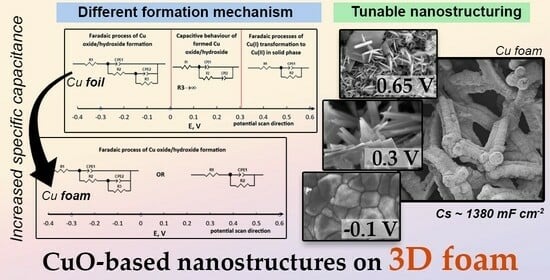
Insights into the Electrochemical Synthesis and Supercapacitive Behaviour of 3D Copper Oxide-Based Nanostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Electrochemical Impedance Spectroscopy
2.3. Structure and Morphology
2.4. Capacitive Properties
3. Results
3.1. Evaluation of Electrochemical Synthesis of Copper Oxide-Based Nanostructures
- -
- Peak I, observed at −0.4 V, corresponds to the oxidation of metallic copper (Equation (5)).
- -
- Peak II, at −0.16 V, is associated with the formation of CuO from Cu2O (Equation (6)).
- -
- R1 = 1.474 Ω cm2; R2 = 113.1 Ω cm2; R3 = 7930 Ω cm2; CPE1 = 3.737·10−5 (F cm−2)0.853; n1 = 0.853; CPE2 = 0.422 (F cm−2)0.422; n2 = 0.422 (Figure 3a,b).
- R1 = 1.703 Ω cm2; R2 = 6431 Ω cm2; CPE1 = 1.01·10−4 (F cm−2)0.938; n1 = 0.938; CPE2 = 0.422 (F cm−2)0.804; n2 = 0.804 (Figure 3c,d).
- R1 = 9.502 Ω cm2; R2 = 2452 Ω cm2; CPE1 = 2.155·10−5 (F cm−2)0.666; n1 = 0.666; CPE2 = 4.003·10−5 (F cm−2)0.775; n2 = 0.775 (Figure 3e,f).
3.2. Evaluation of Morphology and Structure of the Obtained Films
3.3. Evaluation of Capacitive Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delivering the European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal/delivering-european-green-deal_en (accessed on 21 January 2025).
- Sisakyan, N.; Chilingaryan, G.; Manukyan, A.; Mukasyan, A.S. Combustion Synthesis of Materials for Application in Supercapacitors: A Review. Nanomaterials 2023, 13, 3030. [Google Scholar] [CrossRef] [PubMed]
- Gurav, K.V.; Patil, U.M.; Shin, S.W.; Agawane, G.L.; Suryawanshi, M.P.; Pawar, S.M.; Patil, P.S.; Lokhande, C.D.; Kim, J.H. Room Temperature Chemical Synthesis of Cu(OH)2 Thin Films for Supercapacitor Application. J. Alloys Compd. 2013, 573, 27–31. [Google Scholar] [CrossRef]
- Majumdar, D.; Ghosh, S. Recent Advancements of Copper Oxide Based Nanomaterials for Supercapacitor Applications. J. Energy Storage 2021, 34, 101995. [Google Scholar] [CrossRef]
- Patake, V.D.; Joshi, S.S.; Lokhande, C.D.; Joo, O.-S. Electrodeposited Porous and Amorphous Copper Oxide Film for Application in Supercapacitor. Mater. Chem. Phys. 2009, 114, 6–9. [Google Scholar] [CrossRef]
- Sadale, S.B.; Patil, S.B.; Teli, A.M.; Masegi, H.; Noda, K. Effect of Deposition Potential and Annealing on Performance of Electrodeposited Copper Oxide Thin Films for Supercapacitor Application. Solid State Sci. 2022, 123, 106780. [Google Scholar] [CrossRef]
- Patil, S.S.; Pawar, S.M.; Ghatage, S.V.; Patil, A.P.; Redekar, R.S.; Yadav, H.M.; Tarwal, N.L.; Patil, P.S. Charge Storage Dynamics and Time Series Analysis of Binder Free Rapidly Synthesized Copper Oxide for Supercapacitors. Mater. Sci. Semicond. Process. 2024, 184, 108769. [Google Scholar] [CrossRef]
- Xu, P.; Liu, J.; Liu, T.; Ye, K.; Cheng, K.; Yin, J.; Cao, D.; Wang, G.; Li, Q. Preparation of Binder-Free CuO/Cu2 O/Cu Composites: A Novel Electrode Material for Supercapacitor Applications. RSC Adv. 2016, 6, 28270–28278. [Google Scholar] [CrossRef]
- Singh, B.K.; Shaikh, A.; Dusane, R.O.; Parida, S. Copper Oxide Nanosheets and Nanowires Grown by One-Step Linear Sweep Voltammetry for Supercapacitor Application. J. Energy Storage 2020, 31, 101631. [Google Scholar] [CrossRef]
- Zheng, D.; Sun, X.; An, C.; Pan, F.; Qin, C.; Wang, Z.; Deng, Q.; Song, Y.; Li, Y. Flexible Multi-Layered Porous CuxO/NiO (x = 1, 2) Photo-Assisted Electrodes for Hybrid Supercapacitors: Design and Mechanism Insight. Chem. Eng. J. 2023, 473, 145289. [Google Scholar] [CrossRef]
- Dubal, D.P.; Dhawale, D.S.; Salunkhe, R.R.; Jamdade, V.S.; Lokhande, C.D. Fabrication of Copper Oxide Multilayer Nanosheets for Supercapacitor Application. J. Alloys Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Viswanathan, A.; Shetty, A.N. Facile In-Situ Single Step Chemical Synthesis of Reduced Graphene Oxide-Copper Oxide-Polyaniline Nanocomposite and Its Electrochemical Performance for Supercapacitor Application. Electrochim. Acta 2017, 257, 483–493. [Google Scholar] [CrossRef]
- Shinde, S.; Dhaygude, H.; Kim, D.-Y.; Ghodake, G.; Bhagwat, P.; Dandge, P.; Fulari, V. Improved Synthesis of Copper Oxide Nanosheets and Its Application in Development of Supercapacitor and Antimicrobial Agents. J. Ind. Eng. Chem. 2016, 36, 116–120. [Google Scholar] [CrossRef]
- Pendashteh, A.; Mousavi, M.F.; Rahmanifar, M.S. Fabrication of Anchored Copper Oxide Nanoparticles on Graphene Oxide Nanosheets via an Electrostatic Coprecipitation and Its Application as Supercapacitor. Electrochim. Acta 2013, 88, 347–357. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Xiao, J.; Jiang, H.; Hu, T.; Meng, C. Copper Oxide/Cuprous Oxide/Hierarchical Porous Biomass-Derived Carbon Hybrid Composites for High-Performance Supercapacitor Electrode. J. Alloys Compd. 2019, 782, 1103–1113. [Google Scholar] [CrossRef]
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-Functional Reactively-Sputtered Copper Oxide Electrodes for Supercapacitor and Electro-Catalyst in Direct Methanol Fuel Cell Applications. Sci. Rep. 2016, 6, 21310. [Google Scholar] [CrossRef]
- Majumdar, D.; Baugh, N.; Bhattacharya, S.K. Ultrasound Assisted Formation of Reduced Graphene Oxide-Copper (II) Oxide Nanocomposite for Energy Storage Applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 158–170. [Google Scholar] [CrossRef]
- Morariu (Popescu), M.-I.; Nicolaescu, M.; Hulka, I.; Duţeanu, N.; Orha, C.; Lăzău, C.; Bandas, C. Fabrication of Cu2O/CuO Nanowires by One-Step Thermal Oxidation of Flexible Copper Mesh for Supercapacitor Applications. Batteries 2024, 10, 246. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Lokhande, C.D.; Holze, R. CuO Cauliflowers for Supercapacitor Application: Novel Potentiodynamic Deposition. Mater. Res. Bull. 2013, 48, 923–928. [Google Scholar] [CrossRef]
- Sayyed, S.G.; Shaikh, A.V.; Shinde, U.P.; Hiremath, P.; Naik, N. Copper Oxide-Based High-Performance Symmetric Flexible Supercapacitor: Potentiodynamic Deposition. J. Mater. Sci. Mater. Electron. 2023, 34, 1361. [Google Scholar] [CrossRef]
- Xu, W.; Dai, S.; Liu, G.; Xi, Y.; Hu, C.; Wang, X. CuO Nanoflowers Growing on Carbon Fiber Fabric for Flexible High-Performance Supercapacitors. Electrochim. Acta 2016, 203, 1–8. [Google Scholar] [CrossRef]
- Xi, Y.; Xiao, Z.; Lv, H.; Sun, H.; Zhai, S.; An, Q. Construction of CuO/Cu-Nanoflowers Loaded on Chitosan-Derived Porous Carbon for High Energy Density Supercapacitors. J. Colloid Interface Sci. 2023, 630, 525–534. [Google Scholar] [CrossRef]
- Luan, V.H.; Han, J.H.; Kang, H.W.; Lee, W. Highly Porous and Capacitive Copper Oxide Nanowire/Graphene Hybrid Carbon Nanostructure for High-Performance Supercapacitor Electrodes. Compos. Part B Eng. 2019, 178, 107464. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, H. Improvement in Flexibility and Volumetric Performance for Supercapacitor Application and the Effect of Ni–Fe Ratio on Electrode Behaviour. J. Mater. Chem. A 2015, 3, 7607–7615. [Google Scholar] [CrossRef]
- Singh, B.K.; Shaikh, A.; Badrayyana, S.; Mohapatra, D.; Dusane, R.O.; Parida, S. Nanoporous Gold–Copper Oxide Based All-Solid-State Micro-Supercapacitors. RSC Adv. 2016, 6, 100467–100475. [Google Scholar] [CrossRef]
- Shu, X.; Zheng, H.; Xu, G.; Zhao, J.; Cui, L.; Cui, J.; Qin, Y.; Wang, Y.; Zhang, Y.; Wu, Y. The Anodization Synthesis of Copper Oxide Nanosheet Arrays and Their Photoelectrochemical Properties. Appl. Surf. Sci. 2017, 412, 505–516. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, X.; Sun, H.; Li, Y.; Zhang, K.; Wu, Y. Corrosion Behavior of Copper at Elevated Temperature. Int. J. Electrochem. Sci. 2012, 7, 7902–7914. [Google Scholar] [CrossRef]
- Teo, W.Z.; Ambrosi, A.; Pumera, M. Direct Electrochemistry of Copper Oxide Nanoparticles in Alkaline Media. Electrochem. Commun. 2013, 28, 51–53. [Google Scholar] [CrossRef]
- Caballero-Briones, F.; Artés, J.M.; Díez-Pérez, I.; Gorostiza, P.; Sanz, F. Direct Observation of the Valence Band Edge by in Situ ECSTM-ECTS in p-Type Cu2O Layers Prepared by Copper Anodization. J. Phys. Chem. C 2009, 113, 1028–1036. [Google Scholar] [CrossRef]
- Vidhyadharan, B.; Misnon, I.I.; Aziz, R.A.; Padmasree, K.P.; Yusoff, M.M.; Jose, R. Superior Supercapacitive Performance in Electrospun Copper Oxide Nanowire Electrodes. J. Mater. Chem. A 2014, 2, 6578–6588. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. The Study of Thin Films by Electrochemical Impedance Spectroscopy. In Nanostructures and Thin Films for Multifunctional Applications; Tiginyanu, I., Topala, P., Ursaki, V., Eds.; NanoScience and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–42. ISBN 978-3-319-30197-6. [Google Scholar]
- Dupont, M.; Hollenkamp, A.F.; Donne, S.W. Electrochemically Active Surface Area Effects on the Performance of Manganese Dioxide for Electrochemical Capacitor Applications. Electrochim. Acta 2013, 104, 140–147. [Google Scholar] [CrossRef]
- Vainoris, M.; Cesiulis, H.; Tsyntsaru, N. Metal Foam Electrode as a Cathode for Copper Electrowinning. Coatings 2020, 10, 822. [Google Scholar] [CrossRef]
- Vainoris, M.; Tsyntsaru, N.; Cesiulis, H. Modified Electrodeposited Cobalt Foam Coatings as Sensors for Detection of Free Chlorine in Water. Coatings 2019, 9, 306. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Vivier, V.; Orazem, M.E.; Pébère, N.; Tribollet, B. The Apparent Constant-Phase-Element Behavior of a Disk Electrode with Faradaic Reactions. J. Electrochem. Soc. 2007, 154, C99. [Google Scholar] [CrossRef]
- Bae, J.H.; Han, J.-H.; Chung, T.D. Electrochemistry at Nanoporous Interfaces: New Opportunity for Electrocatalysis. Phys. Chem. Chem. Phys. 2012, 14, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Schalenbach, M.; Selmert, V.; Kretzschmar, A.; Raijmakers, L.; Durmus, Y.E.; Tempel, H.; Eichel, R.-A. How Microstructures, Oxide Layers, and Charge Transfer Reactions Influence Double Layer Capacitances. Part 1: Impedance Spectroscopy and Cyclic Voltammetry to Estimate Electrochemically Active Surface Areas (ECSAs). Phys. Chem. Chem. Phys. 2024, 26, 14288–14304. [Google Scholar] [CrossRef]
- Oyarzún, D.P.; Tello, A.; Sánchez, J.; Boulett, A.; Linarez Pérez, O.E.; Martin-Trasanco, R.; Pizarro, G.D.C.; Flores, M.; Zúñiga, C. Exploration of Copper Oxide Nanoneedle Electrosynthesis Applied in the Degradation of Methylene Blue. Nanomaterials 2021, 11, 2994. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, M.; Kong, M.; Tao, S.; Zhang, C.; Wang, K. Synthesis and Growth Features of Copper Hydroxide Iodide Nanoneedles. Mater. Lett. 2007, 61, 846–849. [Google Scholar] [CrossRef]
- Kumar, S.K.; Murugesan, S.; Suresh, S. Anodization Assisted Preparation of Diverse Nanostructured Copper Oxide Films for Solar Selective Absorber. Opt. Mater. 2023, 135, 113304. [Google Scholar] [CrossRef]
- Rajani, C.; Anuradha, V.; Sunandamma, Y. Copper Oxide/Hydroxide Nanomaterial Synthesized from Simple Copper Salt. Int. J. Nanosci. 2020, 19, 1950028. [Google Scholar] [CrossRef]
- Dörner, L.; Cancellieri, C.; Rheingans, B.; Walter, M.; Kägi, R.; Schmutz, P.; Kovalenko, M.V.; Jeurgens, L.P.H. Cost-Effective Sol-Gel Synthesis of Porous CuO Nanoparticle Aggregates with Tunable Specific Surface Area. Sci. Rep. 2019, 9, 11758. [Google Scholar] [CrossRef]
- He, D.; Xing, S.; Sun, B.; Cai, H.; Suo, H.; Zhao, C. Design and Construction of Three-Dimensional Flower-like CuO Hierarchical Nanostructures on Copper Foam for High Performance Supercapacitor. Electrochim. Acta 2016, 210, 639–645. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “Supercapacitor” to “Battery” Behavior in Electrochemical Energy Storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Wang, B.; Cao, B.; Wang, C.; Zhang, Y.; Yao, H.; Wang, Y. The Optical and Electrical Performance of CuO Synthesized by Anodic Oxidation Based on Copper Foam. Materials 2020, 13, 5411. [Google Scholar] [CrossRef]
- He, D.; Wang, G.; Liu, G.; Suo, H.; Zhao, C. Construction of Leaf-like CuO–Cu2 O Nanocomposites on Copper Foam for High-Performance Supercapacitors. Dalton Trans. 2017, 46, 3318–3324. [Google Scholar] [CrossRef] [PubMed]













| Crystallite Size, nm | ||||||
|---|---|---|---|---|---|---|
| Foil | Foam | |||||
| Emax, V | Cu2O | Cu(OH)2 | CuO | Cu2O | Cu(OH)2 | CuO |
| 0.65 | - | - | 10.9 | - | - | 9.1 |
| 0.6 | - | 27.6 | 13.3 | - | - | 9.1 |
| 0.3 | 35.1 | 35.0 | 10.2 | 23.2 | 23.4 | 9.6 |
| −0.1 | 37.0 | 35.0 | - | 39.8 | 37.8 | - |
| −0.3 | 17.7 | - | - | 6.4 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonkus, G.; Levinas, R.; Tsyntsaru, N.; Cesiulis, H. Insights into the Electrochemical Synthesis and Supercapacitive Behaviour of 3D Copper Oxide-Based Nanostructures. Solids 2025, 6, 15. https://doi.org/10.3390/solids6020015
Jonkus G, Levinas R, Tsyntsaru N, Cesiulis H. Insights into the Electrochemical Synthesis and Supercapacitive Behaviour of 3D Copper Oxide-Based Nanostructures. Solids. 2025; 6(2):15. https://doi.org/10.3390/solids6020015
Chicago/Turabian StyleJonkus, Gintautas, Ramunas Levinas, Natalia Tsyntsaru, and Henrikas Cesiulis. 2025. "Insights into the Electrochemical Synthesis and Supercapacitive Behaviour of 3D Copper Oxide-Based Nanostructures" Solids 6, no. 2: 15. https://doi.org/10.3390/solids6020015
APA StyleJonkus, G., Levinas, R., Tsyntsaru, N., & Cesiulis, H. (2025). Insights into the Electrochemical Synthesis and Supercapacitive Behaviour of 3D Copper Oxide-Based Nanostructures. Solids, 6(2), 15. https://doi.org/10.3390/solids6020015