Salivary Biomarkers Identification: Advances in Standard and Emerging Technologies
Abstract
1. Introduction
2. Literature Review
2.1. Material and Method
2.2. Types of Salivary Biomarkers
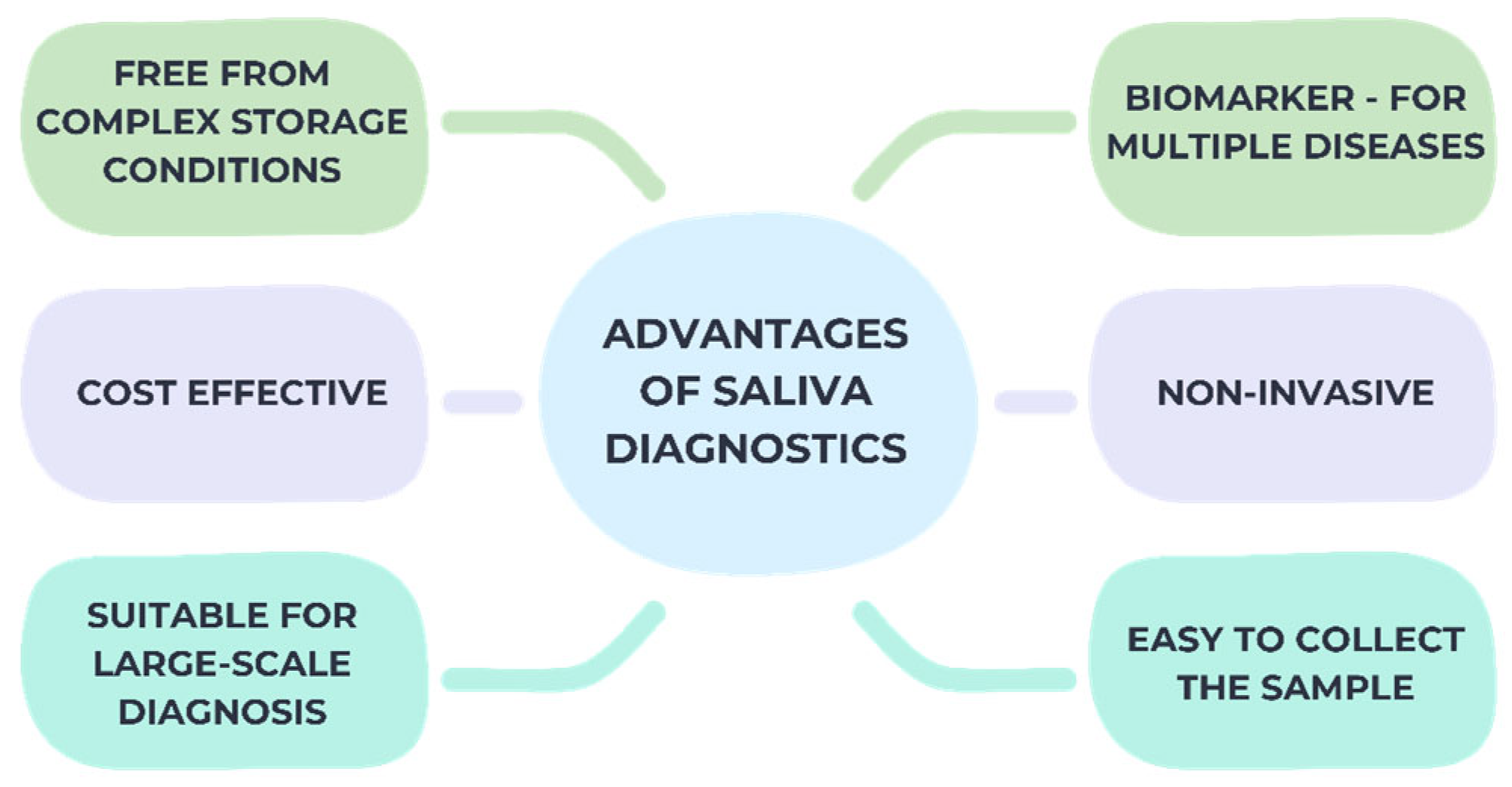
2.3. Saliva Collection
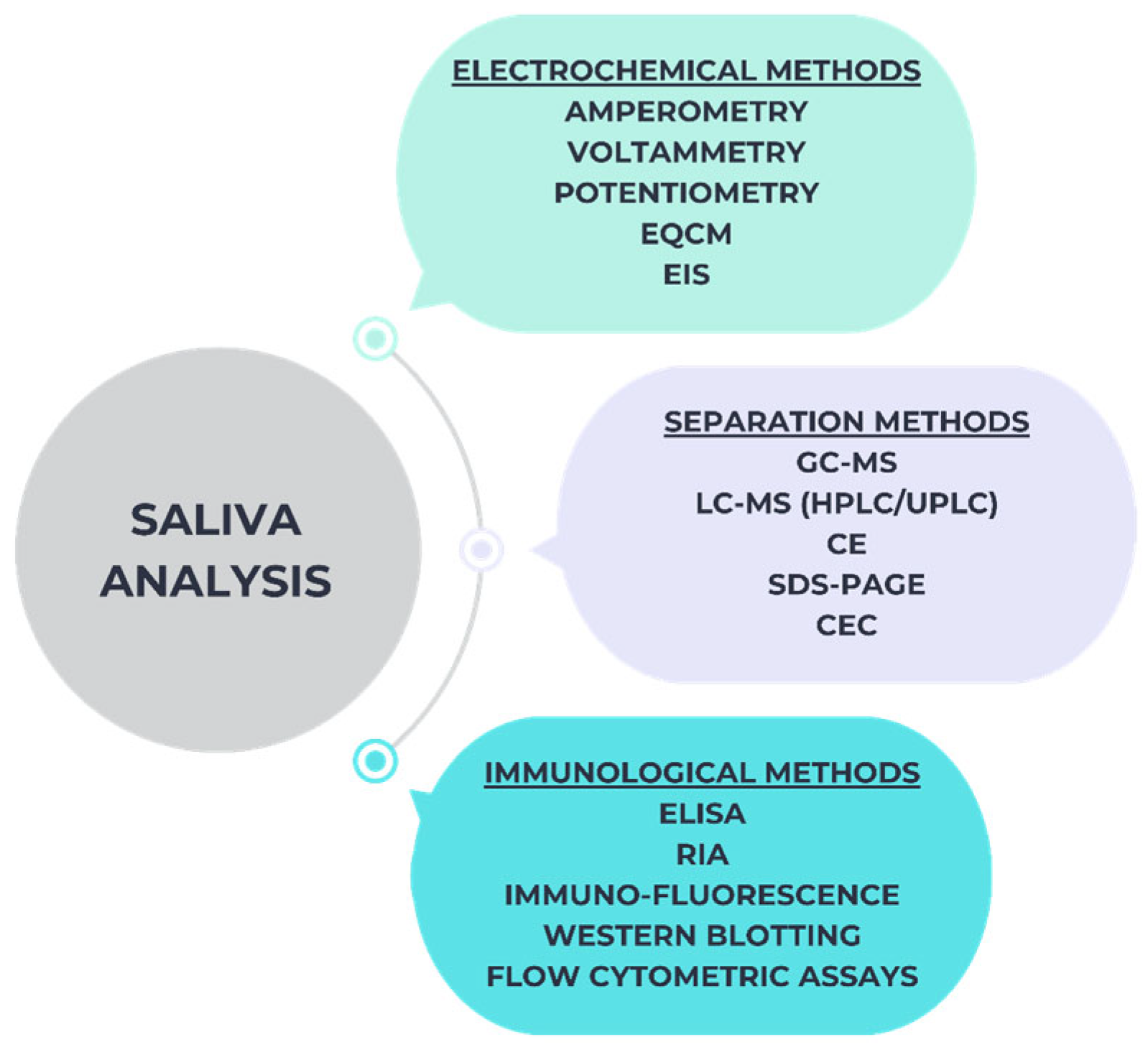
2.4. Approaches for the Detection of Salivary Biomarkers
2.4.1. ELISAs Method
2.4.2. Western-Blotting Method
2.4.3. ICG Method
2.4.4. NMR Method
2.4.5. CE-MS Method
2.4.6. Fluorescence or Chemiluminescence Based Methods
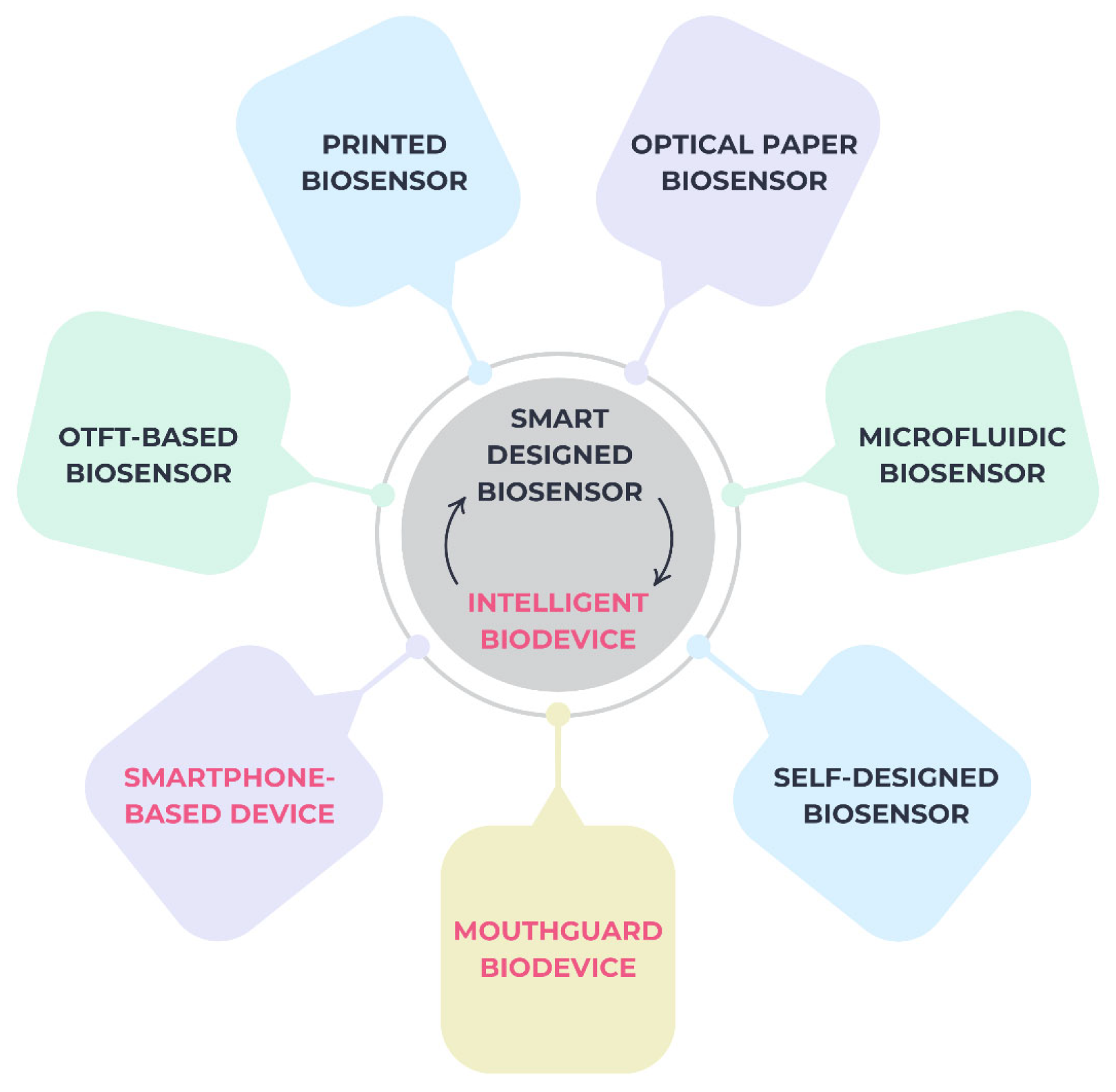
2.5. Smart Biosensors and Intelligent Devices
3. Current Trends and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular Disease |
| RNA | Ribonucleic Acid |
| DNA | Deoxyribonucleic Acid |
| AI | Artificial Intelligence |
| mRNA | Messenger RNA |
| TNF-α | Tumor Necrosis Factor-alpha. |
| IL-6 | Interleukin 6 |
| MMPs | Matrix Metalloproteinases |
| miRNA | MicroRNA |
| MDA | Malonedialdehyde |
| CRP | Protein C Reactive |
| ELISA | Enzyme Linked Immunosorbent Assays |
| ICG | Immunochromatography |
| NMR | Nuclear Magnetic Resonance |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| CE-MS | Capillary Electrophoresis-Mass Spectrometry |
| MS | Mass Spectrometry |
| µL | Microliter |
| OTFTs | Organic Thin Film Transistors |
| POC | Point-of-Care |
References
- Zhang, C.-Z.; Cheng, X.Q.; Li, J.-Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.-D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241–248. [Google Scholar] [PubMed]
- Lamy, E.; Capela-Silva, F.; Tvarijonaviciute, A. Research on Saliva Secretion and Composition. BioMed Res. Int. 2018, 2018, 7406312. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, N.; Åkerman, S.; Klinge, B.; Lundegren, N.; Jansson, H.; Tryselius, Y.; Sorsa, T.; Gustafsson, A. Salivary Biomarkers for Detection of Systemic Diseases. PLoS ONE 2013, 8, e61356. [Google Scholar] [CrossRef]
- Gohel, V.; Jones, J.; Wehler, C. Salivary biomarkers and cardiovascular disease: A systematic review. Clin. Chem. Lab. Med. 2018, 56, 1432–1442. [Google Scholar] [CrossRef]
- Surdu, A.; Foia, L.G.; Luchian, I.; Trifan, D.; Tatarciuc, M.S.; Scutariu, M.M.; Ciupilan, C.; Budala, D.G. Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina 2025, 61, 243. [Google Scholar] [CrossRef]
- Roca, C.; Alkhateeb, A.A.; Deanhardt, B.K.; Macdonald, J.K.; Chi, D.L.; Wang, J.R.; Wolfgang, M.C. Saliva sampling method influences oral microbiome composition and taxa distribution associated with oral diseases. PLoS ONE 2024, 19, e0301016. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Wang, Y.; Cui, J.; Liu, M.; Du, W.; Xu, Y. Computational prediction of human salivary proteins from blood circulation and application to diagnostic biomarker identification. PLoS ONE 2013, 8, e80211. [Google Scholar] [CrossRef]
- Miller, C.S.; Foley, J.D.; Bailey, A.L.; Campell, C.L.; Humphries, R.L.; Christodoulides, N.; Floriano, P.N.; Simmons, G.; Bhagwandin, B.; Jacobson, J.W.; et al. Current developments in salivary diagnostics. Biomark. Med. 2010, 4, 171–189. [Google Scholar] [CrossRef]
- Roi, A.; Rusu, L.C.; Roi, C.I.; Luca, R.E.; Boia, S.; Munteanu, R.I. A New Approach for the Diagnosis of Systemic and Oral Diseases Based on Salivary Biomolecules. Dis. Markers 2019, 2019, 8761860. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, M.; Olianas, A.; Cabras, T.; Manconi, B.; Fanni, D.; Faa, G.; Desiderio, C.; Messana, I.; Castagnola, M. Saliva, a bodily fluid with recognized and potential diagnostic applications. J. Sep. Sci. 2021, 44, 3677–3690. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Jankowski, J.; Gruszczyński, D.; Surdacka, A. Salivary Alterations of Myeloperoxidase in Patients with Systemic Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12078. [Google Scholar] [CrossRef] [PubMed]
- Tappia, P.S.; Ramjiawan, B. Biomarkers for Early Detection of Cancer: Molecular Aspects. Int. J. Mol. Sci. 2023, 24, 5272. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Slowey, P.D.; Almas, K. Human Saliva Collection Devices for Proteomics: An Update. Int. J. Mol. Sci. 2016, 17, 846. [Google Scholar] [CrossRef]
- Géli, V.; Nabet, N. Saliva, a molecular reflection of the human body? Implications for diagnosis and treatment. Cell Stress 2024, 8, 59–68. [Google Scholar] [CrossRef]
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Menini, M.; De Giovanni, E.; Bagnasco, F.; Delucchi, F.; Pera, F.; Baldi, D.; Pesce, P. Salivary Micro-RNA and Oral Squamous Cell Carcinoma: A Systematic Review. J. Pers. Med. 2021, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, W.; Birenzweig, Y.; Sharav, Y.; Aframian, D.J.; Rettman, A.; Hanut, A.; Brotman, Y.; Haviv, Y. Salivary Metabolomics as a Diagnostic Tool: Distinct Metabolic Profiles Across Orofacial Pain Subtypes. Int. J. Mol. Sci. 2025, 26, 2260. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, M.; Lin, Z.; Mahmoodi, S.R.; Javanmard, M. Automated Electrical Detection of Proteins for Oral Squamous Cell Carcinoma in an Integrated Microfluidic Chip Using Multi-Frequency Impedance Cytometry and Machine Learning. Sensors 2025, 25, 1566. [Google Scholar] [CrossRef]
- Hu, S.; Gao, K.; Pollard, R.; Arellano-Garcia, M.; Zhou, H.; Zhang, L.; Elashoff, D.; Kallenberg, C.G.; Vissink, A.; Wong, D.T. Preclinical validation of salivary biomarkers for primary Sjögren’s syndrome. Arthritis Care Res. 2010, 62, 1633–1638. [Google Scholar] [CrossRef]
- Sande López, L.; García-Mato, E.; de Coo, A.; Cruz, R.; Antequera, D.; Diz, P.; Carro, E.; Rivas, B. Salivary Lactoferrin Levels and Polymorphisms in Down Syndrome Individuals with Periodontitis. J. Clin. Med. 2025, 14, 1815. [Google Scholar] [CrossRef]
- Hu, S.; Vissink, A.; Arellano, M.; Roozendaal, C.; Zhou, H.; Kallenberg, C.G.; Wong, D.T. Identification of autoantibody biomarkers for primary Sjögren’s syndrome using protein microarrays. Proteomics 2011, 11, 1499–1507. [Google Scholar] [CrossRef]
- Lee, W.; Barbosa, A.D.; Lee, A.H.-Y.; Currie, A.; Martino, D.; Stenos, J.; Long, M.; Beaman, M.; Harvey, N.T.; Kresoje, N.; et al. From Local to Systemic: The Journey of Tick Bite Biomarkers in Australian Patients. Int. J. Mol. Sci. 2025, 26, 1520. [Google Scholar] [CrossRef]
- Onim, M.S.H.; Thapliyal, H.; Rhodus, E.K. Utilizing Machine Learning for Context-Aware Digital Biomarker of Stress in Older Adults. Information 2024, 15, 274. [Google Scholar] [CrossRef]
- Acharya, S.; Hegde, U.; Acharya, A.B.; Nitin, P. Dysbiosis linking periodontal disease and oral squamous cell carcinoma—A brief narrative review. Heliyon 2024, 10, 32259. [Google Scholar] [CrossRef]
- Budală, D.G.; Luchian, I.; Tatarciuc, M.; Butnaru, O.; Armencia, A.O.; Virvescu, D.I.; Scutariu, M.M.; Rusu, D. Are Local Drug Delivery Systems a Challenge in Clinical Periodontology? J. Clin. Med. 2023, 12, 4137. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Badran, Z.; Boghossian, A.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The increasing importance of the oral microbiome in periodontal health and disease. Future Sci. OA 2023, 9, FSO856. [Google Scholar] [CrossRef] [PubMed]
- Calixto, P.S.; Ferraz, F.C.; Dutra, G.C.; Pelozzo, M.J.B.; Trovão, M.E.; Rego, F.G.d.M.; Picheth, G.; Campelo, P.M.S.; Sari, M.H.M. Exploring Saliva as a Sample for Non-Invasive Glycemic Monitoring in Diabetes: A Scoping Review. Biomedicines 2025, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Chundru, V.N.S.; Madhavan, R.N.; Chintala, L.; Boyapati, R.; Srikar, M. Evaluation of salivary biomarker interleukin-6 in oral squamous cell carcinoma and oral potentially malignant disorders—A comparative cross-sectional South Indian study. J. Oral Maxillofac. Pathol. 2024, 28, 387–392. [Google Scholar] [CrossRef]
- Parra Meder, Á.; Costa, R.; López-Jarana, P.; Ríos-Carrasco, B.; Relvas, M.; Salazar, F. Inflammatory Mediators in the Oral Fluids and Blood Samples of Type 1 Diabetic Patients, According to Periodontal Status—A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2552. [Google Scholar] [CrossRef]
- van der Meulen, T.A.; Harmsen, H.J.M.; Vila, A.V.; Kurilshikov, A.; Liefers, S.C.; Zhernakova, A.; Fu, J.; Wijmenga, C.; Weersma, R.K.; de Leeuw, K.; et al. Shared gut, but distinct oral microbiota composition in primary Sjögren’s syndrome and systemic lupus erythematosus. J. Autoimmun. 2019, 97, 77–87. [Google Scholar] [CrossRef]
- van der Meulen, T.A.; Harmsen, H.J.M.; Bootsma, H.; Liefers, S.C.; Vich Vila, A.; Zhernakova, A.; Weersma, R.K.; Spijkervet, F.K.L.; Kroese, F.G.M.; Vissink, A. Reduced salivary secretion contributes more to changes in the oral microbiome of patients with primary Sjögren’s syndrome than underlying disease. Ann. Rheum. Dis. 2018, 77, 1542–1544. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, P.; Gao, W. Microbial dysbiosis in periodontitis and peri-implantitis: Pathogenesis, immune responses, and therapeutic. Front. Cell. Infect. Microbiol. 2025, 15, 1517154. [Google Scholar] [CrossRef]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva—Are our assays good enough? Ann. Clin. Biochem. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef]
- Lee, J.M.; Garon, E.; Wong, D.T. Salivary diagnostics. Orthod. Craniofac. Res. 2009, 12, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, P.; Cheng, F.; Wang, X.; Duan, Y. Measurement of salivary metabolite biomarkers for early monitoring of oral cancer with ultra performance liquid chromatography–mass spectrometry. Talanta 2014, 119, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.F.; Salgado, P.A.; Molgatini, S.L.; Gliosca, L.A.; Squassi, A.F. Saliva sampling methods. Cariogenic streptococci count using two different methods of saliva collection in children. Acta Odontol. Latinoam. 2022, 35, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Kim, H.R.; Chae, H.J. Compliance with Saliva Collection Protocol in Healthy Volunteers: Strategies for Managing Risk and Errors. Int. J. Med. Sci. 2018, 15, 823–831. [Google Scholar] [CrossRef]
- Armstrong, A.J.S.; Parmar, V.; Blaser, M.J. Assessing saliva microbiome collection and processing methods. NPJ Biofilms Microbiomes 2021, 7, 81. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Zhu, J.; Liao, Z.; Wang, S.; Liu, W. Correlations of Salivary and Blood Glucose Levels among Six Saliva Collection Methods. Int. J. Environ. Res. Public Health 2022, 19, 4122. [Google Scholar] [CrossRef]
- Fernandes, A.; Skinner, M.L.; Woelfel, T.; Carpenter, T.; Haggerty, K.P. Implementing Self-collection of Biological Specimens with a Diverse Sample. Field Methods 2013, 25, 10. [Google Scholar] [CrossRef]
- Yau, J.C.Y.; Fong, T.C.T.; Wan, A.H.Y.; Ho, R.T.H. Comparison of passive drool and cotton-based collection methods for salivary C-reactive protein measurement. Am. J. Hum. Biol. 2022, 34, e23782. [Google Scholar] [CrossRef]
- Li, Y.; Ou, Y.; Fan, K.; Liu, G. Salivary diagnostics: Opportunities and challenges. Theranostics 2024, 14, 6969–6990. [Google Scholar] [CrossRef]
- Fey, J.M.H.; Bikker, F.J.; Hesse, D. Saliva Collection Methods Among Children and Adolescents: A Scoping Review. Mol. Diagn. Ther. 2024, 28, 15–26. [Google Scholar] [CrossRef]
- Gomar-Vercher, S.; Simón-Soro, A.; Montiel-Company, J.M.; Almerich-Silla, J.M.; Mira, A. Stimulated and unstimulated saliva samples have significantly different bacterial profiles. PLoS ONE 2018, 13, e0198021. [Google Scholar] [CrossRef] [PubMed]
- Wetterö, J.; von Löhneysen, S.; Cobar, F.; Kristenson, M.; Garvin, P.; Sjöwall, C. Pronounced Diurnal Pattern of Salivary C-Reactive Protein (CRP) With Modest Associations to Circulating CRP Levels. Front. Immunol. 2021, 11, 607166. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Sharma, P.; Berhane, S.; van Wyk, S.S.; Nyaaba, N.; Domen, J.; Taylor, M.; Cunningham, J.; Davenport, C.; Dittrich, S.; et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2022, 7, CD013705. [Google Scholar] [PubMed]
- Davenport, C.; Arevalo-Rodriguez, I.; Mateos-Haro, M.; Berhane, S.; Dinnes, J.; Spijker, R.; Buitrago-Garcia, D.; Ciapponi, A.; Takwoingi, Y.; Deeks, J.J.; et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. The effect of sample site and collection procedure on identification of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2024, 12, CD014780. [Google Scholar]
- Szabo, Y.Z.; Slavish, D.C. Measuring salivary markers of inflammation in health research: A review of methodological considerations and best practices. Psychoneuroendocrinology 2021, 124, 105069. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Mortazavi, H.; Yousefi-Koma, A.A.; Yousefi-Koma, H. Extensive comparison of salivary collection, transportation, preparation, and storage methods: A systematic review. BMC Oral Health 2024, 24, 168. [Google Scholar] [CrossRef]
- Albagieh, H.; Alshehri, A.Z.; Alduraywishi, A.S.; Aldaws, A.; AlBalawi, S.S.; Abu Shaqqaf, H.F.; Almubayi, R.A. Evaluation of Salivary Diagnostics: Applications, Benefits, Challenges, and Future Prospects in Dental and Systemic Disease Detection. Cureus 2025, 17, e77520. [Google Scholar] [CrossRef]
- Costa, M.M.; Benoit, N.; Saby, F.; Pradines, B.; Granjeaud, S.; Almeras, L. Optimization and Standardization of Human Saliva Collection for MALDI-TOF MS. Diagnostics 2021, 11, 1304. [Google Scholar] [CrossRef]
- Poljak, M.; Cuschieri, K.; Alemany, L.; Vorsters, A. Testing for Human Papillomaviruses in Urine, Blood, and Oral Specimens: An Update for the Laboratory. J. Clin. Microbiol. 2023, 61, e0140322. [Google Scholar] [CrossRef]
- Gottfried-Blackmore, A.; Rubin, S.J.S.; Bai, L.; Aluko, S.; Yang, Y.; Park, W.; Habtezion, A. Effects of processing conditions on stability of immune analytes in human blood. Sci. Rep. 2020, 10, 17328. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bai, H.; Zhang, P.; Zhou, X.; Ying, B. Promising applications of human-derived saliva biomarker testing in clinical diagnostics. Int. J. Oral Sci. 2023, 15, 2. [Google Scholar] [CrossRef]
- Gardner, A.; Parkes, H.G.; Carpenter, G.H.; So, P.W. Developing and Standardizing a Protocol for Quantitative Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy of Saliva. J. Proteome Res. 2018, 17, 1521–1531. [Google Scholar] [CrossRef]
- Masutomi, K.; Bando, M.; Inagaki, Y.; Kido, R.; Uemura, Y.; Hatada, Y.; Kido, J.I.; Fukui, M.; Hinode, D.; Yumoto, H. Relationship between oral hypofunction and salivary biomarkers in older adults: A cross-sectional study. BMC Oral Health 2024, 24, 766. [Google Scholar] [CrossRef]
- Czégény, Z.S.; Chicharro, J.L.; Fernández, P.; Gutiérrez, A.; Cámara, C. Homogeneity and stability studies on sodium, calcium, magnesium, and manganese in human saliva. Biol. Trace Elem. Res. 2001, 79, 131–137. [Google Scholar]
- Luchian, I.; Budală, D.G.; Baciu, E.-R.; Ursu, R.G.; Diaconu-Popa, D.; Butnaru, O.; Tatarciuc, M. The Involvement of Photobiology in Contemporary Dentistry—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 3985. [Google Scholar] [CrossRef]
- Gavião, M.B.; Bilt, A.V. Salivary secretion and chewing: Stimulatory effects from artificial and natural foods. J. Appl. Oral Sci. 2004, 12, 159–163. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Casati, S.; Goldoni, R.; Thomaz, D.V.; Kehr, N.S.; Galimberti, D.; Del Fabbro, M.; Tartaglia, G.M. Salivary biomarkers: Novel noninvasive tools to diagnose chronic inflammation. Int. J. Oral Sci. 2023, 15, 27. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Aydin, S.; Emre, E.; Ugur, K.; Aydin, M.A.; Sahin, İ.; Cinar, V.; Akbulut, T. An overview of ELISA: A review and update on best laboratory practices for quantifying peptides and proteins in biological fluids. J. Int. Med. Res. 2025, 53, 3000605251315913. [Google Scholar] [CrossRef] [PubMed]
- MacMullan, M.A.; Ibrayeva, A.; Trettner, K.; Deming, L.; Das, S.; Tran, F.; Moreno, J.R.; Casian, J.G.; Chellamuthu, P.; Kraft, J.; et al. ELISA detection of SARS-CoV-2 antibodies in saliva. Sci. Rep. 2020, 10, 20818. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Di Chiara, C.; Furlan, G.; Gastaldo, S.; Talli, I.; Donà, D.; Basso, D.; Giaquinto, C.; Plebani, M. Clinical and Analytical Performance of ELISA Salivary Serologic Assay to Detect SARS-CoV-2 IgG in Children and Adults. Antibodies 2024, 13, 6. [Google Scholar] [CrossRef]
- Thomas, S.N.; Karger, A.B.; Altawallbeh, G.; Nelson, K.M.; Jacobs, D.R., Jr.; Gorlin, J.; Barcelo, H.; Thyagarajan, B. Ultrasensitive detection of salivary SARS-CoV-2 IgG antibodies in individuals with natural and COVID-19 vaccine-induced immunity. Sci. Rep. 2022, 12, 8890. [Google Scholar] [CrossRef]
- Hayrapetyan, H.; Tran, T.; Tellez-Corrales, E.; Madiraju, C. Enzyme-Linked Immunosorbent Assay: Types and Applications. Methods Mol. Biol. 2023, 2612, 1–17. [Google Scholar]
- Sule, R.; Rivera, G.; Gomes, A.V. Western blotting (immunoblotting): History, theory, uses, protocol and problems. Biotechniques 2023, 75, 99–114. [Google Scholar] [CrossRef]
- Vilela, A.C.S.; Costa, C.A.; Oliveira, S.A.; Souza, M.B.L.D.; Fiaccadori, F.S.; Leles, C.R.; Costa, N.L. Validity and reliability of immunochromatographic IgM/IgG rapid tests for COVID-19 salivary diagnosis. Oral Dis. 2022, 28, 2465–2473. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kwon, J.; Shin, S.; Eun, Y.G.; Shin, J.H.; Lee, G.J. Optimization of saliva collection and immunochromatographic detection of salivary pepsin for point-of-care testing of laryngopharyngeal reflux. Sensors 2020, 20, 325. [Google Scholar] [CrossRef]
- Gerothanassis, I.P.; Troganis, A.; Exarchou, V.; Barbarossou, K. Nuclear magnetic resonance (NMR) spectroscopy: Basic principles and phenomena, and their applications to chemistry, biology and medicine. Chem. Educ. Res. Pract. 2002, 3, 229–252. [Google Scholar] [CrossRef]
- Tsikas, D.; Zoerner, A.A. Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: A historical retrospect and a discussion. J. Chromatogr. B 2014, 964, 79–88. [Google Scholar] [CrossRef]
- Wakayama, M.; Hirayama, A.; Soga, T. Capillary electrophoresis-mass spectrometry. Methods Mol. Biol. 2015, 1277, 113–122. [Google Scholar] [PubMed]
- Seyfinejad, B.; Jouyban, A. Capillary electrophoresis-mass spectrometry in pharmaceutical and biomedical analyses. J. Pharm. Biomed. Anal. 2022, 221, 115059. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.A.; Akter, F. Capillary Electrophoresis Mass Spectrometry: Developments and Applications for Enantioselective Analysis from 2011–2020. Molecules 2022, 27, 4126. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Arnold, F.H. Engineering new catalytic activities in enzymes. Nat. Catal. 2020, 3, 203–213. [Google Scholar] [CrossRef]
- Ornelas-González, A.; Ortiz-Martínez, M.; González-González, M.; Rito-Palomares, M. Enzymatic Methods for Salivary Biomarkers Detection: Overview and Current Challenges. Molecules 2021, 26, 7026. [Google Scholar] [CrossRef]
- Van Gool, A.; Corrales, F.; Čolović, M.; Krstić, D.; Oliver-Martos, B.; Martínez-Cáceres, E.; Jakasa, I.; Gajski, G.; Brun, V.; Kyriacou, K.; et al. Analytical Techniques for Multiplex Analysis of Protein Biomarkers. Expert Rev. Proteom. 2020, 17, 257–273. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-McAuley, C.; Compton, R.G. Chemical Analysis in Saliva and the Search for Salivary Biomarkers—A Tutorial Review. Analyst 2017, 143, 81–99. [Google Scholar] [CrossRef]
- Toyo’oka, T. Derivatization-based High-throughput Bioanalysis by LC-MS. Anal. Sci. 2017, 33, 555–564. [Google Scholar] [CrossRef]
- Wróblewski, K.; Petruczynik, A.; Tuzimski, T.; Przygodzka, D.; Buszewicz, G.; Kołodziejczyk, P.; Tutka, P. Comparison of Various Chromatographic Systems for Analysis of Cytisine in Human Serum, Saliva and Pharmaceutical Formulation by HPLC with Diode Array, Fluorescence or Mass Spectrometry Detection. Molecules 2019, 24, 2580. [Google Scholar] [CrossRef]
- Carlier, J.; Guitton, J.; Romeuf, L.; Bévalot, F.; Boyer, B.; Fanton, L.; Gaillard, Y. Screening approach by ultra-high performance liquid chromatography-tandem mass spectrometry for the blood quantification of thirty-four toxic principles of plant origin. Application to forensic toxicology. J. Chromatogr. B 2015, 975, 65–76. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, C. Microscale Thermophoresis (MST) to Detect the Interaction Between Purified Protein and Small Molecule. Methods Mol. Biol. 2021, 2213, 187–193. [Google Scholar]
- El Deeb, S.; Al-Harrasi, A.; Khan, A.; Al-Broumi, M.; Al-Thani, G.; Alomairi, M.; Elumalai, P.; Sayed, R.A.; Ibrahim, A.E. Microscale thermophoresis as a powerful growing analytical technique for the investigation of biomolecular interaction and the determination of binding parameters. Methods Appl. Fluoresc. 2022, 10, 042001. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Garcia, P.N.; de Souza, M.M.; Izidoro, M.A.; Juliano, L.; Lourenço, S.V.; Camillo, C.M.C. Saliva metabolomics: Concepts and applications in oral disorders. Clin. Oral Investig. 2024, 28, 579. [Google Scholar] [CrossRef]
- Kwasnik, A.; Tonry, C.; Ardle, A.M.; Butt, A.Q.; Inzitari, R.; Pennington, S.R. Proteomes, Their Compositions and Their Sources. Adv. Exp. Med. Biol. 2016, 919, 3–21. [Google Scholar]
- Cuevas-Córdoba, B.; Santiago-García, J. Saliva: A fluid of study for OMICS. OMICS 2014, 18, 87–97. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl. Biochem. Biotechnol. 2012, 168, 1718–1727. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Luchian, I.; Vata, I.; Martu, I.; Tatarciuc, M.; Valeria, P.; Martu, S. Challenges in ortho-perio and general dentistry interrelationship. Limits and perspectives. Rom. J. Oral Rehabil. 2016, 8, 80–88. [Google Scholar]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Choi, S. Powering point-of-care diagnostic devices. Biotechnol. Adv. 2016, 34, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019, 9, 6793–6803. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Fujita, T.; Kato, S.; Yoshimitsu, Y.; Ito, Y.M.; Yano, R. Utility of salivary cortisol profile as a predictive biomarker in nurses’ turnover risk: A preliminary study. J. Physiol. Anthropol. 2024, 43, 1. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, S.; Qin, Y.; Xia, X.; Sun, Y.; Han, G.; Shu, T.; Hu, L.; Zhang, Q. Flexible and Wearable Biosensors for Monitoring Health Conditions. Biosensors 2023, 13, 630. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef]
- Ye, S.; Feng, S.; Huang, L.; Bian, S. Recent Progress in Wearable Biosensors: From Healthcare Monitoring to Sports Analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef]
- Manoharan Nair Sudha Kumari, S.; Thankappan Suryabai, X. Sensing the Future-Frontiers in Biosensors: Exploring Classifications, Principles, and Recent Advances. ACS Omega 2024, 9, 48918–48987. [Google Scholar] [CrossRef]
- Kumar, S.; Panwar, S.; Kumar, S.; Augustine, S.; Malhotra, B.D. Biofunctionalized Nanostructured Yttria Modified Non-Invasive Impedometric Biosensor for Efficient Detection of Oral Cancer. Nanomaterials 2019, 9, 1190. [Google Scholar] [CrossRef]
- Chao-Shi, Z.O.U.; Mi, Z.H.O.U.; Guo-Ming, X.I.E.; Peng, L.U.O.; Xiong, X.L.; Hua-Jian, X.U.; Zheng, J. Preparation of disposable saliva α-amylase biosensor. Chin. J. Anal. Chem. 2008, 36, 1217–1220. [Google Scholar]
- Xu, Z.; Coriand, L.; Loeffler, R.; Geis-Gerstorfer, J.; Zhou, Y.; Scheideler, L.; Fleischer, M.; Gehring, F.K.; Rupp, F. Saliva-coated titanium biosensor detects specific bacterial adhesion and bactericide caused mass loading upon cell death. Biosens. Bioelectron. 2019, 129, 198–207. [Google Scholar] [CrossRef]
- Soni, A.; Surana, R.K.; Jha, S.K. Smartphone based optical biosensor for the detection of urea in saliva. Sens. Actuators B Chem. 2018, 269, 346–353. [Google Scholar] [CrossRef]
- Elkington, D.; Cooling, N.; Belcher, W.; Dastoor, P.C.; Zhou, X. Organic Thin-Film Transistor (OTFT)-Based Sensors. Electronics 2014, 3, 234–254. [Google Scholar] [CrossRef]
- Wang, N.; Yang, A.; Fu, Y.; Li, Y.; Yan, F. Functionalized organic thin film transistors for biosensing. Acc. Chem. Res. 2019, 52, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Sasaki, Y.; Minamiki, T.; Minami, T. Chemical sensing platforms based on organic thin-film transistors functionalized with artificial receptors. ACS Sens. 2019, 4, 2571–2587. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-Based Biosensors for Detection of Biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Patil, P.O.; Pandey, G.R.; Patil, A.G.; Borse, V.B.; Deshmukh, P.K.; Patil, D.R.; Tade, R.S.; Nangare, S.N.; Khan, Z.G.; Patil, A.M.; et al. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: A review. Biosens. Bioelectron. 2019, 139, 111324. [Google Scholar] [CrossRef]
- Song, Y.; Xu, T.; Xu, L.-P.; Zhang, X. Nanodendritic gold/graphene-based biosensor for tri-mode miRNA sensing. Chem. Commun. 2019, 55, 1742–1745. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, T.; Wang, W.; Wen, Y.; Li, K.; Qian, L.; Zhang, X.; Liu, G. Lateral flow biosensors based on the use of micro- and nanomaterials: A review on recent developments. Microchim. Acta 2019, 187, 70. [Google Scholar] [CrossRef]
- Garcia-Junior, M.A.; Andrade, B.S.; Lima, A.P.; Soares, I.P.; Notário, A.F.O.; Bernardino, S.S.; Guevara-Vega, M.F.; Honório-Silva, G.; Munoz, R.A.A.; Jardim, A.C.G.; et al. Artificial-Intelligence Bio-Inspired Peptide for Salivary Detection of SARS-CoV-2 in Electrochemical Biosensor Integrated with Machine Learning Algorithms. Biosensors 2025, 15, 75. [Google Scholar] [CrossRef]
- Guido, R.; Ferrisi, S.; Lofaro, D.; Conforti, D. An Overview on the Advancements of Support Vector Machine Models in Healthcare Applications: A Review. Information 2024, 15, 235. [Google Scholar] [CrossRef]
- Carrillo-Rodriguez, P.; Selheim, F.; Hernandez-Valladares, M. Mass Spectrometry-Based Proteomics Workflows in Cancer Research: The Relevance of Choosing the Right Steps. Cancers 2023, 15, 555. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, Q.; Xiong, N.N.; Jiang, S.; Chen, L. Surrogate-Assisted Evolutionary Algorithm for Expensive Constrained Multi-Objective Discrete Optimization Problems. Complex Intell. Syst. 2022, 8, 2699–2718. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Hwan Shin, T.; Lee, G. Machine Intelligence in Peptide Therapeutics: A Next-generation Tool for Rapid Disease Screening. Med. Res. Rev. 2020, 40, 1276–1314. [Google Scholar] [CrossRef]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.-Y. HPEPDOCK: A Web Server for Blind Peptide–Protein Docking Based on a Hierarchical Algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]
- Guevara-Vega, M.; Rosa, R.B.; Caixeta, D.C.; Costa, M.A.; de Souza, R.C.; Ferreira, G.M.; Mundim Filho, A.C.; Carneiro, M.G.; Jardim, A.C.G.; Sabino-Silva, R. Salivary Detection of Chikungunya Virus Infection Using a Portable and Sustainable Biophotonic Platform Coupled with Artificial Intelligence Algorithms. Sci. Rep. 2024, 14, 21546. [Google Scholar] [CrossRef]
- Adeoye, J.; Su, Y.X. Artificial intelligence in salivary biomarker discovery and validation for oral diseases. Oral Dis. 2024, 30, 23–37. [Google Scholar] [CrossRef]
- Budala, D.G.; Surlari, Z.; Bida, F.C.; Ciocan-Pendefunda, A.A.; Agop-Forna, D. Digital instruments in dentistry-back to the future. Rom. J. Oral Rehab. 2023, 15, 310–318. [Google Scholar]
- Arya, S.S.; Dias, S.B.; Jelinek, H.F.; Hadjileontiadis, L.J.; Pappa, A.M. The convergence of traditional and digital biomarkers through AI-assisted biosensing: A new era in translational diagnostics? Biosens. Bioelectron. 2023, 235, 115387. [Google Scholar] [CrossRef]
- Jasim, H.; Carlsson, A.; Hedenberg-Magnusson, B.; Ghafouri, B.; Ernberg, M. Saliva as a medium to detect and measure biomarkers related to pain. Sci. Rep. 2018, 8, 3220. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Fenniri, H. Cutting Edge Methods for Non-Invasive Disease Diagnosis Using E-Tongue and E-Nose Devices. Biosensors 2017, 7, 59. [Google Scholar] [CrossRef]
- Semerci, Z.M.; Yardımcı, S. Empowering Modern Dentistry: The Impact of Artificial Intelligence on Patient Care and Clinical Decision Making. Diagnostics 2024, 14, 1260. [Google Scholar] [CrossRef]
| Technique | Sensitivity | Specificity | Cost | Time Efficiency | Equipment Requirement | Clinical Applicability |
|---|---|---|---|---|---|---|
| ELISA | High | High | Moderate | Moderate | Moderate | Routine clinical use, protein biomarkers |
| Western blotting | High | Very High | High | Low | High | Protein identification and quantification |
| Immunochromatography (ICG) | Moderate | Moderate | Low | High | Low | Rapid, portable diagnostics |
| Nuclear Magnetic Resonance (NMR) | Moderate | High | Very High | Low | Very High | Structural and quantitative biomarker analysis |
| Capillary Electrophoresis-Mass Spectrometry (CE-MS) | Very High | Very High | Very High | Low | High | Small molecule separation, metabolomics |
| Smart Biosensors and AI-Integrated Detection | Very High | High | High | Very High | Moderate | Emerging real-time diagnostics, home-use potential |
| Biosensors | Main Action | Benefits |
|---|---|---|
| Biosensor for cancer detection [108] | Detects the cancer biomarker CYFRA-21–1 in saliva | Can diagnose cancer at an early stage without invasive surgery or expensive therapies—ELISA is used for identifying purposes. |
| Alpha Amylase Biosensor [109] | Salivary alpha amylase level detection | Gives information about an individual’s diet |
| Gives information on the acidity levels in the mouth cavity as a result of digesting complex carbs | ||
| Titanium biosensor [110] | Assessing periodontal health and identifying hazardous amounts of streptococcus Gordonii | Titanium has longevity, resistance to corrosion, and excellent quality |
| The ability to employ CHX as a protective oral health aid expands its application | ||
| Urea Smartphone Biosensor [111] | Measuring urea levels from saliva by linking a transducer to a mobile app that calculates an array of urea levels | Real, tangible thing to experiment with and manipulate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, V.; Luchian, I.; Goriuc, A.; Budala, D.G.; Bida, F.C.; Cojocaru, C.; Butnaru, O.-M.; Virvescu, D.I. Salivary Biomarkers Identification: Advances in Standard and Emerging Technologies. Oral 2025, 5, 26. https://doi.org/10.3390/oral5020026
Constantin V, Luchian I, Goriuc A, Budala DG, Bida FC, Cojocaru C, Butnaru O-M, Virvescu DI. Salivary Biomarkers Identification: Advances in Standard and Emerging Technologies. Oral. 2025; 5(2):26. https://doi.org/10.3390/oral5020026
Chicago/Turabian StyleConstantin, Vlad, Ionut Luchian, Ancuta Goriuc, Dana Gabriela Budala, Florinel Cosmin Bida, Cristian Cojocaru, Oana-Maria Butnaru, and Dragos Ioan Virvescu. 2025. "Salivary Biomarkers Identification: Advances in Standard and Emerging Technologies" Oral 5, no. 2: 26. https://doi.org/10.3390/oral5020026
APA StyleConstantin, V., Luchian, I., Goriuc, A., Budala, D. G., Bida, F. C., Cojocaru, C., Butnaru, O.-M., & Virvescu, D. I. (2025). Salivary Biomarkers Identification: Advances in Standard and Emerging Technologies. Oral, 5(2), 26. https://doi.org/10.3390/oral5020026










