Auxin-Producing Bacteria Used as Microbial Biostimulants Improve the Growth of Tomato (Solanum lycopersicum L.) Seedlings in Hydroponic Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains
2.2. Preparation and Germination of Tomato Seeds
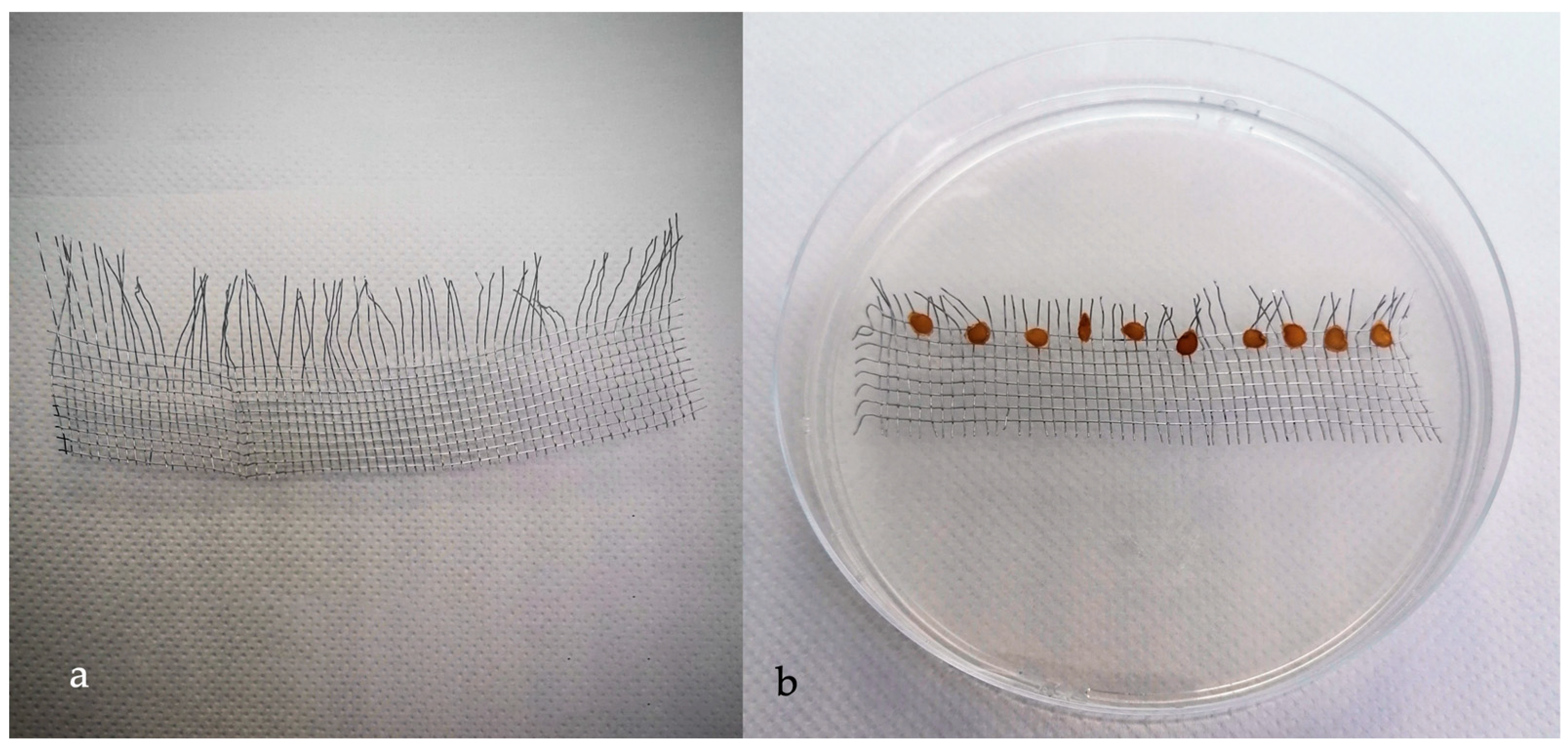
2.3. Acclimation
2.4. Co-Inoculation
2.5. Analysis of Indole-3-Acetic Acid (IAA) Production
2.6. Data Analysis
3. Results
3.1. The Germination and Survival Rate of Tomato Seeds
3.2. Effect of the Bacterial Strains Inoculation on Roots and Stems
3.3. Analysis of Indole-3-Acetic Acid (IAA) Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz-Rodríguez, A.M.; Salcedo Gastelum, L.A.; Félix Pablos, C.M.; Parra-Cota, F.I.; Santoyo, G.; Puente, M.L.; Bhattacharya, D.; Mukherjee, J.; de los Santos-Villalobos, S. The Current and Future Role of Microbial Culture Collections in Food Security Worldwide. Front. Sustain. Food Syst. 2021, 4, 614739. [Google Scholar] [CrossRef]
- Jan, S.; Rashid, Z.; Ahngar, T.A.; Iqbal, S.; Naikoo, M.A.; Majeed, S.; Bhat, T.A.; Gul, R.; Nazir, I. Hydroponics—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1779–1787. [Google Scholar] [CrossRef]
- Orsini, F.; Kahane, R.; Nono-Womdim, R.; Gianquinto, G. Urban Agriculture in the Developing World: A Review. Agron. Sustain. Dev. 2013, 33, 695–720. [Google Scholar] [CrossRef]
- Khan, S.; Purohit, A.; Vadsaria, N. Hydroponics: Current and Future State of the Art in Farming. J. Plant Nutr. 2020, 44, 1515–1538. [Google Scholar] [CrossRef]
- Suryaningprang, A.; Suteja, J.; Mulyaningrum, M.; Herlinawati, E. Hydroponic: Empowering Local Farmer Knowhow to Gain Value Added on Agriculture Commodity. Bp. Int. Res. Critic. Inst. J. Humanit. Soc. Sci. 2021, 4, 787–796. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Beneficial Bacteria and Fungi in Hydroponic Systems: Types and Characteristics of Hydroponic Food Production Methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]
- Pomoni, D.I.; Koukou, M.K.; Vrachopoulos, M.G.; Vasiliadis, L. A Review of Hydroponics and Conventional Agriculture Based on Energy and Water Consumption, Environmental Impact, and Land Use. Energies 2023, 16, 1960. [Google Scholar] [CrossRef]
- Nederhoff, E.; Stanghellini, C. Water Use Efficiency of Tomatoes’, Practical Hydroponics and Greenhouses. North Manly NSW Aust. Casper Public 2010, 115, 52–59. [Google Scholar]
- Oxford Analytica—Fertiliser and Food Prices Could Be High for Years. Available online: https://dailybrief.oxan.com/Analysis/DB268415/Fertiliser-and-food-prices-could-be-high-for-years#:~:text=The%20fertiliser%20market%20imbalance%2C%20like,GDP%20growth%20and%20living%20standards (accessed on 24 February 2024).
- Nerlich, A.; Karlowsky, S.; Schwarz, D.; Förster, N.; Dannehl, D. Soilless Tomato Production: Effects of Hemp Fiber and Rock Wool Growing Media on Yield, Secondary Metabolites, Substrate Characteristics and Greenhouse Gas Emissions. Horticulturae 2022, 8, 272. [Google Scholar] [CrossRef]
- Halbert-Howard, A.; Häfner, F.; Karlowsky, S.; Schwarz, D.; Krause, A. Evaluating Recycling Fertilizers for Tomato Cultivation in Hydroponics, and Their Impact on Greenhouse Gas Emissions. Environ. Sci. Pollut. Res. 2021, 28, 59284–59303. [Google Scholar] [CrossRef]
- Karlowsky, S.; Gläser, M.; Henschel, K.; Schwarz, D. Seasonal Nitrous Oxide Emissions from Hydroponic Tomato and Cucumber Cultivation in a Commercial Greenhouse Company. Front. Sustain. Food Syst. 2021, 5, 626053. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. The Use of PGPB to Promote Plant Hydroponic Growth. Plants 2022, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, M.R.; Afrilandha, N.; Hindersah, R.; Suryatmana, P.; Fitriatin, B.N.; Kamaluddin, N.N. The Effect of Beneficial Microorganism as Biofertilizer Application in Hydroponic-Grown Tomato. Sains Tanah 2023, 20, 66–77. [Google Scholar] [CrossRef]
- Gul, A.; Ozaktan, H.; Yolageldi, L.; Cakir, B.; Sahin, M.; Akat, S. Effect of Rhizobacteria on Yield of Hydroponically Grown Tomato Plants. Int. Symp. Adv. Technol. Manag. Towards Sustain. Greenh. Ecosyst. Greensys 2011, 952, 777–784. [Google Scholar]
- Aini, N.; Yamika, W.S.D.; Pahlevi, R.W. The Effect of Nutrient Concentration and Inoculation of PGPR and AMF on the Yield and Fruit Quality of Hydroponic Cherry Tomatoes (Lycopersicon esculentum Mill. Var. Cerasiforme). J. Appl. Hortic. 2019, 21, 116–122. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Yilmaz, D.; Zikaria, K.; Ikiz, B.; Gruda, N.S. Enhancing the yield, quality and antioxidant content of lettuce through innovative and eco-friendly biofertilizer practices in hydroponics. Horticulturae 2023, 9, 1274. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything You Must Know about Azospirillum and Its Impact on Agriculture and Beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Torres Vera, R.; Bernabé García, A.J.; Carmona Álvarez, F.J.; Martínez Ruiz, J.; Fernández Martín, F. Application and Effectiveness of Methylobacterium symbioticum as a Biological Inoculant in Maize and Strawberry Crops. Folia Microbiol. 2024, 69, 121–131. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ros, M.; Martínez, J.; Carmona, F.; Bernabé, A.; Torres, R.; Lucena, T.; Aznar, R.; Arahal, D.R.; Fernández, F. Methylobacterium symbioticum Sp. Nov., a New Species Isolated from Spores of Glomus iranicum Var. Tenuihypharum. Curr. Microbiol. 2020, 77, 2031–2041. [Google Scholar] [CrossRef]
- Baset, M.M.; Shamsuddin, Z.H.; Wahab, Z.; Marziah, M. Effect of Plant Growth Promoting Rhizobacterial (PGPR) Inoculation on Growth and Nitrogen Incorporation of Tissue-Cultured Musa Plantlets under Nitrogen-Free Hydroponics Condition. Aust. J. Crop Sci. 2010, 4, 85–90. [Google Scholar]
- Aini, N.; Yamika, W.S.D.; Ulum, B. Effect of Nutrient Concentration, PGPR and AMF on Plant Growth, Yield and Nutrient Uptake of Hydroponic Lettuce. Int. J. Agric. Biol. 2019, 21, 175–183. [Google Scholar]
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watanabe, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial Mineralization of Organic Nitrogen into Nitrate to Allow the Use of Organic Fertilizer in Hydroponics. Soil. Sci. Plant Nutr. 2011, 57, 190–203. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial Modulation of Plant Ethylene Levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour Moghaddam, M.J.; Emtiazi, G.; Salehi, Z. Enhanced Auxin Production by Azospirillum Pure Cultures from Plant Root Exudates. J. Agric. Sci. Technol. 2012, 14, 985–994. [Google Scholar]
- El-Khawas, H.; Adachi, K. Identification and Quantification of Auxins in Culture Media of Azospirillum and Klebsiella and Their Effect on Rice Roots; Springer: Berlin/Heidelberg, Germany, 1999; Volume 28. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A Universal Cell Factory for Industry, Agriculture, Biomaterials and Medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef]
- Poveda, J.; González-Andrés, F. Bacillus as a Source of Phytohormones for Use in Agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 1–17. [Google Scholar] [CrossRef]
- Bochow, H. Phytosanitary Effects of Bacillus subtilis as Biocontrol Agent. Meded.-Fac. Landbouwwet. Rijksuniv. 1992, 57, 387–393. [Google Scholar]
- Böhme, M. Effects of Lactate, Humate and Bacillus subtilis on the Growth of Tomato Plants in Hydroponic Systems. Int. Symp. Grow. Media Hydroponics 1997, 481, 231–240. [Google Scholar]
- Gül, A.; Kidoglu, F.; Tüzel, Y.; Tüzel, I.H. Effects of Nutrition and Bacillus amyloliquefaciens on Tomato (Solanum lycopersicum L.) Growing in Perlite. Span. J. Agric. Res. 2008, 3, 422–429. [Google Scholar]
- Antonio Lucas García, J.; Probanza, A.; Ramos, B.; Palomino, M.; Javier Gutiérrez Mañero, F.; Ruiz Palomino, M. Effect of Inoculation of Bacillus licheniformis on Tomato and Pepper. Agronomie 2004, 24, 169–176. [Google Scholar] [CrossRef]
- Kaur, J.; Pethani, B.P.; Kumar, S.; Kim, M.; Sunna, A.; Kautto, L.; Penesyan, A.; Paulsen, I.T.; Nevalainen, H. Pseudomonas aeruginosa Inhibits the Growth of Scedosporium aurantiacum, an Opportunistic Fungal Pathogen Isolated from the Lungs of Cystic Fibrosis Patients. Front. Microbiol. 2015, 6, 866. [Google Scholar] [CrossRef]
- Baldani, V.L.D.; Alvarez, M.A.D.B.; Baldani, J.I. Establishment of Inoculated Azospirillum Spp. in the Rhizosphere and in Roots of Field Grown Wheat and Sorghum. Plant Soil 1986, 90, 35–46. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Vande Broek, A.; Vanderleyden, J. Phytostimulatory Effect of Azospirillum brasilense Wild Type and Mutant Strains Altered in IAA Production on Wheat. Plant Soil 1999, 212, 153–162. [Google Scholar] [CrossRef]
- Ferreira, N.D.S.; Sant’ Anna, F.H.; Reis, V.M.; Ambrosini, A.; Volpiano, C.G.; Rothballer, M.; Schwab, S.; Baura, V.A.; Balsanelli, E.; de Oliveira Pedrosa, F.; et al. Genome-Based Reclassification of Azospirillum brasilense Sp245 as the Type Strain of Azospirillum baldaniorum Sp. Nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 6203–6212. [Google Scholar] [CrossRef]
- Filippi, C.; Bagnoli, G.; Volterrani, M.; Picci, G. Plant and Soil Antagonistic Effects of Soil Bacteria on Fusarium oxysporum Schlecht f. Sp. Dianthi (Prill and Del.) Snyd. and Hans IlL Relation between Protection against Fusarium Wilt in Carnation and Bacterial Antagonists Colonization on Roots. Plant Soil 1987, 98, 161–167. [Google Scholar] [CrossRef]
- Citernesi, A.S.; Filippi, C.; Bagnoli, G.; Giovannetti, M. Effects of the Antimycotic Molecule Iturin A2, Secreted By Bacillus subtilis strain M51, on Arbuscular Mycorrhizal Fungi. Microbiol. Res. 1994, 149, 241–246. [Google Scholar] [CrossRef]
- Nathoo, N.; Bernards, M.A.; MacDonald, J.; Yuan, Z.C. A Hydroponic Co-Cultivation System for Simultaneous and Systematic Analysis of Plant/Microbe Molecular Interactions and Signaling. J. Vis. Exp. 2017, 125, e55955. [Google Scholar] [CrossRef]
- ISTA (International Seed Testing Association). International Rules for Seed Testing. Seed Sci. Tech. 1976, 46, 52–70. [Google Scholar]
- Kaur, H.; Rakesh, S.; Sharma, P. Effect of Hoagland Solution for Growing Tomato Hydroponically in Green House. HortFlora Res. Spectr. 2016, 5, 310–315. [Google Scholar]
- Souza, G.L.O.D.D.; DA Silva, D.F.; Nietsce, S.; Xavier, A.A.; Pereira, M.C.T. Endophytic Bacteria Used as Bioinoculants in Micropropagated Banana Seedlings. Rev. Bras. Frutic. 2017, 39, e-324. [Google Scholar] [CrossRef]
- Saijai, S.; Ando, A.; Inukai, R.; Shinohara, M.; Ogawa, J. Analysis of Microbial Community and Nitrogen Transition with Enriched Nitrifying Soil Microbes for Organic Hydroponics. Biosci. Biotechnol. Biochem. 2016, 80, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Kontopoulou, C.-K.; Giagkou, S.; Stathi, E.; Savvas, D.; Iannetta, P.P.M. Responses of Hydroponically Grown Common Bean Fed with Nitrogen-Free Nutrient Solution to Root Inoculation with N 2-Fixing Bacteria. HortScience 2015, 50, 579–602. [Google Scholar] [CrossRef]
- Pereira, L.C.; Pereira, C.B.; Correia, L.V.; Matera, T.C.; Dos Santos, R.F.; de Carvalho, C.; Osipi, E.A.F.; Braccini, A.L. Corn Responsiveness to Azospirillum: Accessing the Effect of Root Exudates on the Bacterial Growth and Its Ability to Fix Nitrogen. Plants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for Help with Root Exudates: Adaptive Mechanisms by Which Stressed Plants Assemble Health-Promoting Soil Microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of Indole-3-Acetic Acid (IAA) Production in Klebsiella by LC-MS/MS and the Salkowski Method. Bio Protoc. 2019, 9, e3230. [Google Scholar] [CrossRef]
- Bartolini, S.; Pappalettere, L.; Toffanin, A. Azospirillum baldaniorum Sp245 Induces Anatomical Changes in Cuttings of Olive (Olea europaea L., Cultivar Leccino): Preliminary Results. Agronomy 2023, 13, 301. [Google Scholar] [CrossRef]
- Osburn, R.M.; Schroth, M.N. Effect of Osmopriming Sugar Beet Seed on Exudation and Subsequent Damping-off Caused by Pythium ultimum. Phytopathology 1988, 78, 1246–1250. [Google Scholar]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed Biopriming with Plant Growth Promoting Rhizobacteria: A Review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef]
- Gan, Y.; Stulen, I.; Van Keulen, H.; Kuiper, P.J.C. Low Concentrations of Nitrate and Ammonium Stimulate Nodulation and N2 Fixation While Inhibiting Specific Nodulation (Nodule DW g−1 Root Dry Weight) and Specific N2 Fixation (N2 Fixed g−1 Root Dry Weight) in Soybean. Plant Soil 2004, 258, 281–292. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yu, C.B.; Cheng, X.; Li, C.J.; Sun, J.H.; Zhang, F.S.; Lambers, H.; Li, L. Intercropping Alleviates the Inhibitory Effect of N Fertilization on Nodulation and Symbiotic N2 Fixation of Faba Bean. Plant Soil 2009, 323, 295–308. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic solutions for soilless production systems: Issues and opportunities in a smart agriculture perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Burdman, S.; Volpin, H.; Kigel, J.; Kapulnik, Y.; Okon, Y. Promotion of Nod Gene Inducers and Nodulation in Common Bean (Phaseolus vulgaris) Roots Inoculated with Azospirillum brasilense Cd. Appl. Environ. Microbiol. 1996, 62, 3030–3033. [Google Scholar] [CrossRef]
- Woitke, M.; Junge, H.; Schnitzler, W.H. Bacillus subtilis as Growth Promotor in Hydroponically Grown Tomatoes under Saline Conditions. VII Int. Symp. Prot. Cultiv. Mild Winter Clim. Prod. Pest Manag. Glob. Compet. 2004, 659, 363–369. [Google Scholar]
- Peterson, P.S.; de Medeiros, F.H.V.; de Oliveira, T.S.; de Almeida Zago, J.R.; Bettiol, W. Bacillus subtilis and Bacillus licheniformis Promote Tomato Growth. Braz. J. Microbiol. 2023, 54, 397–406. [Google Scholar] [CrossRef]
- Dilworth, L.L.; Riley, C.K.; Stennett, D.K. Plant Constituents: Carbohydrates, Oils, Resins, Balsams, and Plant Hormones. In Pharmacognosy; McCreath, S.B., Clement, Y.N., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 62–80. [Google Scholar] [CrossRef]
- Arrobas, M.; Correia, C.M.; Rodrigues, M.Â. Methylobacterium symbioticum Applied as a Foliar Inoculant Was Little Effective in Enhancing Nitrogen Fixation and Lettuce Dry Matter Yield. Sustainability 2024, 16, 4512. [Google Scholar] [CrossRef]
- Valente, F.; Panozzo, A.; Bozzolin, F.; Barion, G.; Bolla, P.K.; Bertin, V.; Potestio, S.; Visioli, G.; Wang, Y.; Vamerali, T. Growth, Photosynthesis and Yield Responses of Common Wheat to Foliar Application of Methylobacterium symbioticum under Decreasing Chemical Nitrogen Fertilization. Preprints 2024, 2024080878. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil Microbial Inoculants for Sustainable Agriculture: Limitations and Opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Cerozi, B.d.S.; Fitzsimmons, K. Use of Bacillus Spp. to Enhance Phosphorus Availability and Serve as a Plant Growth Promoter in Aquaponics Systems. Sci. Hortic. 2016, 211, 277–282. [Google Scholar] [CrossRef]
- Gerk, L.P.; Gilchrist, K.; Kennedy, I.R. Mutants with Enhanced Nitrogenase Activity in Hydroponic Azospirillum brasilense-Wheat Associations. Appl. Environ. Microbiol. 2000, 66, 2175–2184. [Google Scholar] [CrossRef]
- Tortora, M.L.; Díaz-Ricci, J.C.; Pedraza, R.O. Azospirillum brasilense Siderophores with Antifungal Activity against Colletotrichum acutatum. Arch. Microbiol. 2011, 193, 275–286. [Google Scholar] [CrossRef]
- Escalante, F.M.E.; Cortés-Jiménez, D.; Tapia-Reyes, G.; Suárez, R. Immobilized Microalgae and Bacteria Improve Salt Tolerance of Tomato Seedlings Grown Hydroponically. J. Appl. Phycol. 2015, 27, 1923–1933. [Google Scholar] [CrossRef]






| Species | Strain | Reference/Source | Isolated from |
|---|---|---|---|
| Azospirillum baldaniorum | Sp245 | Baldani et al., 1986 [36]; Dobbelaere et al., 1999 [37]; dos Santos Ferreira 2020 [38] | Triticum aestivum—Brazil |
| Azospirillum brasilense | Sp7 | DSMZ * | Digitaria decumbens roots—Brazil |
| Azospirillum brasilense | Cd | DSMZ | Cynodon dactylon roots—USA |
| Bacillus amyloliquefaciens | Fukumoto strain F | DSMZ | soil—unknown county |
| Bacillus licheniformis | Gibson 46 | DSMZ | country of unknown origin |
| Bacillus subtilis | 101BS | Filippi et al., 1987 [39]; Citernesi et al., 1994 [40] | carnation rhizosphere |
| Methylobacterium symbioticum | SB0023/3T | Pascual et al., 2020 [21] Symborg Inc. (Murcia, Spain) ** (EP Application No. EP3747267A1) | spores of Glomus iranicum—Spain |
| MIX A | Azospirillum baldaniorum Sp245; Azospirillum brasilense Sp7; Azospirillum brasilense Cd, Methylobacterium symbioticum SB0023/3T | ||
| MIX B | Bacillus amyloliquefaciens Fukumoto strain F, Bacillus licheniformis Gibson 46, Bacillus subtilis 101BS | ||
| Treatment | Survival (%) |
|---|---|
| Azospirillum baldaniorum Sp245 | 98.5 ± 0.5 a |
| Azospirillum brasilense Sp7 | 92.4 ± 1.2 a |
| Azospirillum brasilense Cd | 95.2 ± 2.4 a |
| Methylobacterium symbioticum SB0023/3T | 95.1 ± 1.5 a |
| MIX A | 96.8 ± 1.7 a |
| Avg | 95.6 ± 1.8 |
| Control | 50 ± 1.1 b |
| Bacillus amyloliquefaciens | 91.6 ± 0.4 a |
| Bacillus licheniformis | 88.9 ± 1.0 a |
| Bacillus subtilis 101BS | 89.2 ± 1.2 a |
| MIX B | 90.1 ± 1.3 a |
| Avg | 89.9 ± 1.1 |
| Control | 66.1 ± 2.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pappalettere, L.; Bartolini, S.; Toffanin, A. Auxin-Producing Bacteria Used as Microbial Biostimulants Improve the Growth of Tomato (Solanum lycopersicum L.) Seedlings in Hydroponic Systems. BioTech 2024, 13, 32. https://doi.org/10.3390/biotech13030032
Pappalettere L, Bartolini S, Toffanin A. Auxin-Producing Bacteria Used as Microbial Biostimulants Improve the Growth of Tomato (Solanum lycopersicum L.) Seedlings in Hydroponic Systems. BioTech. 2024; 13(3):32. https://doi.org/10.3390/biotech13030032
Chicago/Turabian StylePappalettere, Livia, Susanna Bartolini, and Annita Toffanin. 2024. "Auxin-Producing Bacteria Used as Microbial Biostimulants Improve the Growth of Tomato (Solanum lycopersicum L.) Seedlings in Hydroponic Systems" BioTech 13, no. 3: 32. https://doi.org/10.3390/biotech13030032






