generation in landfills. Renew. Energy 2007, 32, 1243–1257. [Google Scholar] [CrossRef]
- ISO 11348-3; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test). International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Neale, P.A.; Antony, A.; Bartkow, M.E.; Farré, M.J.; Heitz, A.; Kristiana, I.; Tang, J.Y.M.; Escher, B.I. Bioanalytical assessment of the formation of disinfection byproducts in a drinking water treatment plant. Environ. Sci. Technol. 2012, 46, 10317–10325. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Cózar, Z.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernández León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Sebille, E.V.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Cao, D.; Xiao, W.; Luo, X.; Liu, G.; Zheng, H. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 12148. [Google Scholar] [CrossRef]
- Ng, E.L.; Lwanga, E.H.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, B.; Shukla, P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 2020, 17, 100567. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Bikiaris, D.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Biobased poly(ethylene furanoate-co-ethylene succinate) copolyesters: Solid state structure, melting point depression and biodegradability. R. Soc. Chem. Adv. 2016, 6, 84003–84015. [Google Scholar] [CrossRef]
- Allen Field: Innovative Design & Manufacturing. Available online: https://www.allenfield.com/wp-content/uploads/2020/07/AF_EcoOne.pdf (accessed on 12 January 2023).
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Beckham, G. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef]
- Sivan, A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426. [Google Scholar] [CrossRef]
- Wiesinger, H.; Klotz, M.; Wang, Z.; Zhao, W.; Haupt, M.; Hellweg, S. The Identity of Oxo-Degradable Plastics and Their Use in Switzerland; Federal Office for the Environment (FOEN): Zurich, Switzerland, 2020. [Google Scholar]
- ASTM D5338-21; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials under Controlled Composting Conditions, Incorporating Thermophilic Temperatures. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM G160-12; Standard Practice for Evaluating Microbial Susceptibility of Nonmetallic Material by Laboratory Soil Burial. ASTM International: West Conshohocken, PA, USA, 2019.
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]


| Type of Enzyme/Bacteria | Polymer Type | Biodegradation Mechanism | Mode of Action and Mechanisms |
|---|---|---|---|
| Proteases | Proteins | Hydrolysis | Catalyze the cleavage of peptide bonds in proteins, breaking them down into smaller peptides and eventually amino acids. |
| Lipases | Lipids | Hydrolysis | Break down ester bonds in lipids, producing free fatty acids and glycerol. |
| Amylases | Starch | Hydrolysis | Break down the α-1,4-glycosidic bonds in starch, producing glucose. |
| Cellulases | Cellulose | Hydrolysis | Break down the β-1,4-glycosidic bonds in cellulose, producing glucose. |
| Chitinases | Chitin | Hydrolysis | Break down the β-1,4-glycosidic bonds in chitin, producing N-acetylglucosamine. |
| Laccases | Lignin | Oxidation | Oxidize the phenolic and non-phenolic structures in lignin, breaking down the polymer into smaller fragments. |
| Peroxidases | Lignin | Oxidation | Catalyze the oxidation of lignin by hydrogen peroxide or oxygen, breaking it down into smaller fragments. |
| Kosakonia sp. | Polyethylene | Anaerobic metabolism | Production of extracellular enzymes to break down polyethylene into smaller fragments for cellular uptake and utilization as carbon and energy sources. |
| Aspergillus sp. | Various | Aerobic metabolism | Produce reactive oxygen species and a range of extracellular enzymes, e.g., cellulases, hemicellulases, and ligninases. |
| Environment | Standard or Test Method | Analysis Time (Months) | Parameters Monitored | Interpretation of Results and Validity Criteria |
|---|---|---|---|---|
| Soil | ASTM D5988-18 | 6 | CO2 evolution | The reference material should have undergone 70% biodegradation, and the amount of CO2 released from the control reactors should be within 20% of the average. |
| ISO 17556:2019 | 6 or until 24 | BOD; CO2 evolution | The reference material should biodegrade above 60%, and the amount of CO2 produced should be within 20% of the average. | |
| Landfilling | ASTM D5526-94D | Until no significant gas production | CH4 and CO2 evolution | The test method measures the percentage conversion of organic carbon in the sample to carbon in gaseous form, with a minimum test duration of 7 days. The level of biodegradation is compared to a cellulose-positive control when it reaches 70% biodegradation. |
| Compost | ASTM D6400-21 | 3–6 | CO2 evolution | After 180 days, at least 90% of the sample’s organic carbon (either absolute or relative) should have transformed into CO2. |
| ASTM D5338:15 | 4 | Cumulative CO2 production, DMR, CMR, GMR | The sample should produce less than 2 g of volatile fatty acids per kilogram of dry matter, achieve 70% biodegradation according to the reference material, and the deviation of the biodegradation percentage from the positive reference should be less than 20%. | |
| ISO 14855-2012 | 6 | CO2 evolution | According to the reference material, the sample must biodegrade at least 70% after 45 days. The difference between the percent biodegradation of the reference material in different vessels must be less than 20% at the end of the test, and the blank inoculum should produce between 50 mg and 150 mg of carbon dioxide per gram of volatile solids after 10 days of incubation. | |
| ISO 17088:2021 | 6 | CO2 evolution | After 180 days, at least 90% of the sample’s organic carbon (either absolute or relative) should have transformed into CO2. | |
| ISO 14855-2:2018 | 6 | CO2 evolution | After 45 days, the reference material must exhibit biodegradation above 70%. | |
| Aquatic systems | ISO 18830:2016 | 24 | BOD; static test conditions | The reference material must exhibit biodegradation above 60% after 180 days. The difference between the percentage of biodegradation of the reference material in different vessels should be less than 20% of the mean at the end of the test. |
| ISO 19679:2020 | 24 | CO2 evolution; static test conditions | The reference material must exhibit biodegradation above 60% after 180 days. The CO2 released from the blank at the end of the test should not exceed 3.5 mg CO2/g wet sediment after 6 months. | |
| ASTM D6691-17 | 3 | CO2 evolution; static test conditions | The reference material must exhibit biodegradation above 70%. | |
| ASTM D7991-22 | 24 | CO2 evolution; static test conditions | The reference material must exhibit biodegradation above 60% after 180 days. | |
| ISO 14853:2016 | 3 | CH4 and CO2 evolution, DIC; static test conditions | The determination of the ultimate anaerobic biodegradability of plastics by anaerobic microorganisms requires degradation greater than 70% of the reference material, while the pH of the medium must remain between 6 and 8. |
| Polymer | Degradation Test Condition | Tests Used to Assess Biodegradation | Reference |
|---|---|---|---|
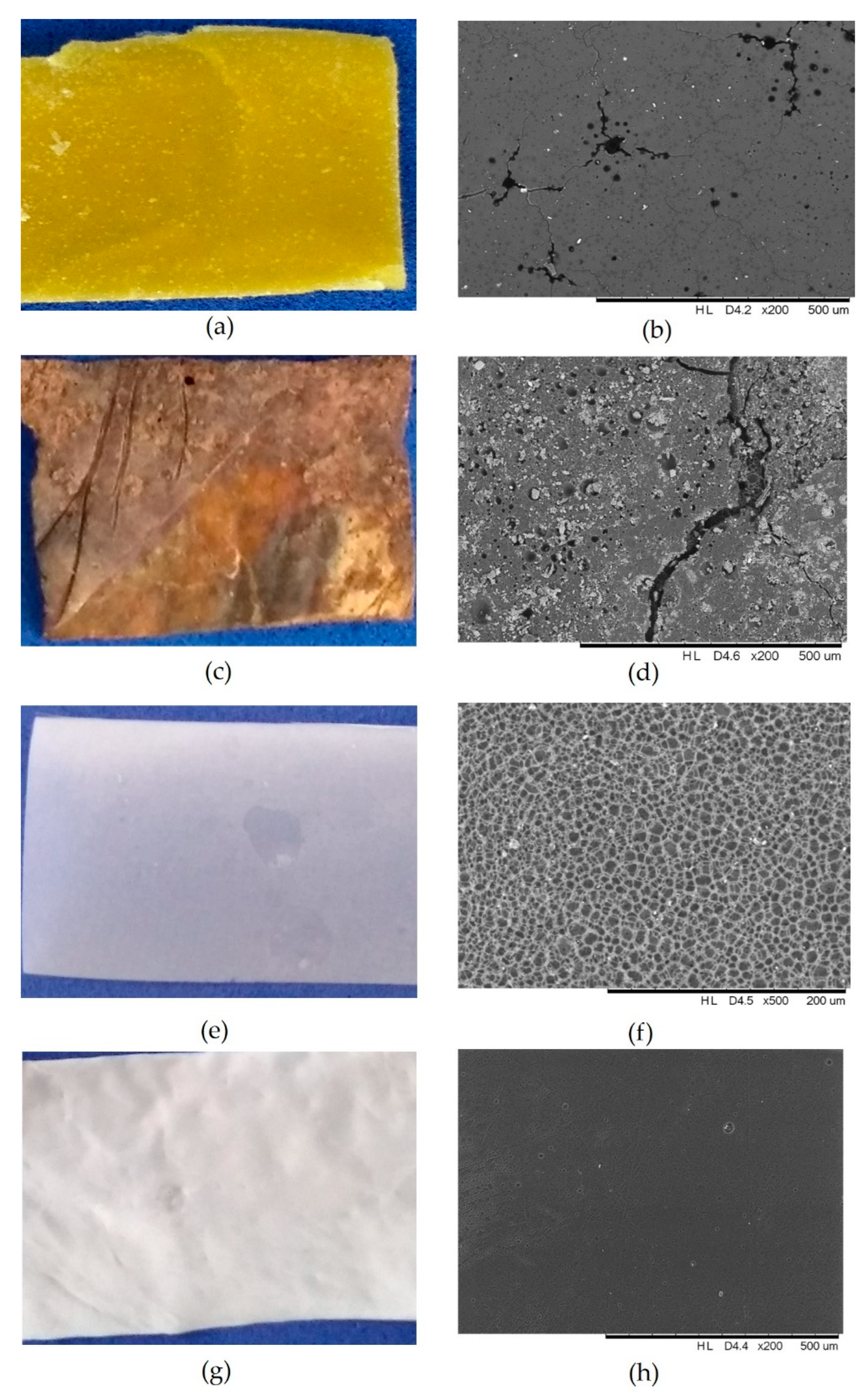
| Poly(butylene adipate-co-terephthalate) (PBAT) | Soil (6 weeks at 25 °C) | SEM and isotope-specific quantification of 13CO2 through NanoSIMS | [55] |
| Cellulose acetate (DS 2.5) | Several aqueous environments (12 months) | SEM, mass loss, FTIR, UV-Vis spectroscopy, TG, DSC, XRD, NMR, SEC | [28] |
| Polyhydroxyalkanoate (PHA), polybutylene succinate (PBS), polybutylene adipate-terephthalate and polylactic acid blend (PBAT/PLA), and polyester | Soil (25 °C and 37 °C up to 270 days) | SEM, CO2 evolution by titration and gas analyzer | [33,38,39] |
| Poly(lactic acid) and chitosan composite | Active soil and sterile soil (25 °C up to 200 days) | Mass loss, tensile testing, molecular weight by GPC, FTIR, SEM, DSC, contact angle | [56] |
| Thermoplastic starch-graphene composites | Soil (23 °C, 120 days) and aerobic composting process | SEM, mass loss, CO2 evolution | [57] |
| Polyethylene (PE), compostable bags (at least 60% of starch), and cellulosic plates | Anaerobic conditions | Biochemical methane potential and visual observation | [58] |
| PE and PE-modified with oxo-biodegradable compound | Accelerated weathering (UV irradiation 8 h/70 °C followed by a steam condensation 4 h/55 °C), and soil burial (30 °C) | Tensile testing, mass loss, contact angle, FTIR | [59] |
| PLA-clay composites | Aerobic composting | Mass loss, cumulative CO2, visual observation | [60] |
| Low-density polyethylene (LDPE) | Solid mineral salt medium (Petri dish) | Tensile testing, SEM, mass loss | [61] |
| Polyvinyl alcohol (PVOH) incorporated with cellulose nanocrystals | Soil burial (3 months) | Tensile testing, FTIR, mass loss, DSC, SEM | [32] |
| Poly-d-lactic acid (PDLA) and cellulose microfibers | Solid and liquid mineral salt medium (Petri dish) | Mass loss, visual observation, FTIR, SEM, NMR | [62] |
| Starch and gelatin bioplastics | Soil respiration chambers | Respirometry (OxiTop) | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371-399. https://doi.org/10.3390/macromol3020023
Silva RRA, Marques CS, Arruda TR, Teixeira SC, de Oliveira TV. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol. 2023; 3(2):371-399. https://doi.org/10.3390/macromol3020023
Chicago/Turabian StyleSilva, Rafael Resende Assis, Clara Suprani Marques, Tarsila Rodrigues Arruda, Samiris Cocco Teixeira, and Taíla Veloso de Oliveira. 2023. "Biodegradation of Polymers: Stages, Measurement, Standards and Prospects" Macromol 3, no. 2: 371-399. https://doi.org/10.3390/macromol3020023
APA StyleSilva, R. R. A., Marques, C. S., Arruda, T. R., Teixeira, S. C., & de Oliveira, T. V. (2023). Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol, 3(2), 371-399. https://doi.org/10.3390/macromol3020023