Pristine and Reassembled Nanosheets of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) as Photocatalysts for Hydrogen Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Precursors for Exfoliation
2.2. Exfoliation into Nanosheets and Their Reassembly
2.3. Investigation of Photocatalytic Activity and Stability
2.4. Instrumentation and Data Processing
2.4.1. XRD
2.4.2. Raman Spectroscopy
2.4.3. TG
2.4.4. CHN-Analysis
2.4.5. DRS
2.4.6. TR-PLS
2.4.7. SEM
2.4.8. BET
2.4.9. Laser Granulometry
2.4.10. UV-Vis Spectrophotometry
2.4.11. pH-Metry
3. Results and Discussion
3.1. Characterization of the Initial Titanates, Their Pristine and Reassembled Nanosheets
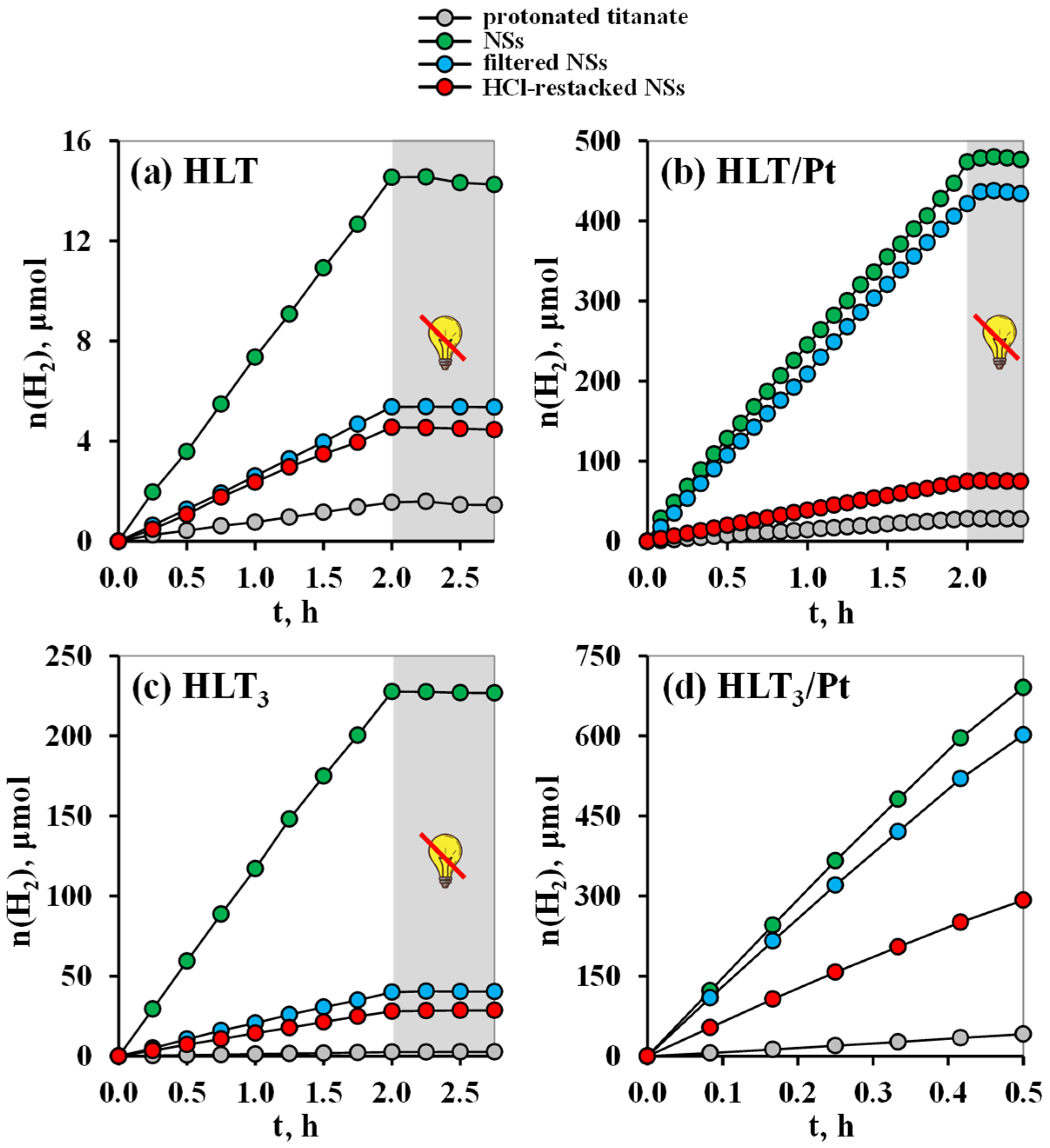
3.2. Photocatalytic Activity in the Reactions of Hydrogen Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Recent Progress on Semiconductor Heterogeneous Photocatalysts in Clean Energy Production and Environmental Remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, R.; Raizada, P.; Ahamad, T.; Alshehri, S.M.; Nguyen, V.H.; Thakur, S.; Nguyen, C.C.; Kim, S.Y.; Le, Q.; et al. An Overview on Recent Progress in Photocatalytic Air Purification: Metal-Based and Metal-Free Photocatalysis. Environ. Res. 2022, 214, 113995. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Kim, M.K.; Danish, M.; Jo, W.K. State-of-the-Art Review on Photocatalysis for Efficient Wastewater Treatment: Attractive Approach in Photocatalyst Design and Parameters Affecting the Photocatalytic Degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Rozhkova, E.; Ariga, K. From Molecules to Materials: Pathways to Artificial Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Ismail, A.A.; Bahnemann, D.W. Photochemical Splitting of Water for Hydrogen Production by Photocatalysis: A Review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from Photo-Catalytic Water Splitting Process: A Review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Kausar, F.; Varghese, A.; Pinheiro, D.; Devi, K.R.S. Recent Trends in Photocatalytic Water Splitting Using Titania Based Ternary Photocatalysts—A Review. Int. J. Hydrogen Energy 2022, 47, 22371–22402. [Google Scholar] [CrossRef]
- Zhao, H.; Mao, Q.; Jian, L.; Dong, Y.; Zhu, Y. Photodeposition of Earth-Abundant Cocatalysts in Photocatalytic Water Splitting: Methods, Functions, and Mechanisms. Chin. J. Catal. 2022, 43, 1774–1804. [Google Scholar] [CrossRef]
- Moridon, S.N.F.; Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Photocatalytic Water Splitting Performance of TiO2 Sensitized by Metal Chalcogenides: A Review. Ceram. Int. 2022, 48, 5892–5907. [Google Scholar] [CrossRef]
- Li, R.; Li, C. Scalable Solar Water Splitting Using Particulate Photocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 33, 100577. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Kearney, L.; Brandon, M.P.; Pryce, M.T. Design Components of Porphyrin-Based Photocatalytic Hydrogen Evolution Systems: A Review. Coord. Chem. Rev. 2022, 467, 214599. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Palmisano, L. Photocatalytic Formation of H2 and Value-Added Chemicals in Aqueous Glucose (Pt)-TiO2 Suspension. Int. J. Hydrogen Energy 2016, 41, 5934–5947. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Peng, S.; Lu, G.; Li, S. Effect of Epimerization of D-Glucose on Photocatalytic Hydrogen Generation over Pt/TiO2. Catal. Commun. 2012, 18, 21–25. [Google Scholar] [CrossRef]
- Bahadori, E.; Ramis, G.; Zanardo, D.; Menegazzo, F.; Signoretto, M.; Gazzoli, D.; Pietrogiacomi, D.; Michele, A. Di Photoreforming of Glucose over CuO/TiO2. Catalysts 2020, 10, 477. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Nasillo, G.; Palmisano, L. Photocatalytic Solar Light H2 Production by Aqueous Glucose Reforming. Eur. J. Inorg. Chem. 2018, 2018, 4522–4532. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Murcia, J.J.; Rizzo, L.; Ventre, G.; Pepe, G.; Campiglia, P.; Hidalgo, M.C.; Navío, J.A.; Sannino, D. Photocatalytic Hydrogen Production from Degradation of Glucose over Fluorinated and Platinized TiO2 Catalysts. J. Catal. 2016, 339, 47–56. [Google Scholar] [CrossRef]
- Bello, M.O.; Prabhakar, S.; Abdus-salam, N.; Adekola, F.A.; Shobha, C.; Sesha, A.V.; Pal, U. Na-Y Zeolite Supported TiO2/Pd Nanoparticles for Enhanced Photoredox Catalytic Properties and Green Hydrogen Generation. Catal. Commun. 2024, 186, 106817. [Google Scholar] [CrossRef]
- Jiang, Z.; Ye, Z.; Shangguan, W. Recent Advances of Hydrogen Production through Particulate Semiconductor Photocatalytic Overall Water Splitting. Front. Energy 2022, 16, 49–63. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Meng, X. Recent Advances on Electrocatalytic and Photocatalytic Seawater Splitting for Hydrogen Evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Q. Redox-Mediated Electrocatalytic and Photocatalytic Hydrogen Production. Curr. Opin. Electrochem. 2022, 35, 101097. [Google Scholar] [CrossRef]
- Sahani, S.; Malika Tripathi, K.; Il Lee, T.; Dubal, D.P.; Wong, C.P.; Chandra Sharma, Y.; Young Kim, T. Recent Advances in Photocatalytic Carbon-Based Materials for Enhanced Water Splitting under Visible-Light Irradiation. Energy Convers. Manag. 2022, 252, 115133. [Google Scholar] [CrossRef]
- Chen, J.; Abazari, R.; Adegoke, K.A.; Maxakato, N.W.; Bello, O.S.; Tahir, M.; Tasleem, S.; Sanati, S.; Kirillov, A.M.; Zhou, Y. Metal–Organic Frameworks and Derived Materials as Photocatalysts for Water Splitting and Carbon Dioxide Reduction. Coord. Chem. Rev. 2022, 469, 214664. [Google Scholar] [CrossRef]
- Jaryal, R.; Kumar, R.; Khullar, S. Mixed Metal-Metal Organic Frameworks (MM-MOFs) and Their Use as Efficient Photocatalysts for Hydrogen Evolution from Water Splitting Reactions. Coord. Chem. Rev. 2022, 464, 214542. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, P.; Bansal, N.K.; Paul, M.; Dixit, H.; Porwal, S.; Mishra, S.; Singh, T. Recent Progress in Perovskite Transition Metal Oxide-Based Photocatalyst and Photoelectrode Materials for Solar-Driven Water Splitting. J. Environ. Chem. Eng. 2022, 10, 108429. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; He, H.; Yang, S.; Duan, X.; Wang, S. Bismuth-Based Complex Oxides for Photocatalytic Applications in Environmental Remediation and Water Splitting: A Review. Sci. Total Environ. 2022, 804, 150215. [Google Scholar] [CrossRef]
- Subramanyam, P.; Meena, B.; Biju, V.; Misawa, H.; Challapalli, S. Emerging Materials for Plasmon-Assisted Photoelectrochemical Water Splitting. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100472. [Google Scholar] [CrossRef]
- Weng, Y.C.; Li, Y.H.; Yuan, W.L.; Huang, L.W. Enhanced Photocatalytic Activity of Amphiphilic Single-Walled Carbon Nanohorn–In0.2Cd0.8S Composites for Water Splitting. Catal. Commun. 2024, 186, 106818. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic Production of Hydrogen from Biomass-Derived Feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Liu, R.; Yoshida, H.; Fujita, S.; Arai, M. Photocatalytic Hydrogen Production from Glycerol and Water with NiOx/TiO2 Catalysts. Appl. Catal. B Environ. 2014, 144, 41–45. [Google Scholar] [CrossRef]
- Taboada, E.; Angurell, I.; Llorca, J. Hydrogen Photoproduction from Bio-Derived Alcohols in an Optical Fiber Honeycomb Reactor Loaded with Au/TiO2. J. Photochem. Photobiol. A Chem. 2014, 281, 35–39. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The Roles of Metal Co-Catalysts and Reaction Media in Photocatalytic Hydrogen Production: Performance Evaluation of M/TiO2 Photocatalysts (M = Pd, Pt, Au) in Different Alcohol-Water Mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Dosado, A.G.; Chen, W.T.; Chan, A.; Sun-Waterhouse, D.; Waterhouse, G.I.N. Novel Au/TiO2 Photocatalysts for Hydrogen Production in Alcohol-Water Mixtures Based on Hydrogen Titanate Nanotube Precursors. J. Catal. 2015, 330, 238–254. [Google Scholar] [CrossRef]
- Wang, X.; Dong, H.; Hu, Z.; Qi, Z.; Li, L. Fabrication of a Cu2O/Au/TiO2 Composite Film for Efficient Photocatalytic Hydrogen Production from Aqueous Solution of Methanol and Glucose. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 219, 10–19. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Ming, J.; Liu, M.; Fang, P. Hydrogen Generation by Photocatalytic Reforming of Glucose with Heterostructured CdS/MoS2 Composites under Visible Light Irradiation. Int. J. Hydrogen Energy 2017, 42, 16968–16978. [Google Scholar] [CrossRef]
- Jaswal, R.; Shende, R.; Nan, W.; Shende, A. Photocatalytic Reforming of Pinewood (Pinus Ponderosa) Acid Hydrolysate for Hydrogen Generation. Int. J. Hydrogen Energy 2017, 42, 2839–2848. [Google Scholar] [CrossRef]
- Irshad, M.; Ain, Q.; Zaman, M.; Aslam, M.Z.; Kousar, N.; Asim, M.; Rafique, M.; Siraj, K.; Tabish, A.N.; Usman, M.; et al. Photocatalysis and Perovskite Oxide-Based Materials: A Remedy for a Clean and Sustainable Future. RSC Adv. 2022, 12, 7009–7039. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Perovskites by Design: A Toolbox of Solid-State Reactions. Chem. Mater. 2002, 14, 1455–1471. [Google Scholar] [CrossRef]
- Uppuluri, R.; Sen Gupta, A.; Rosas, A.S.; Mallouk, T.E. Soft Chemistry of Ion-Exchangeable Layered Metal Oxides. Chem. Soc. Rev. 2018, 47, 2401–2430. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Komori, Y.; Hayashi, S.; Sugahara, Y. Local Environments and Dynamics of Hydrogen Atoms in Protonated Forms of Ion-Exchangeable Layered Perovskites Estimated by Solid-State 1 H NMR. J. Solid. State Chem. 2006, 179, 3357–3364. [Google Scholar] [CrossRef]
- Nishimoto, S.; Matsuda, M.; Miyake, M. Novel Protonated and Hydrated Ruddlesden–Popper Phases, HxNa1−xLaTiO4·yH2O, Formed by Ion-Exchange/Intercalation Reaction. J. Solid. State Chem. 2005, 178, 811–818. [Google Scholar] [CrossRef]
- Nishimoto, S.; Matsuda, M.; Harjo, S.; Hoshikawa, A.; Kamiyama, T.; Ishigaki, T.; Miyake, M. Structure Determination of n=1 Ruddlesden–Popper Compound HLaTiO4 by Powder Neutron Diffraction. J. Eur. Ceram. Soc. 2006, 26, 725–729. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Lewandowski, J.T.; Johnson, J.W. Ion Exchange of the Layered Perovskite KCa2Nb3O10 by Protons. J. Less Common. Met. 1986, 116, 137–146. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J.T. Interlayer Chemistry between Thick Transition-Metal Oxide Layers: Synthesis and Intercalation Reactions of K[Ca2Nan−3NbnO3n+1] (3 ≤ n ≤ 7). Inorg. Chem. 1985, 24, 3727–3729. [Google Scholar] [CrossRef]
- Silyukov, O.I.; Kurnosenko, S.A.; Minich, I.A.; Rodionov, I.A.; Zvereva, I.A. Protonated Forms of Layered Perovskite-Like Titanate NaNdTiO4: Neutron and X-Ray Diffraction Structural Analysis. Solids 2021, 2, 265–277. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Arakawa, H. Substitution Effects of In3+ by Fe3+ on Photocatalytic and Structural Properties of Bi2InNbO7 Photocatalysts. J. Mol. Catal. 2001, 168, 289–297. [Google Scholar] [CrossRef]
- Reddy, V.; Hwang, D.; Lee, J. Effect of Zr Substitution for Ti in KLaTiO4 for Photocatalytic Water Splitting. Catal. Lett. 2003, 90, 39–44. [Google Scholar] [CrossRef]
- Kumar, V.; Govind; Uma, S. Investigation of Cation (Sn2+) and Anion (N3−) Substitution in Favor of Visible Light Photocatalytic Activity in the Layered Perovskite K2La2Ti3O10. J. Hazard. Mater. 2011, 189, 502–508. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, T.; Guo, Y.; Yang, B.; Wang, Y. Controllable Doping of Nitrogen and Tetravalent Niobium Affords Yellow and Black Calcium Niobate Nanosheets for Enhanced Photocatalytic Hydrogen Evolution. RSC Adv. 2016, 6, 64930–64936. [Google Scholar] [CrossRef]
- Kawashima, K.; Hojamberdiev, M.; Chen, S.; Yubuta, K.; Wagata, H.; Domen, K.; Teshima, K. Understanding the Effect of Partial N3−-to-O2− Substitution and H+-to-K+ Exchange on Photocatalytic Water Reduction Activity of Ruddlesden–Popper Layered Perovskite KLaTiO4. Mol. Catal. 2017, 432, 250–258. [Google Scholar] [CrossRef]
- Cui, W.; Qi, Y.; Liu, L.; Rana, D.; Hu, J.; Liang, Y. Synthesis of PbS–K2La2Ti3O10 Composite and Its Photocatalytic Activity for Hydrogen Production. Prog. Nat. Sci. Mater. Int. 2012, 22, 120–125. [Google Scholar] [CrossRef][Green Version]
- Cui, W.; Liu, L.; Ma, S.; Liang, Y.; Zhang, Z. CdS-Sensitized K2La2Ti3O10 Composite: A New Photocatalyst for Hydrogen Evolution under Visible Light Irradiation. Catal. Today 2013, 207, 44–49. [Google Scholar] [CrossRef]
- Cui, W.; Guo, D.; Liu, L.; Hu, J.; Rana, D.; Liang, Y. Preparation of ZnIn2S4/K2La2Ti3O10 Composites and Their Photocatalytic H2 Evolution from Aqueous Na2S/Na2SO3 under Visible Light Irradiation. Catal. Commun. 2014, 48, 55–59. [Google Scholar] [CrossRef]
- Saito, K.; Kozeni, M.; Sohmiya, M.; Komaguchi, K.; Ogawa, M.; Sugahara, Y.; Ide, Y. Unprecedentedly Enhanced Solar Photocatalytic Activity of a Layered Titanate Simply Integrated with TiO2 Nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 30920–30925. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Lv, C.; Zhang, C.; Jin, X.; Meng, Q.; Chen, G. Construction of 2D-Composite HCa2Nb3O10/CaNb2O6 Heterostructured Photocatalysts with Enhanced Hydrogen Production Performance. New J. Chem. 2018, 42, 681–687. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Zhang, L.; Wong, K.H.; Chen, Z.; Yu, J.C.; Zhao, J.; Hu, C.; Chan, C.Y.; Wong, P.K. AgBr-Ag-Bi2WO6 Nanojunction System: A Novel and Efficient Photocatalyst with Double Visible-Light Active Components. Appl. Catal. A Gen. 2009, 363, 221–229. [Google Scholar] [CrossRef]
- Kim, H.G.; Jeong, E.D.; Borse, P.H.; Jeon, S.; Yong, K.; Lee, J.S.; Li, W.; Oh, S.H. Photocatalytic Ohmic Layered Nanocomposite for Efficient Utilization of Visible Light Photons. Appl. Phys. Lett. 2006, 89, 2012–2015. [Google Scholar] [CrossRef]
- Kim, H.G.; Borse, P.H.; Choi, W.; Lee, J.S. Photocatalytic Nanodiodes for Visible-Light Photocatalysis. Angew. Chem. 2005, 117, 4661–4665. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Xiong, Z.; Tang, H.; Jiang, C. Fabrication of Flower-like Direct Z-Scheme β-Bi2O3/g-C3N4 photocatalyst with Enhanced Visible Light Photoactivity for Rhodamine B Degradation. Appl. Surf. Sci. 2018, 436, 162–171. [Google Scholar] [CrossRef]
- Youngblood, W.J.; Anna Lee, S.H.; Maeda, K.; Mallouk, T.E. Visible Light Water Splitting Using Dye-Sensitized Oxide Semiconductors. Acc. Chem. Res. 2009, 42, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Mitsuyama, T.; Ikeue, K.; Matsushima, S.; Arai, M. Photocatalytic Property and Electronic Structure of Triple-Layered Perovskite Tantalates, MCa2Ta3O10 (M = Cs, Na, H, and C6H13NH3). J. Phys. Chem. B 2005, 109, 7801–7806. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, L.; Hao, Q.; Zhu, X.; Chen, X.; Tang, K. Preparation of Interlayer Surface Tailored Protonated Double-Layered Perovskite H2CaTa2O7 with n-Alcohols, and Their Photocatalytic Activity. RSC Adv. 2014, 4, 4047–4054. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Maksimova, E.A.; Pozhidaev, A.Y.; Kurnosenko, S.A.; Silyukov, O.I.; Zvereva, I.A. Layered Titanate H2Nd2Ti3O10 Intercalated with n-Butylamine: A New Highly Efficient Hybrid Photocatalyst for Hydrogen Production from Aqueous Solutions of Alcohols. Front. Chem. 2019, 7, 863. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Gruzdeva, E.O.; Mazur, A.S.; Kurnosenko, S.A.; Silyukov, O.I.; Zvereva, I.A. Photocatalytic Hydrogen Generation from Aqueous Methanol Solution over n-Butylamine-Intercalated Layered Titanate H2La2Ti3O10: Activity and Stability of the Hybrid Photocatalyst. Catalysts 2022, 12, 1556. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Voytovich, V.V.; Silyukov, O.I.; Rodionov, I.A.; Kirichenko, S.O.; Minich, I.A.; Malygina, E.N.; Khramova, A.D.; Zvereva, I.A. Photocatalytic Activity of n-Alkylamine and n-Alkoxy Derivatives of Layered Perovskite-like Titanates H2Ln2Ti3O10 (Ln = La, Nd) in the Reaction of Hydrogen Production from an Aqueous Solution of Methanol. Catalysts 2021, 11, 1279. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Voytovich, V.V.; Silyukov, O.I.; Rodionov, I.A.; Zvereva, I.A. Photocatalytic Activity and Stability of Organically Modified Layered Perovskite-like Titanates HLnTiO4 (Ln = La, Nd) in the Reaction of Hydrogen Evolution from Aqueous Methanol. Catalysts 2023, 13, 749. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Voytovich, V.V.; Silyukov, O.I.; Rodionov, I.A.; Zvereva, I.A. Photocatalytic Hydrogen Production from Aqueous Solutions of Glucose and Xylose over Layered Perovskite-like Oxides HCa2Nb3O10, H2La2Ti3O10 and Their Inorganic-Organic Derivatives. Nanomaterials 2022, 12, 2717. [Google Scholar] [CrossRef]
- Voytovich, V.V.; Kurnosenko, S.A.; Silyukov, O.I.; Rodionov, I.A.; Minich, I.A.; Zvereva, I.A. Study of n-Alkylamine Intercalated Layered Perovskite-like Niobates HCa2Nb3O10 as Photocatalysts for Hydrogen Production from an Aqueous Solution of Methanol. Front. Chem. 2020, 8, 300. [Google Scholar] [CrossRef]
- Voytovich, V.V.; Kurnosenko, S.A.; Silyukov, O.I.; Rodionov, I.A.; Bugrov, A.N.; Minich, I.A.; Malygina, E.N.; Zvereva, I.A. Synthesis of n-Alkoxy Derivatives of Layered Perovskite-like Niobate HCa2Nb3O10 and Study of Their Photocatalytic Activity for Hydrogen Production from an Aqueous Solution of Methanol. Catalysts 2021, 11, 897. [Google Scholar] [CrossRef]
- Maeda, K.; Mallouk, T.E. Two-Dimensional Metal Oxide Nanosheets as Building Blocks for Artificial Photosynthetic Assemblies. Bull. Chem. Soc. Jpn. 2018, 92, 38–54. [Google Scholar] [CrossRef]
- Hu, Y.; Mao, L.; Guan, X.; Tucker, K.A.; Xie, H.; Wu, X.; Shi, J. Layered Perovskite Oxides and Their Derivative Nanosheets Adopting Different Modification Strategies towards Better Photocatalytic Performance of Water Splitting. Renew. Sustain. Energy Rev. 2020, 119, 109527. [Google Scholar] [CrossRef]
- Ebina, Y.; Sasaki, T.; Harada, M.; Watanabe, M. Restacked Perovskite Nanosheets and Their Pt-Loaded Materials as Photocatalysts. Chem. Mater. 2002, 1, 4390–4395. [Google Scholar] [CrossRef]
- Zheng, B.; Mao, L.; Shi, J.; Chen, Q.; Hu, Y.; Zhang, G.; Yao, J.; Lu, Y. Facile Layer-by-Layer Self-Assembly of 2D Perovskite Niobate and Layered Double Hydroxide Nanosheets for Enhanced Photocatalytic Oxygen Generation. Int. J. Hydrogen Energy 2021, 46, 34276–34286. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Yuan, H.; Nguyen, M.; Hammer, T.; Koster, G.; Rijnders, G.; Ten Elshof, J.E. Synthesis of KCa2Nb3O10 Crystals with Varying Grain Sizes and Their Nanosheet Monolayer Films As Seed Layers for PiezoMEMS Applications. ACS Appl. Mater. Interfaces 2015, 7, 27473–27478. [Google Scholar] [CrossRef]
- Xu, F.F.; Ebina, Y.; Bando, Y.; Sasaki, T. Structural Characterization of (TBA,H)Ca2Nb3O10 Nanosheets Formed by Delamination of a Precursor-Layered Perovskite. J. Phys. Chem. B 2003, 107, 9638–9645. [Google Scholar] [CrossRef]
- Xu, J.; Xia, B.; Wang, M.; Fan, Z.; Zhang, X.; Ma, J.; Liu, L.; Zhang, B.; Zhang, D.; Tong, Z. A Biosensor Consisting of Ca2Nb3O10− Substrates and Functional Molecule Manganese Porphyrins (MnTMPyP) Utilized for the Determinations of Nitrite. Funct. Mater. Lett. 2018, 11, 1850053. [Google Scholar] [CrossRef]
- Ohisa, S.; Hikichi, T.; Pu, Y.J.; Chiba, T.; Kido, J. Two-Dimensional Ca2Nb3O10 Perovskite Nanosheets for Electron Injection Layers in Organic Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 27885–27893. [Google Scholar] [CrossRef] [PubMed]
- Hojamberdiev, M.; Bekheet, M.F.; Zahedi, E.; Wagata, H.; Kamei, Y.; Yubuta, K.; Gurlo, A.; Matsushita, N.; Domen, K.; Teshima, K. New Dion-Jacobson Phase Three-Layer Perovskite CsBa2Ta3O10 and Its Conversion to Nitrided Ba2Ta3O10 Nanosheets via a Nitridation-Protonation-Intercalation-Exfoliation Route for Water Splitting. Cryst. Growth Des. 2016, 16, 2302–2308. [Google Scholar] [CrossRef]
- Wang, T.H.; Henderson, C.N.; Draskovic, T.I.; Mallouk, T.E. Synthesis, Exfoliation, and Electronic/Protonic Conductivity of the Dion-Jacobson Phase Layer Perovskite HLa2TiTa2O10. Chem. Mater. 2014, 26, 898–906. [Google Scholar] [CrossRef]
- Sakaki, M.; Feng, Y.Q.; Kajiyoshi, K. Ultrasonic-Assisted Exfoliation of Ca2Nb3O10− Nano-Sheets. J. Solid. State Chem. 2019, 277, 253–259. [Google Scholar] [CrossRef]
- Oshima, T.; Nishioka, S.; Kikuchi, Y.; Hirai, S.; Yanagisawa, K.I.; Eguchi, M.; Miseki, Y.; Yokoi, T.; Yui, T.; Kimoto, K.; et al. An Artificial Z-Scheme Constructed from Dye-Sensitized Metal Oxide Nanosheets for Visible Light-Driven Overall Water Splitting. J. Am. Chem. Soc. 2020, 142, 8412–8420. [Google Scholar] [CrossRef]
- Nishioka, S.; Hojo, K.; Xiao, L.; Gao, T.; Miseki, Y.; Yasuda, S.; Yokoi, T.; Sayama, K.; Mallouk, T.E.; Maeda, K. Surface-Modified, Dye-Sensitized Niobate Nanosheets Enabling an Efficient Solar-Driven Z-Scheme for Overall Water Splitting. Sci. Adv. 2022, 8, eadc9115. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Song, Y.; Zou, J.; Wu, L. Preparation of Monolayer HSr2Nb3O10 Nanosheets for Photocatalytic Hydrogen Evolution. Dalt. Trans. 2019, 48, 11136–11141. [Google Scholar] [CrossRef]
- Maeda, K.; Mallouk, T.E. Comparison of Two- and Three-Layer Restacked Dion–Jacobson Phase Niobate Nanosheets as Catalysts for Photochemical Hydrogen Evolution. J. Mater. Chem. 2009, 19, 4813–4818. [Google Scholar] [CrossRef]
- Shi, J.; Mao, L.; Cai, C.; Li, G.; Cheng, C.; Zheng, B.; Hu, Y.; Huang, Z.; Hu, X.; Żyła, G. One-Pot Fabrication of 2D/2D HCa2Nb3O10/g-C3N4 Type II Heterojunctions towards Enhanced Photocatalytic H2 Evolution under Visible-Light Irradiation. Catal. Sci. Technol. 2020, 10, 5896–5902. [Google Scholar] [CrossRef]
- Luo, D.; Huang, Y.; Zhao, Y.; Fang, Y.; Li, Z.; Guo, Q.; Wei, Y.; Fan, L.; Wu, J. Visible-Light-Driven HSr2Nb3O10/CdS Heterojunctions for High Hydrogen Evolution Activity. Int. J. Hydrogen Energy 2020, 45, 2896–2908. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Liang, X.; Shi, X.; Song, Q.; Chen, M.; Jiang, D. Noble-Metal-Free Mo2C Co-Catalsyt Modified Perovskite Oxide Nanosheet Photocatalysts with Enhanced Hydrogen Evolution Performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126252. [Google Scholar] [CrossRef]
- Xie, Z.; Liang, X.; Jiang, D.; Chen, M. Noble-Metal-Free CoxP Nanoparticles: Modified Perovskite Oxide Ultrathin Nanosheet Photocatalysts with Significantly Enhanced Photocatalytic Hydrogen Evolution Activity. Nanotechnology 2020, 31, 325401. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, H.; Li, L.; Mao, B.; Chen, M.; Shen, H.; Shi, W.; Jiang, D.; Lei, Y. Graphene-Sensitized Perovskite Oxide Monolayer Nanosheets for Efficient Photocatalytic Reaction. Adv. Funct. Mater. 2018, 28, 1806284. [Google Scholar] [CrossRef]
- Nishioka, S.; Oshima, T.; Hirai, S.; Saito, D.; Hojo, K.; Mallouk, T.E.; Maeda, K. Excited Carrier Dynamics in a Dye-Sensitized Niobate Nanosheet Photocatalyst for Visible-Light Hydrogen Evolution. ACS Catal. 2021, 11, 659–669. [Google Scholar] [CrossRef]
- Maeda, K.; Sahara, G.; Eguchi, M.; Ishitani, O. Hybrids of a Ruthenium(II) Polypyridyl Complex and a Metal Oxide Nanosheet for Dye-Sensitized Hydrogen Evolution with Visible Light: Effects of the Energy Structure on Photocatalytic Activity. ACS Catal. 2015, 5, 1700–1707. [Google Scholar] [CrossRef]
- Greene, W.N.; Roy, N. Photocatalytic Hydrogen Evolution from Hexaniobate Nanoscrolls and Calcium Niobate Nanosheets Sensitized by Ruthenium(II) Bipyridyl Complexes. J. Phys. Chem. C 2009, 113, 7962–7969. [Google Scholar] [CrossRef]
- Ida, S.; Ogata, C.; Eguchi, M.; Youngblood, W.J.; Mallouk, T.E.; Matsumoto, Y. Photoluminescence of Perovskite Nanosheets Prepared by Exfoliation of Layered Oxides, K2Ln2Ti3O10, KLnNb2O7, and RbLnTa2O7 (Ln: Lanthanide Ion). J. Am. Chem. Soc. 2008, 130, 7052–7059. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Prying Apart Ruddlesden-Popper Phases: Exfoliation into Sheets and Nanotubes for Assembly of Perovskite Thin Films. Solid. State Ion. 2000, 12, 3427–3434. [Google Scholar] [CrossRef]
- Kawashima, K.; Hojamberdiev, M.; Wagata, H.; Yubuta, K.; Domen, K.; Teshima, K. Protonated Oxide, Nitrided, and Reoxidized K2La2Ti3O10 Crystals: Visible-Light-Induced Photocatalytic Water Oxidation and Fabrication of Their Nanosheets. ACS Sustain. Chem. Eng. 2017, 5, 232–240. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Silyukov, O.I.; Minich, I.A.; Zvereva, I.A. Exfoliation of Methylamine and n-Butylamine Derivatives of Layered Perovskite-like Oxides HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) into Nanolayers. Glas. Phys. Chem. 2021, 47, 372–381. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Minich, I.A.; Silyukov, O.I.; Zvereva, I.A. Highly Efficient Liquid-Phase Exfoliation of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) into Nanosheets. Nanomaterials 2023, 13, 3052. [Google Scholar] [CrossRef] [PubMed]
- Kurnosenko, S.A.; Voytovich, V.V.; Silyukov, O.I.; Rodionov, I.A.; Malygina, E.N.; Zvereva, I.A. Influence of HB2Nb3O10-Based Nanosheet Photocatalysts (B = Ca, Sr) Preparation Method on Hydrogen Production Efficiency. Catalysts 2023, 13, 614. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, D.; Huang, Y.; Rong, B.; Chen, X.; Wei, Y.; Fan, L.; Wu, J. Microwave-Mechanochemistry-Assisted Synthesis of Z-Scheme HSr2Nb3O10/WO3 Heterojunctions for Improved Simulated Sunlight Driven Photocatalytic Activity. J. Environ. Chem. Eng. 2021, 9, 104624. [Google Scholar] [CrossRef]
- Xiong, J.; Jing, K.; Zou, J.; Liang, S.; Wu, L. A Hybrid of CdS/HCa2Nb3O10 Ultrathin Nanosheets for Promoting Photocatalytic Hydrogen Evolution. Dalt. Trans. 2017, 46, 13935–13942. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, B.; Jiang, D.; Li, D. CdS Nanoparticles Decorated K+Ca2Nb3O10− Nanosheets with Enhanced Photocatalytic Activity. Mater. Lett. 2018, 229, 236–239. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, T.; Xu, Q.; Li, D.; Meng, S.; Chen, M. Perovskite Oxide Ultrathin Nanosheets/g-C3N4 2D-2D Heterojunction Photocatalysts with Significantly Enhanced Photocatalytic Activity towards the Photodegradation of Tetracycline. Appl. Catal. B Environ. 2017, 201, 617–628. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, D.; Xiao, P.; Jin, Y.; Meng, S.; Chen, M. 2D/2D Heterojunctions of WO3 Nanosheet/K+Ca2Nb3O10− Ultrathin Nanosheet with Improved Charge Separation Efficiency for Significantly Boosting Photocatalysis. Catal. Sci. Technol. 2017, 7, 3481–3491. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Voytovich, V.V.; Silyukov, O.I.; Minich, I.A.; Malygina, E.N.; Zvereva, I.A. Inorganic-Organic Derivatives of Layered Perovskite-like Titanates HLnTiO4 (Ln = La, Nd) with n-Amines and n-Alcohols: Synthesis, Thermal, Vacuum and Hydrolytic Stability. Ceram. Int. 2022, 48, 7240–7252. [Google Scholar] [CrossRef]
- Bie, C.; Wang, L.; Yu, J. Challenges for Photocatalytic Overall Water Splitting. Chem 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A Review of Hydrogen Production Processes by Photocatalytic Water Splitting—from Atomistic Catalysis Design to Optimal Reactor Engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Hota, P.; Das, A.; Maiti, D.K. A Short Review on Generation of Green Fuel Hydrogen through Water Splitting. Int. J. Hydrogen Energy 2022, 48, 523–541. [Google Scholar] [CrossRef]


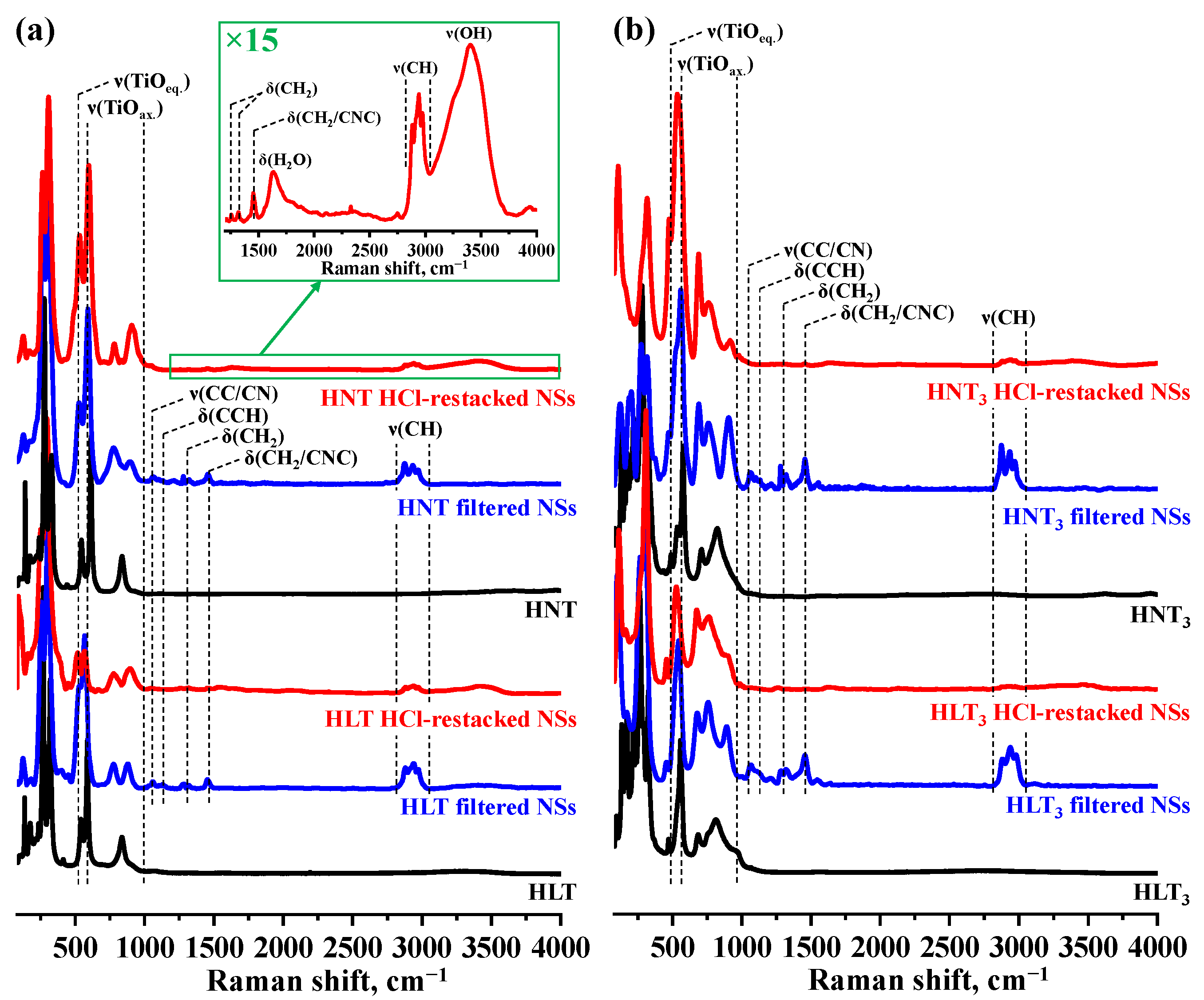
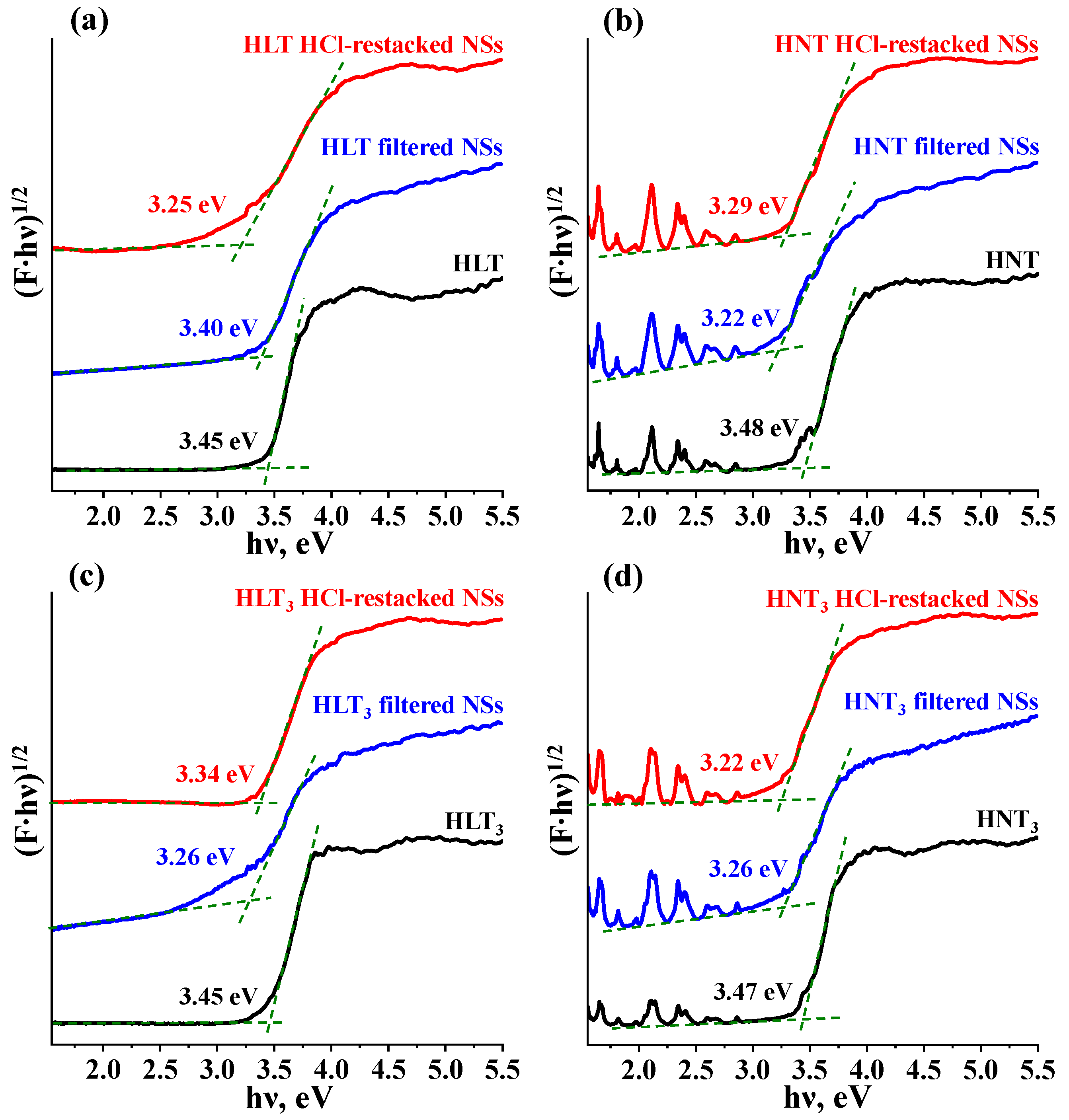

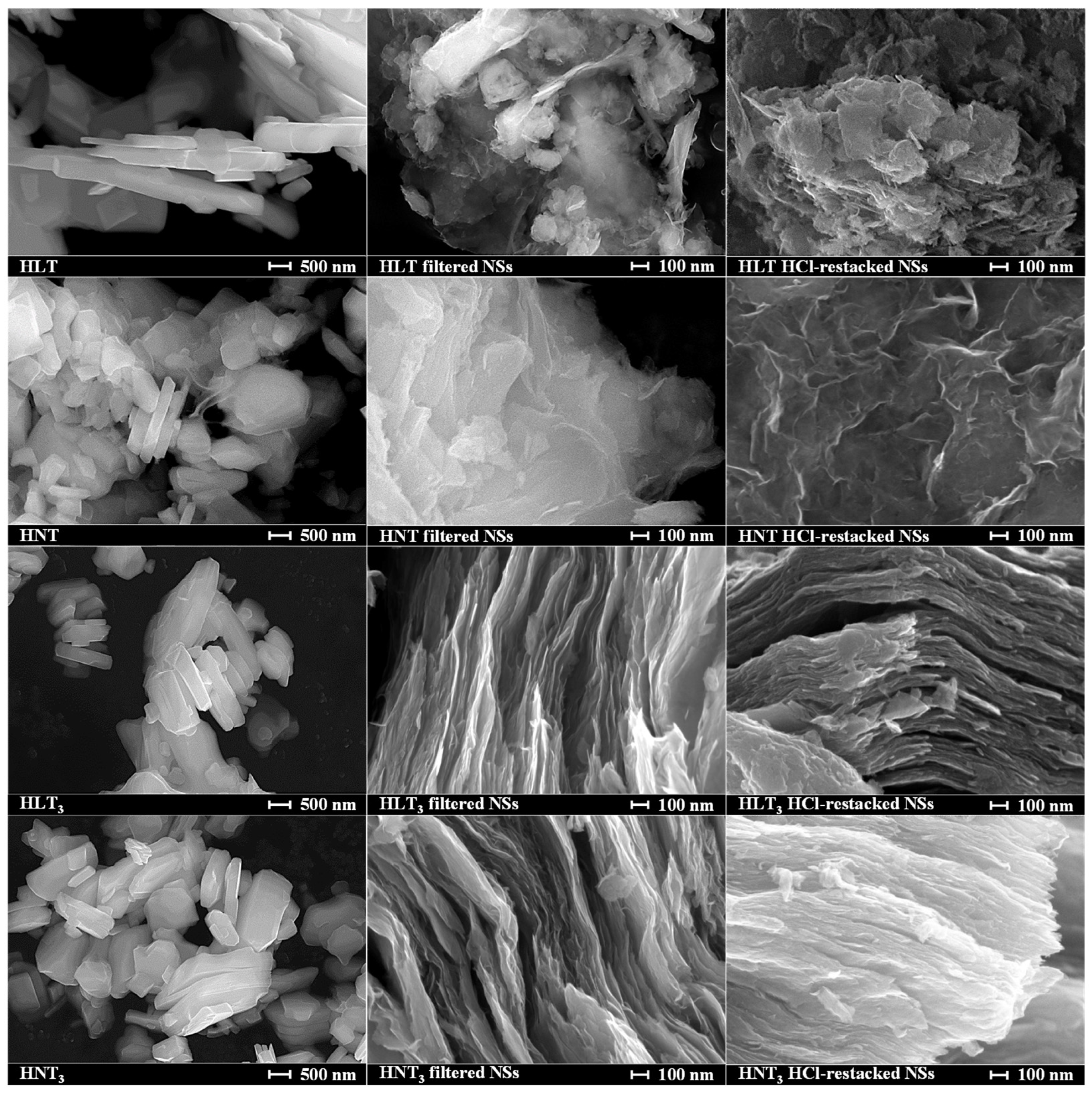
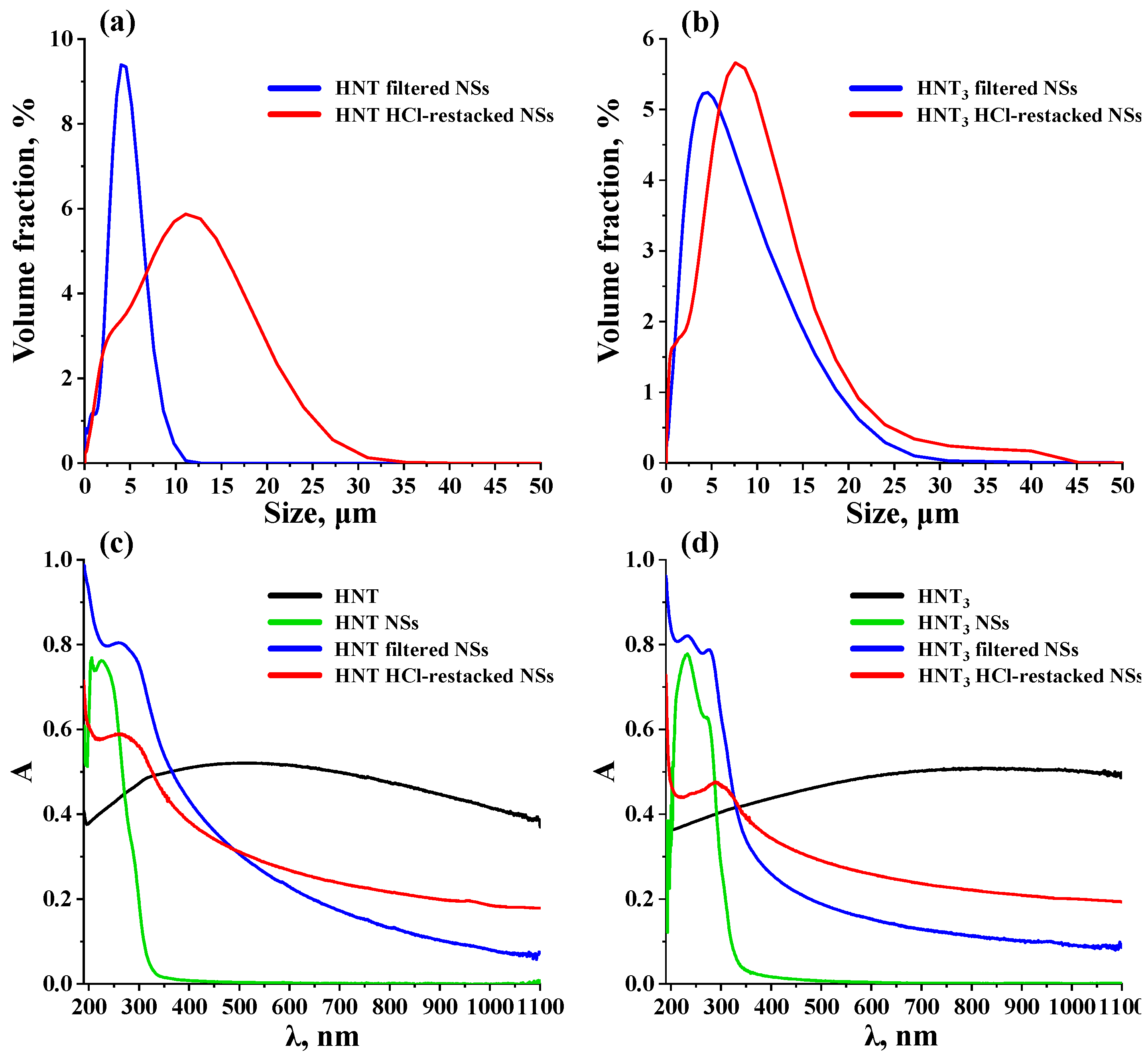

| Sample | a, Å | c, Å | d, Å | x | y | Eg, eV | λabs, nm | τ, μs (λem, nm) | S, m2/g |
|---|---|---|---|---|---|---|---|---|---|
| HLT | 3.7 | 12.2 | 12.2 | − | 0.05 | 3.45 | 359 | 4.70 (360) | 9.7 |
| HLT filtered NSs | ≈3.7 | ≈14.0 | ≈14.0 | 0.05 | 0.40 | 3.40 | 365 | 0.0071 (420) | 54 |
| HLT HCl-restacked NSs | ≈3.7 | ≈14.0 | ≈14.0 | 0.02 | 0.40 | 3.25 | 382 | − | 73 |
| HNT | 3.7 | 12.1 | 12.1 | − | 0.10 | 3.48 | 356 | 4.77 (360) | 8.6 |
| HNT filtered NSs | ≈3.7 | ≈16.0 | ≈16.0 | 0.05 | 0.45 | 3.22 | 385 | 0.0042 (420) | 64 |
| HNT HCl-restacked NSs | ≈3.7 | ≈16.0 | ≈16.0 | 0.01 | 0.75 | 3.29 | 377 | − | 85 |
| HLT3 | 3.8 | 27.2 | 13.6 | − | 0.15 | 3.45 | 359 | 4.73 (370) | 3.2 |
| HLT3 filtered NSs | ≈3.8 | ≈24.0 | ≈24.0 | 0.20 | 0.60 | 3.26 | 380 | 0.0081 (430) | 21 |
| HLT3 HCl-restacked NSs | ≈3.8 | ≈15.5 | ≈15.5 | 0.02 | 1.10 | 3.34 | 371 | 0.0063 (430) | 60 |
| HNT3 | 3.8 | 27.2 | 13.6 | − | 0.15 | 3.47 | 357 | 5.42 (370) | 3.1 |
| HNT3 filtered NSs | ≈3.8 | ≈19.5 | ≈19.5 | 0.20 | 1.20 | 3.26 | 380 | 0.0075 (430) | 15 |
| HNT3 HCl-restacked NSs | ≈3.8 | ≈15.5 | ≈15.5 | 0.02 | 1.20 | 3.22 | 385 | 0.0058 (430) | 47 |
| Photocatalyst | 1 mol.% CH3OH | H2O | ||||
|---|---|---|---|---|---|---|
| ω (H2), μmol/h | φ, % | kPt | ω (H2), μmol/h | φ, % | kPt | |
| HLT | 0.73 | 0.010 | − | 0.03 | 0.0007 | − |
| HLT/Pt | 14.2 | 0.19 | 19 | 0.66 | 0.014 | 22 |
| HLT NSs | 7.21 | 0.12 | − | 0.45 | 0.007 | − |
| HLT NSs/Pt | 225 | 3.72 | 31 | 33.2 | 0.55 | 74 |
| HLT filtered NSs | 2.71 | 0.045 | − | 0.34 | 0.006 | − |
| HLT filtered NSs/Pt | 209 | 3.46 | 77 | 15.7 | 0.26 | 46 |
| HLT HCl-restacked NSs | 2.27 | 0.023 | − | 0.24 | 0.002 | − |
| HLT HCl-restacked NSs/Pt | 37.2 | 0.38 | 16 | 8.48 | 0.087 | 35 |
| HNT | 0.73 | 0.010 | − | 0.04 | 0.0009 | − |
| HNT/Pt | 6.23 | 0.083 | 8.5 | 0.11 | 0.002 | 2.8 |
| HNT NSs | 3.11 | 0.032 | − | 0.37 | 0.004 | − |
| HNT NSs/Pt | 11.2 | 0.12 | 3.6 | 0.81 | 0.008 | 2.2 |
| HNT filtered NSs | 0.94 | 0.010 | − | 0.15 | 0.002 | − |
| HNT filtered NSs/Pt | 9.91 | 0.10 | 11 | 0.48 | 0.005 | 3.2 |
| HNT HCl-restacked NSs | 0.51 | 0.005 | − | 0.03 | 0.0003 | − |
| HNT HCl-restacked NSs/Pt | 7.95 | 0.082 | 16 | 0.25 | 0.003 | 8.3 |
| HLT3 | 1.27 | 0.017 | − | 0.08 | 0.002 | − |
| HLT3/Pt | 85.1 | 1.14 | 67 | 19.7 | 0.43 | 246 |
| HLT3 NSs | 112 | 1.16 | − | 20.9 | 0.21 | − |
| HLT3 NSs/Pt | 1370 | 14.2 | 12 | 139 | 1.42 | 6.7 |
| HLT3 filtered NSs | 19.6 | 0.20 | − | 4.15 | 0.043 | − |
| HLT3 filtered NSs/Pt | 1190 | 12.3 | 61 | 305 | 3.15 | 73 |
| HLT3 HCl-restacked NSs | 14.1 | 0.14 | − | 3.01 | 0.031 | − |
| HLT3 HCl-restacked NSs/Pt | 573 | 5.91 | 41 | 98.6 | 1.02 | 33 |
| HNT3 | 2.00 | 0.027 | − | 0.05 | 0.001 | − |
| HNT3/Pt | 69.0 | 0.92 | 35 | 15.9 | 0.35 | 318 |
| HNT3 NSs | 21.4 | 0.22 | − | 2.10 | 0.022 | − |
| HNT3 NSs/Pt | 1260 | 13.0 | 59 | 70.6 | 0.73 | 34 |
| HNT3 filtered NSs | 9.73 | 0.10 | − | 1.39 | 0.014 | − |
| HNT3 filtered NSs/Pt | 523 | 5.39 | 54 | 142 | 1.46 | 102 |
| HNT3 HCl-restacked NSs | 8.57 | 0.088 | − | 0.58 | 0.006 | − |
| HNT3 HCl-restacked NSs/Pt | 360 | 3.70 | 42 | 15.0 | 0.15 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurnosenko, S.A.; Silyukov, O.I.; Rodionov, I.A.; Minich, I.A.; Zvereva, I.A. Pristine and Reassembled Nanosheets of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) as Photocatalysts for Hydrogen Evolution. Solids 2025, 6, 16. https://doi.org/10.3390/solids6020016
Kurnosenko SA, Silyukov OI, Rodionov IA, Minich IA, Zvereva IA. Pristine and Reassembled Nanosheets of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) as Photocatalysts for Hydrogen Evolution. Solids. 2025; 6(2):16. https://doi.org/10.3390/solids6020016
Chicago/Turabian StyleKurnosenko, Sergei A., Oleg I. Silyukov, Ivan A. Rodionov, Iana A. Minich, and Irina A. Zvereva. 2025. "Pristine and Reassembled Nanosheets of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) as Photocatalysts for Hydrogen Evolution" Solids 6, no. 2: 16. https://doi.org/10.3390/solids6020016
APA StyleKurnosenko, S. A., Silyukov, O. I., Rodionov, I. A., Minich, I. A., & Zvereva, I. A. (2025). Pristine and Reassembled Nanosheets of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) as Photocatalysts for Hydrogen Evolution. Solids, 6(2), 16. https://doi.org/10.3390/solids6020016







