Systemic Effects of Photoactivated 5,10,15,20-tetrakis(N-methylpyridinium-3-yl) Porphyrin on Healthy Drosophila melanogaster
Abstract
1. Introduction
2. Materials and Methods
2.1. TMPyP3 in Nutrient Media
2.2. Light Source and Irradiation Conditions
2.3. TMPyP3 Feeding Procedure
2.4. TMPyP3 Ingestion and Retention in the Tissue after the Oral Administration
2.5. Quantification of the Hydrogen Peroxide (H2O2)
2.6. Negative Geotaxis
2.7. Data Analysis and Statistics
3. Results
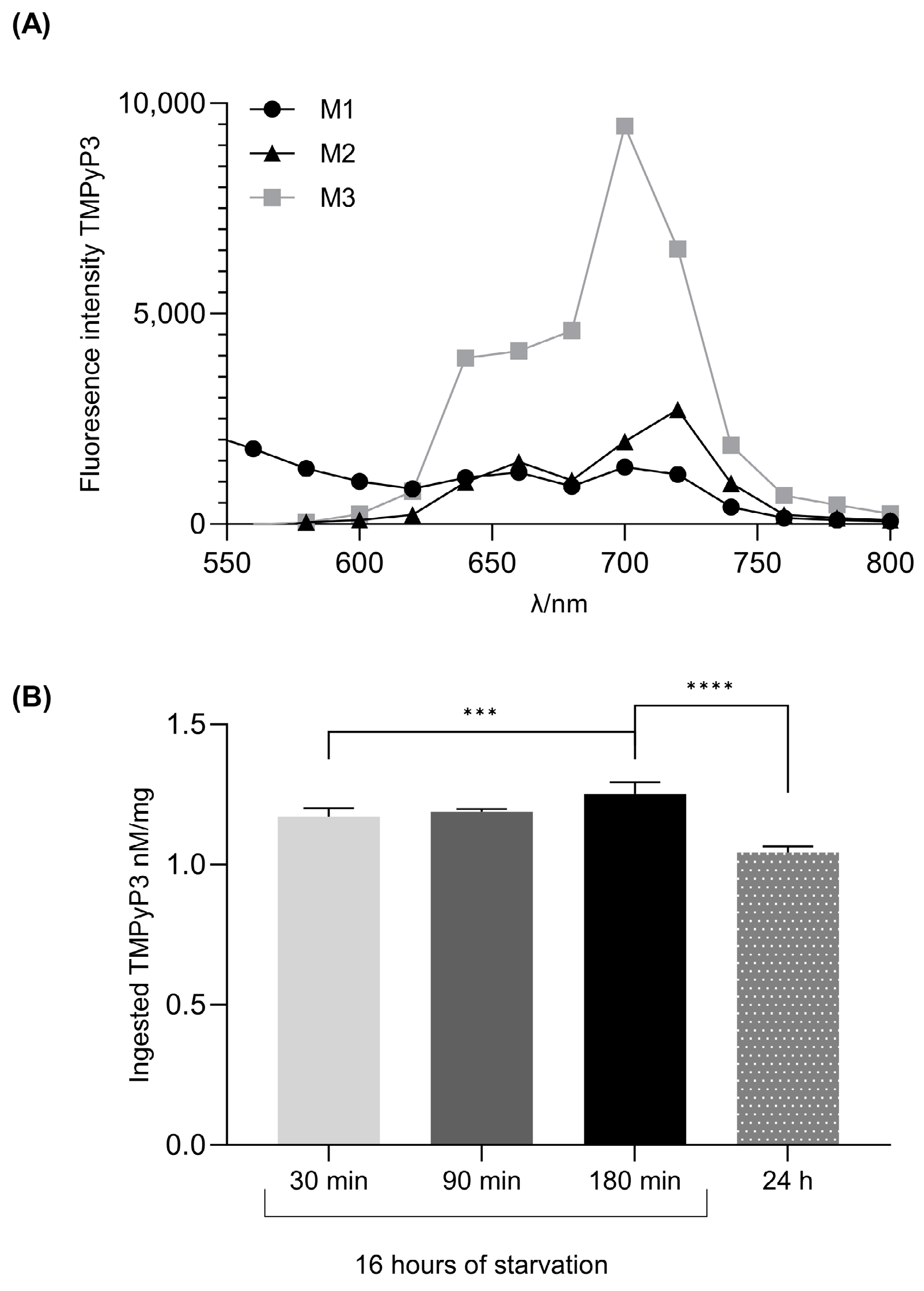
3.1. Starvation in Combination with a Short Duration of Feeding on the Yeast Free Media Leads to Maximal TMPyP3 Ingestion
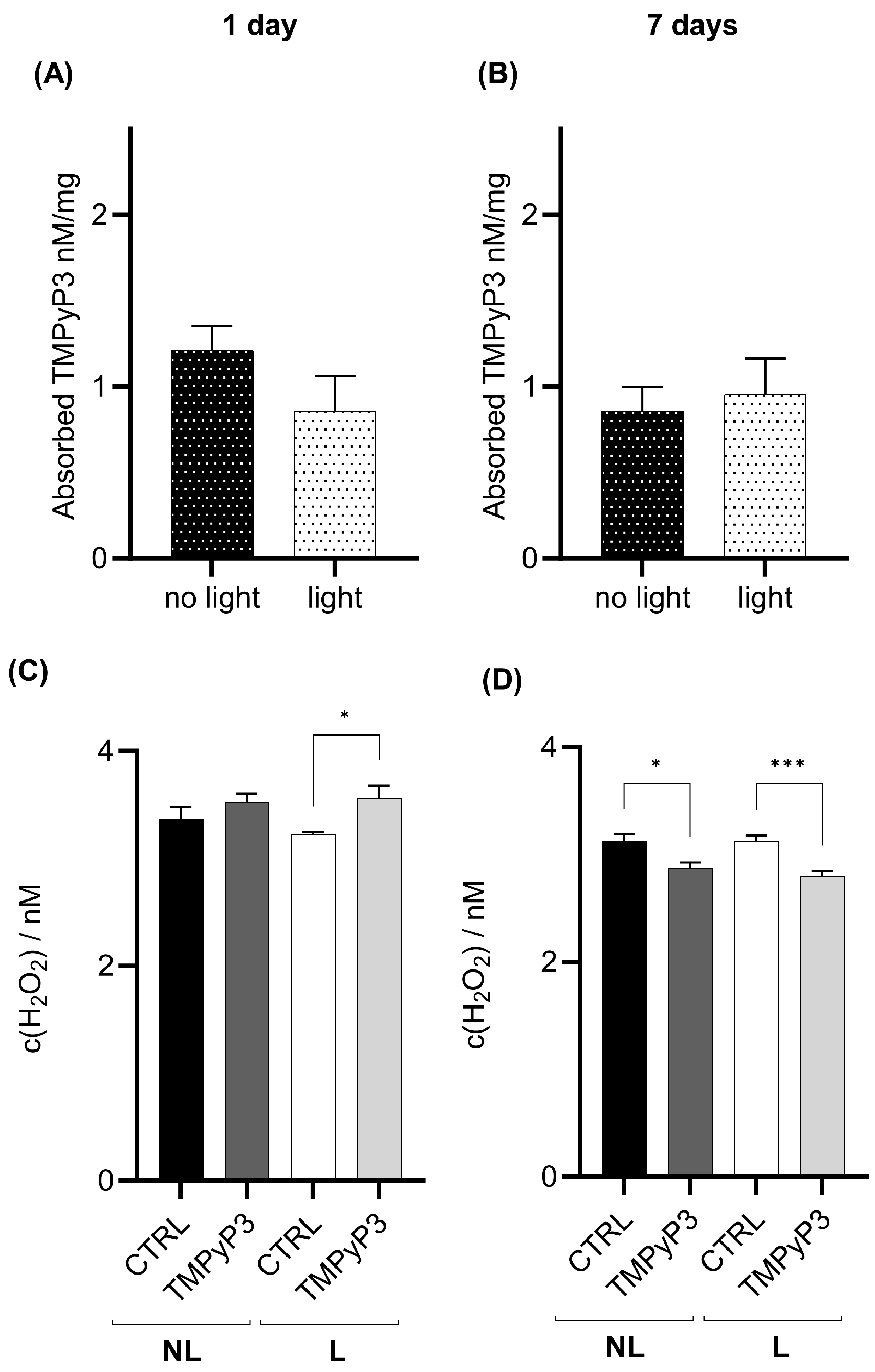
3.2. TMPyP3 Retention in the Body Causes a Light-Independent Decrease in the Levels of H2O2
3.3. Retention of TMPyP3 in the Head Decreases the H2O2 Concentration after Seven Days
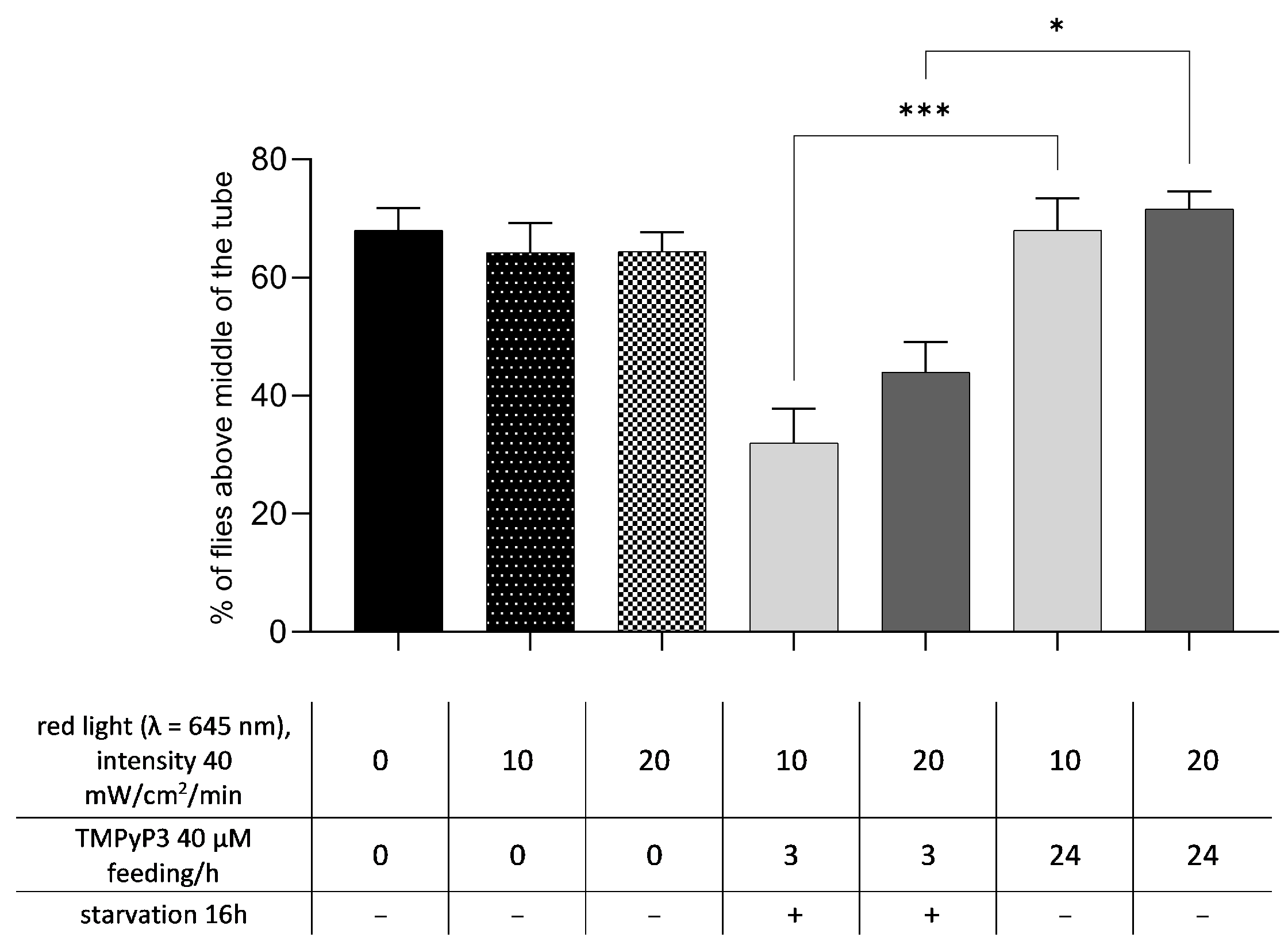
3.4. Retention of TMPyP3 in the Head Decreases Negative Geotaxsis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.-D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; López-Higuera, J.M.; Rodríguez-Cobo, L.; Cobo, A. Advanced Light Source Technologies for Photodynamic Therapy of Skin Cancer Lesions. Pharmaceutics 2023, 15, 2075. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. Available online: https://www.frontiersin.org/articles/10.3389/fchem.2021.686303 (accessed on 12 September 2023). [CrossRef]
- Huang, H.; Song, W.; Rieffel, J.; Lovell, J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015, 3, 23. [Google Scholar] [CrossRef]
- Liu, A.-H.; Sun, X.; Wei, X.-Q.; Zhang, Y.-Z. Efficacy of multiple low-dose photodynamic TMPYP4 therapy on cervical cancer tumour growth in nude mice. Asian Pac. J. Cancer Prev. 2013, 14, 5371–5374. [Google Scholar] [CrossRef][Green Version]
- LIU, H.; LV, C.; DING, B.; WANG, J.; LI, S.; ZHANG, Y. Antitumor activity of G-quadruplex-interactive agent TMPyP4 with photodynamic therapy in ovarian carcinoma cells. Oncol. Lett. 2014, 8, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, X.; Shen, Z.; Mei, Y.; Zhu, J.; Zhang, X.; Liang, G.; Zheng, X. H2O2 enhances the anticancer activity of TMPyP4 by ROS-mediated mitochondrial dysfunction and DNA damage. Med. Oncol. 2021, 38, 59. [Google Scholar] [CrossRef]
- Jurak, I.; Cokarić Brdovčak, M.; Djaković, L.; Bertović, I.; Knežević, K.; Lončarić, M.; Jurak Begonja, A.; Malatesti, N. Photodynamic Inhibition of Herpes Simplex Virus 1 Infection by Tricationic Amphiphilic Porphyrin with a Long Alkyl Chain. Pharmaceutics 2023, 15, 956. [Google Scholar] [CrossRef] [PubMed]
- Jelovica, M.; Grbčić, P.; Mušković, M.; Sedić, M.; Pavelić, S.K.; Lončarić, M.; Malatesti, N. In Vitro Photodynamic Activity of N-Methylated and N-Oxidised Tripyridyl Porphyrins with Long Alkyl Chains and Their Inhibitory Activity in Sphingolipid Metabolism. ChemMedChem 2018, 13, 360–372. [Google Scholar] [CrossRef]
- Ong, C.; Yung, L.-Y.L.; Cai, Y.; Bay, B.-H.; Baeg, G.-H. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 2015, 9, 396–403. [Google Scholar] [CrossRef]
- Yoho, J.; Stroh, C.; Swavey, S.; Kango-Singh, M. Toxicity and localization studies of a potential photodynamic therapy agent in Drosophila. Genesis 2014, 52, 309–314. [Google Scholar] [CrossRef]
- Schuitmaker, J.J.; Vogel, E.W.; Nagelkerke, J.F.; Bos, R.P. Mutagenicity and dark toxicity of the second-generation photosensitizer bacteriochlorin a. J. Photochem. Photobiol. B Biol. 1998, 47, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, E.; Vidal, L.M.; Cruces, M.P.; Janczur, M.K. Action of protoporphyrin-IX (PP-IX) in the lifespan of Drosophila melanogaster deficient in endogenous antioxidants, Sod and Cat. Open J. Anim. Sci. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, Y.; Si, W.; Zhong, X.; Cai, Y.; Zou, J.; Shao, J.; Yang, Z.; Dong, X. Zinc(II) Metalated Porphyrins as Photothermogenic Photosensitizers for Cancer Photodynamic/Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 238–247. [Google Scholar] [CrossRef]
- Lesar, A.; Mušković, M.; Begić, G.; Lončarić, M.; Tomić Linšak, D.; Malatesti, N.; Gobin, I. Cationic Porphyrins as Effective Agents in Photodynamic Inactivation of Opportunistic Plumbing Pathogen Legionella pneumophila. Int. J. Mol. Sci. 2020, 21, 5367. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lee, C.-M.; Lee, L.-C.; Tung, L.-C.; Hsieh-Li, H.-M.; Lee-Chen, G.-J.; Su, M.-T. Mitochondrial Dysfunction and Oxidative Stress Contribute to the Pathogenesis of Spinocerebellar Ataxia Type 12 (SCA12)*. J. Biol. Chem. 2011, 286, 21742–21754. [Google Scholar] [CrossRef]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef]
- Protchenko, O.; Shakoury-Elizeh, M.; Keane, P.; Storey, J.; Androphy, R.; Philpott, C.C. Role of PUG1 in Inducible Porphyrin and Heme Transport in Saccharomyces cerevisiae. Eukaryot. Cell 2008, 7, 859–871. [Google Scholar] [CrossRef]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic Inactivation of Bacterial and Yeast Biofilms With a Cationic Porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, M.; Setnicka, V.; Král, V.; Volka, K. Noncovalent interactions of peptides with porphyrins in aqueous solution: Conformational study using vibrational CD spectroscopy. Biopolymers 2001, 60, 307–316. [Google Scholar] [CrossRef]
- Miguel-Aliaga, I.; Jasper, H.; Lemaitre, B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 2018, 210, 357–396. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.A.; Carvalho, G.B.; Amador, A.; Phillips, A.M.; Hoxha, S.; Lizotte, K.J.; Ja, W.W. Quantifying Drosophila food intake: Comparative analysis of current methodology. Nat. Methods 2014, 11, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Miguel-Aliaga, I. The Digestive Tract of Drosophila melanogaster. Annu. Rev. Genet. 2013, 47, 377–404. [Google Scholar] [CrossRef]
- Wong, R.; Piper, M.D.W.; Wertheim, B.; Partridge, L. Quantification of Food Intake in Drosophila. PLoS ONE 2009, 4, e6063. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Pass, G. The Insect Circulatory System: Structure, Function, and Evolution. Annu. Rev. Entomol. 2020, 65, 121–143. [Google Scholar] [CrossRef]
- Contreras, E.G.; Klämbt, C. The Drosophila blood-brain barrier emerges as a model for understanding human brain diseases. Neurobiol. Dis. 2023, 180, 106071. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.G.; Sierralta, J. The Fly Blood-Brain Barrier Fights Against Nutritional Stress. J. Exp. Neurosci. 2022, 17, 26331055221120252. [Google Scholar] [CrossRef] [PubMed]
- Schirmeier, S.; Klämbt, C. The Drosophila blood-brain barrier as interface between neurons and hemolymph. Mech. Dev. 2015, 138, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Stork, T.; Engelen, D.; Krudewig, A.; Silies, M.; Bainton, R.J.; Klämbt, C. Organization and Function of the Blood–Brain Barrier in Drosophila. J. Neurosci. 2008, 28, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Daneman, R. Formation and maintenance of the BBB. Mech. Dev. 2015, 138, 8–16. [Google Scholar] [CrossRef]
- Samperi, M.; Vittorio, S.; De Luca, L.; Romeo, A.; Monsù Scolaro, L. Interaction of Aggregated Cationic Porphyrins with Human Serum Albumin. Int. J. Mol. Sci. 2023, 24, 2099. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, B.P.; Borozan, S.Z.; Stojanović, S.Đ. π–π and cation–π interactions in protein–porphyrin complex crystal structures. RSC Adv. 2012, 2, 12963–12972. [Google Scholar] [CrossRef]
- Rozinek, S.C.; Thomas, R.J.; Brancaleon, L. Biophysical characterization of the interaction of human albumin with an anionic porphyrin. Biochem. Biophys. Rep. 2016, 7, 295–302. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.R. Drosophila Central Nervous System Glia. Cold Spring Harb. Perspect. Biol. 2015, 7, a020552. [Google Scholar] [CrossRef] [PubMed]
- Limmer, S.; Weiler, A.; Volkenhoff, A.; Babatz, F.; Klämbt, C. The Drosophila blood-brain barrier: Development and function of a glial endothelium. Front. Neurosci. 2014, 8, 365. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2014.00365 (accessed on 24 January 2024). [CrossRef] [PubMed]
- Afonso, S.; Vanore, G.; Batlle, A. Protoporphyrin IX and oxidative stress. Free Radic. Res. 1999, 31, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Oswald, M.C.; Brooks, P.S.; Zwart, M.F.; Mukherjee, A.; West, R.J.; Giachello, C.N.; Morarach, K.; Baines, R.A.; Sweeney, S.T.; Landgraf, M. Reactive oxygen species regulate activity-dependent neuronal plasticity in Drosophila. eLife 2018, 7, e39393. [Google Scholar] [CrossRef] [PubMed]
- Milton, V.J.; Sweeney, S.T. Oxidative stress in synapse development and function. Dev. Neurobiol. 2012, 72, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Kilian, K.; Pęgier, M. Porphyrins as Chelating Agents for Molecular Imaging in Nuclear Medicine. Molecules 2022, 27, 3311. [Google Scholar] [CrossRef]
- Biesaga, M.; Pyrzyńska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Giuntini, F.; Foglietta, F.; Marucco, A.M.; Troia, A.; Dezhkunov, N.V.; Pozzoli, A.; Durando, G.; Fenoglio, I.; Serpe, L.; Canaparo, R. Insight into ultrasound-mediated reactive oxygen species generation by various metal-porphyrin complexes. Free Radic. Biol. Med. 2018, 121, 190–201. [Google Scholar] [CrossRef]
- Hao, J.; Miao, W.; Lu, S.; Cheng, Y.; Jia, G.; Li, C. Controllable stereoinversion in DNA-catalyzed olefin cyclopropanation via cofactor modification. Chem. Sci. 2021, 12, 7918–7923. [Google Scholar] [CrossRef]
- Zagami, R.; Castriciano, M.A.; Romeo, A.; Monsù Scolaro, L. Enhancement of the Rates for Insertion of Zinc(II) Ions into a Cationic Porphyrin Catalyzed by Poly(glutamate). Int. J. Mol. Sci. 2023, 24, 17371. [Google Scholar] [CrossRef]
- Muñoz, P.; Humeres, A.; Elgueta, C.; Kirkwood, A.; Hidalgo, C.; Núñez, M.T. Iron Mediates N-Methyl-d-aspartate Receptor-dependent Stimulation of Calcium-induced Pathways and Hippocampal Synaptic Plasticity. J. Biol. Chem. 2011, 286, 13382–13392. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Manaia, M.N.; Chiorcea-Paquim, A.-M. Cationic porphyrin TMPyP4 redox behaviour and interaction with nucleic acids: Towards a new methodology for screening porphyrin-based anticancer drugs. Electrochim. Acta 2023, 462, 142749. [Google Scholar] [CrossRef]
- Lebedeva, N.S.; Yurina, E.S.; Gubarev, Y.A.; Syrbu, S.A. Interactions of tetracationic porphyrins with DNA and their effects on DNA cleavage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Drugs: Differential Effects as Cancer Therapeutics and Protectors of Normal Tissue Against Oxidative Injury. Antioxid. Redox Signal 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Batinić-Haberle, I. Manganese porphyrins and related compounds as mimics of superoxide dismutase. In Methods in Enzymology; Superoxide Dismutase; Academic Press: Cambridge, MA, USA, 2002; Volume 349, pp. 223–233. [Google Scholar]
- Hanna, M.E.; Bednářová, A.; Rakshit, K.; Chaudhuri, A.; O’Donnell, J.M.; Krishnan, N. Perturbations in dopamine synthesis lead to discrete physiological effects and impact oxidative stress response in Drosophila. J. Insect Physiol. 2015, 73, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Pirooznia, S.K.; Yuan, C.; Khan, M.R.; Karuppagounder, S.S.; Wang, L.; Xiong, Y.; Kang, S.U.; Lee, Y.; Dawson, V.L.; Dawson, T.M. PARIS induced defects in mitochondrial biogenesis drive dopamine neuron loss under conditions of parkin or PINK1 deficiency. Mol. Neurodegener. 2020, 15, 17. [Google Scholar] [CrossRef]
- Vrailas-Mortimer, A.; del Rivero, T.; Mukherjee, S.; Nag, S.; Gaitanidis, A.; Kadas, D.; Consoulas, C.; Duttaroy, A.; Sanyal, S. A muscle-specific p38 MAPK/Mef2/MnSOD pathway regulates stress, motor function, and life span in Drosophila. Dev. Cell 2011, 21, 783–795. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filošević Vujnović, A.; Čabrijan, S.; Mušković, M.; Malatesti, N.; Andretić Waldowski, R. Systemic Effects of Photoactivated 5,10,15,20-tetrakis(N-methylpyridinium-3-yl) Porphyrin on Healthy Drosophila melanogaster. BioTech 2024, 13, 23. https://doi.org/10.3390/biotech13030023
Filošević Vujnović A, Čabrijan S, Mušković M, Malatesti N, Andretić Waldowski R. Systemic Effects of Photoactivated 5,10,15,20-tetrakis(N-methylpyridinium-3-yl) Porphyrin on Healthy Drosophila melanogaster. BioTech. 2024; 13(3):23. https://doi.org/10.3390/biotech13030023
Chicago/Turabian StyleFilošević Vujnović, Ana, Sara Čabrijan, Martina Mušković, Nela Malatesti, and Rozi Andretić Waldowski. 2024. "Systemic Effects of Photoactivated 5,10,15,20-tetrakis(N-methylpyridinium-3-yl) Porphyrin on Healthy Drosophila melanogaster" BioTech 13, no. 3: 23. https://doi.org/10.3390/biotech13030023
APA StyleFilošević Vujnović, A., Čabrijan, S., Mušković, M., Malatesti, N., & Andretić Waldowski, R. (2024). Systemic Effects of Photoactivated 5,10,15,20-tetrakis(N-methylpyridinium-3-yl) Porphyrin on Healthy Drosophila melanogaster. BioTech, 13(3), 23. https://doi.org/10.3390/biotech13030023





