Optimizing the Mechanical Properties and Corrosion Performance of Low-Alloyed Mg-Zn-Ca Alloy by Regulating Zn/Ca Atomic Ratios
Abstract
1. Introduction
2. Experimental Section
2.1. Material Preparation
2.2. Microstructural Analysis
2.3. Mechanical Performance
2.4. Electrochemical Performance Testing
2.5. In Vitro Immersion Testing
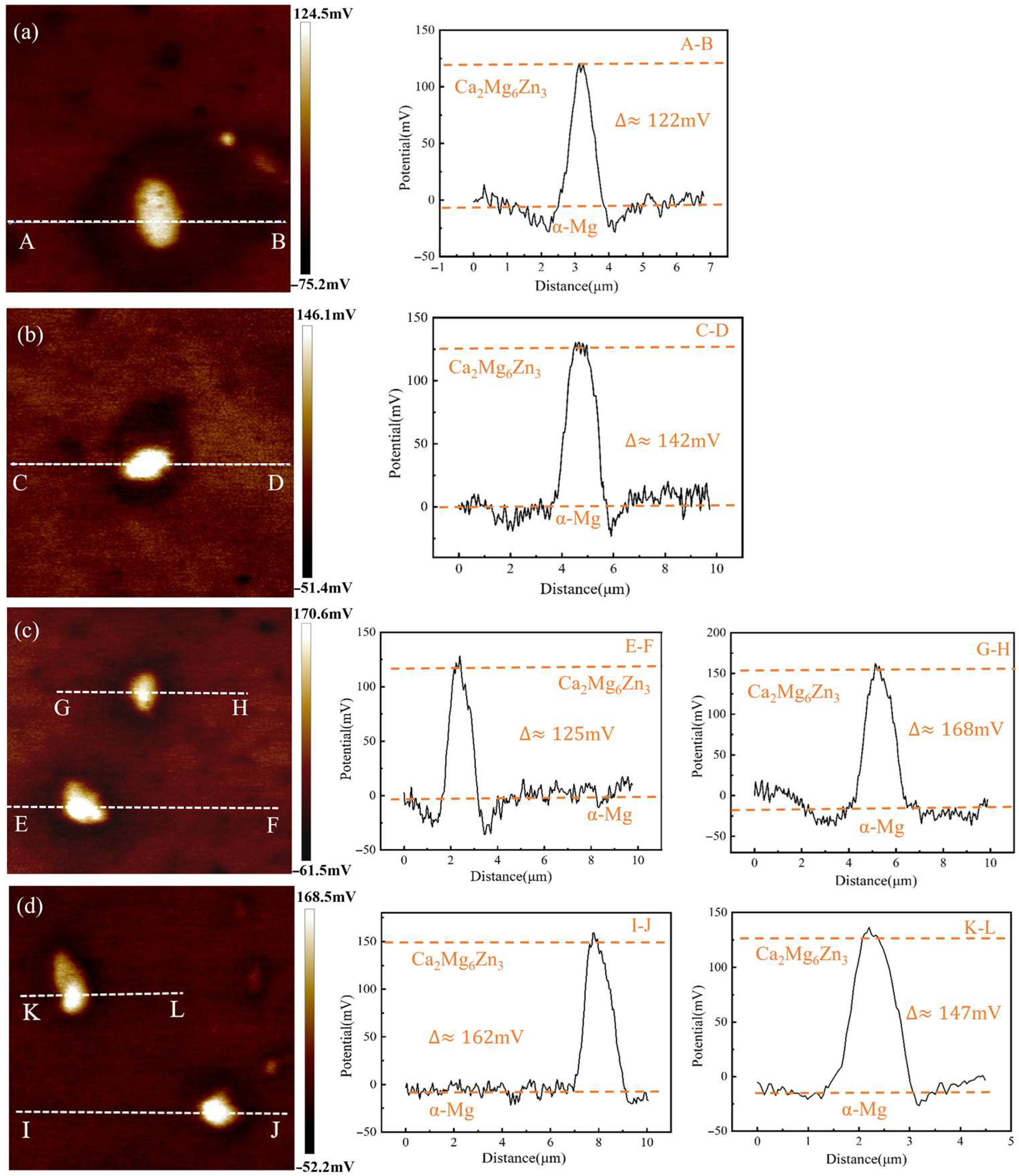
2.6. Second-Phase Potential Testing
2.7. Quasi-In Situ Corrosion Testing
3. Results
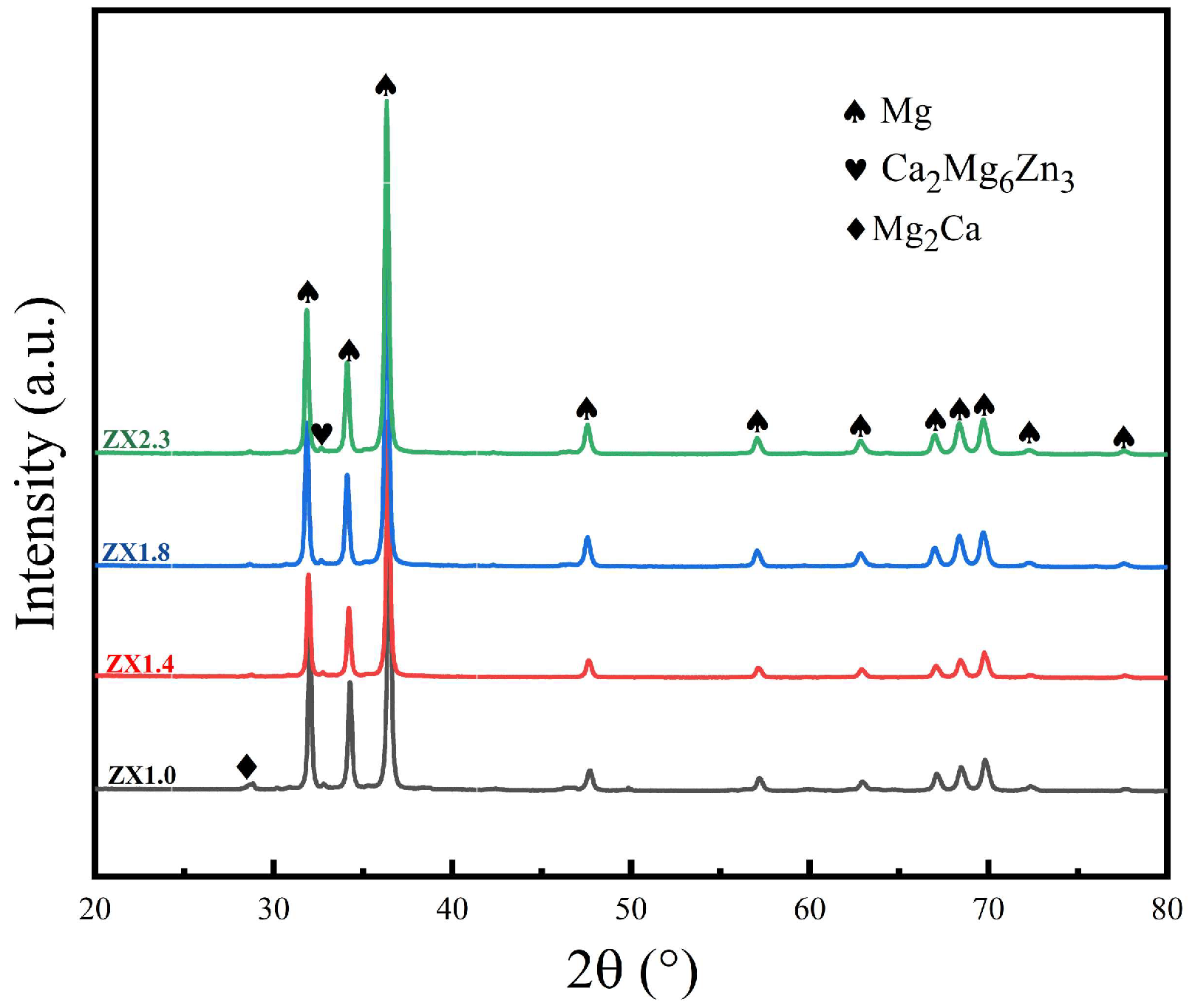
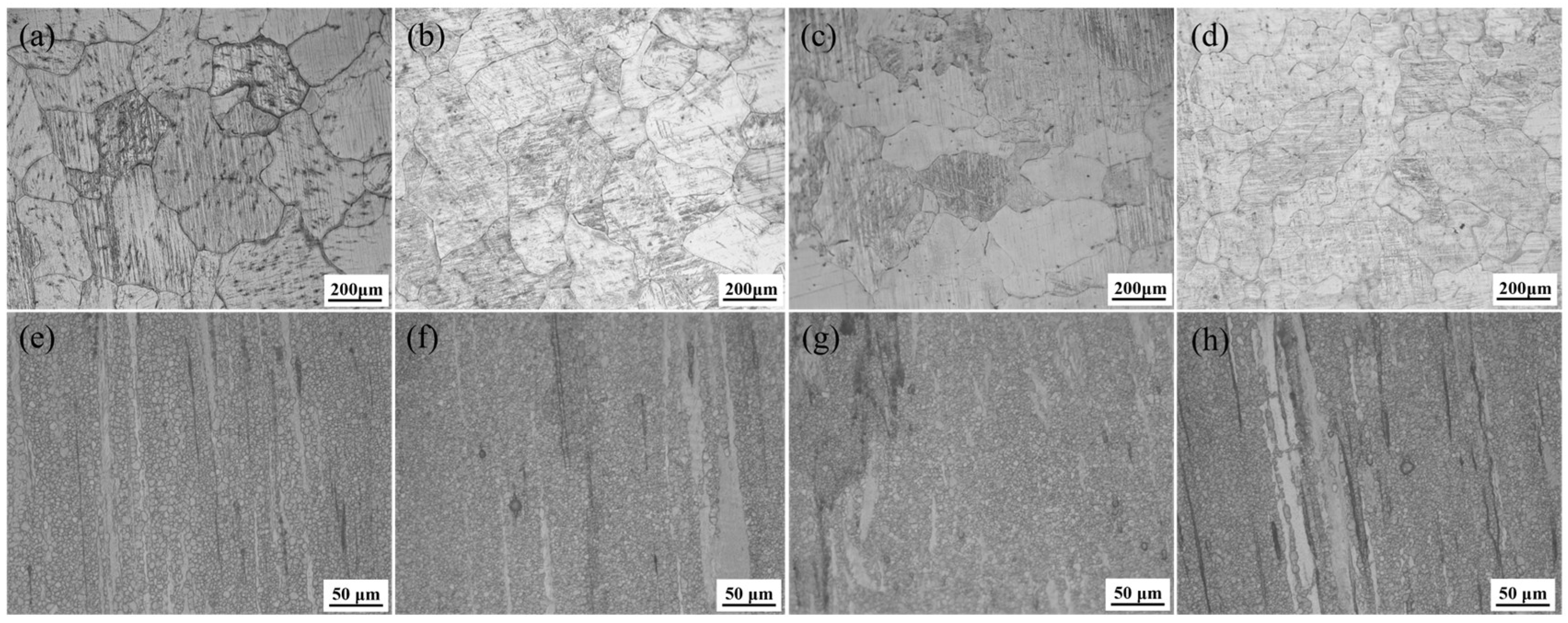
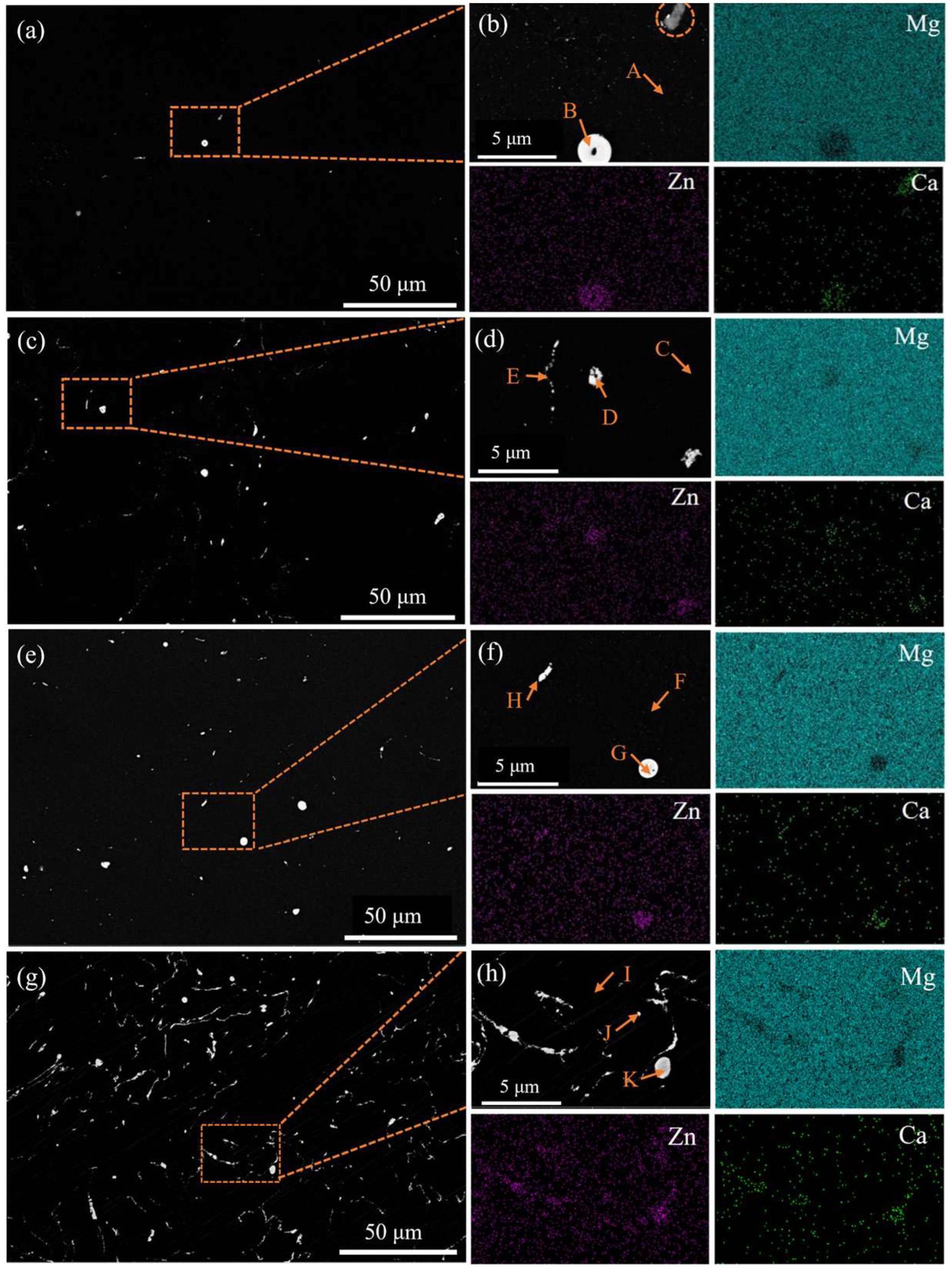
3.1. Microstructural Characterization
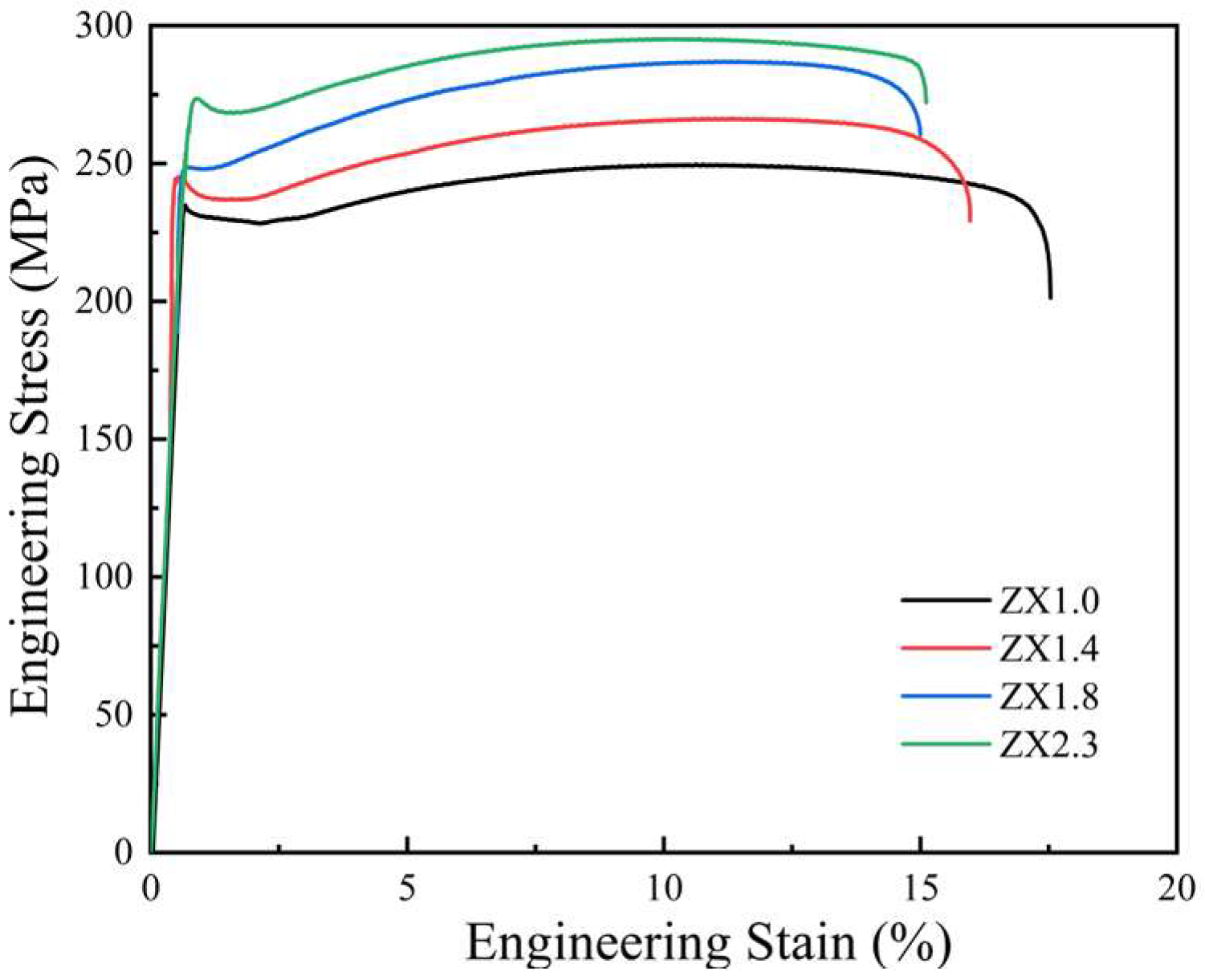
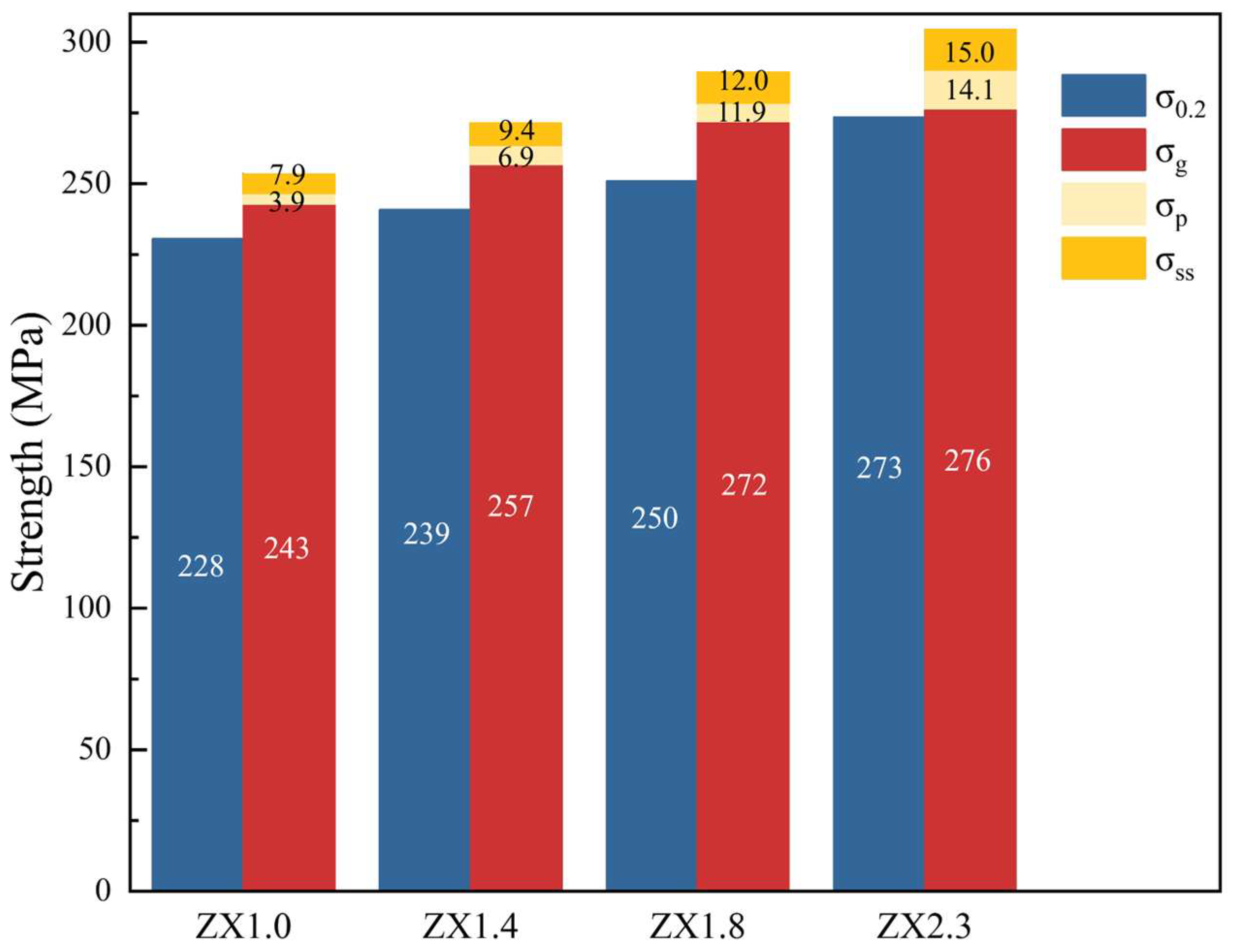
3.2. Mechanical Properties
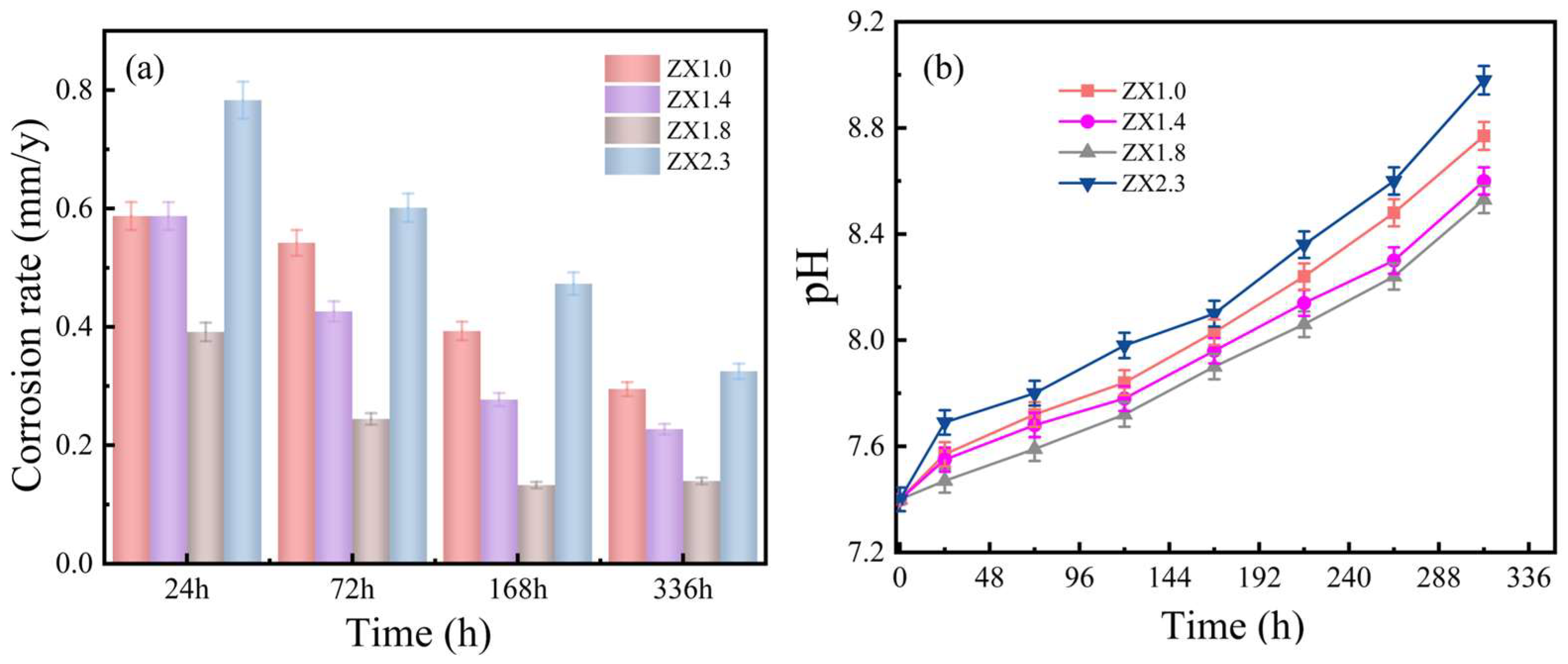
3.3. Electrochemical Testing
4. Discussion
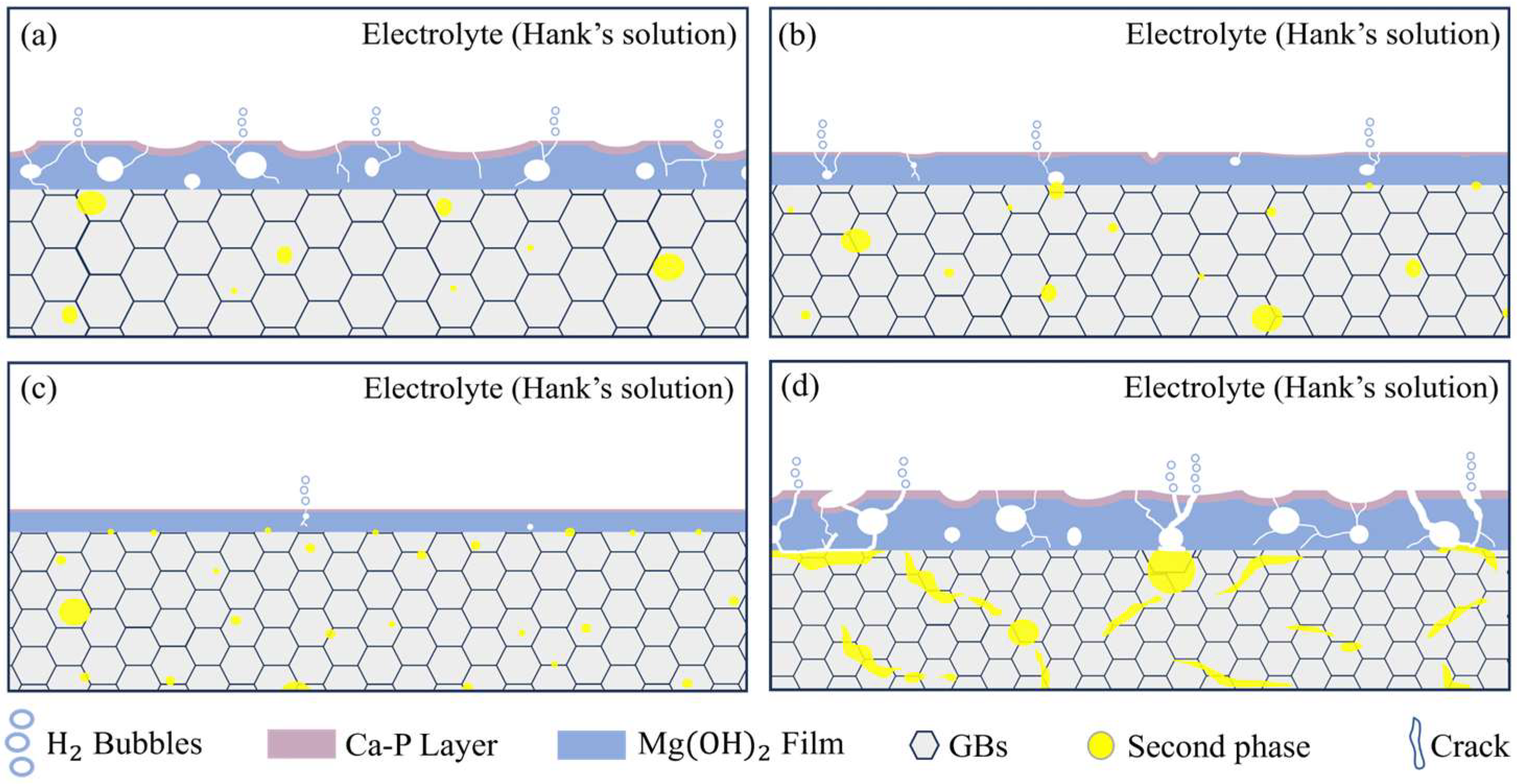
4.1. Impact of Zn/Ca Ratio on the Microstructure
4.2. Influence of the Zn/Ca Ratio on the Mechanical Properties
4.3. Effect of Zn/Ca Ratio on Corrosion Resistance of Alloys
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Zhu, J.; Ge, N.; Liu, Y.; Yan, Z.; Liu, G.; Li, Y.; Wang, Y.; Wu, G.; Qiu, T.; et al. A biodegradable magnesium alloy promotes subperiosteal osteogenesis via interleukin-10-dependent macrophage immunomodulation. Biomaterials 2025, 318, 122992. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Hashmi, A.W.; Ahmad, S.; Tian, Y. Critical review on biodegradable and biocompatibility magnesium alloys: Progress and prospects in bio-implant applications. Inorg. Chem. Commun. 2024, 169, 113111. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Wang, J.; Lai, Y.; Qin, L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2012, 100B, 1691–1701. [Google Scholar] [CrossRef]
- Baigonakova, G.; Marchenko, E.; Zhukov, I.; Vorozhtsov, A. Structure, cytocompatibility and biodegradation of nanocrystalline coated Mg-Ca-Zn alloys. Vacuum 2022, 207, 111630. [Google Scholar] [CrossRef]
- Kumar Rout, P.; Roy, S.; Rathore, D. Recent advances in the development of Mg-Ca-Zn alloys as biodegradable orthopedic implants. Mater. Today Proc. 2024, 115, 24–30. [Google Scholar] [CrossRef]
- Eide, D.J. Transcription factors and transporters in zinc homeostasis: Lessons learned from fungi. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 88–110. [Google Scholar] [CrossRef]
- Bowen, P.K.; Guillory, R.J.; Shearier, E.R.; Seitz, J.-M.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C 2015, 56, 467–472. [Google Scholar] [CrossRef]
- Freeman, S.P.H.T.; Beck, B.; Bierman, J.M.; Caffee, M.W.; Heaney, R.P.; Holloway, L.; Marcus, R.; Southon, J.R.; Vogel, J.S. The study of skeletal calcium metabolism with 41Ca and 45Ca. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 2000, 172, 930–933. [Google Scholar] [CrossRef]
- Zareian, Z.; Emamy, M.; Malekan, M.; Mirzadeh, H.; Kim, W.; Bahmani, A. Tailoring the mechanical properties of Mg-Zn magnesium alloy by calcium addition and hot extrusion process. Mater. Sci. Eng. A 2020, 774, 138929. [Google Scholar] [CrossRef]
- Jiang, P.; Blawert, C.; Hou, R.; Bohlen, J.; Konchakova, N.; Zheludkevich, M.L. A comprehensive comparison of the corrosion performance, fatigue behavior and mechanical properties of micro-alloyed MgZnCa and MgZnGe alloys. Mater. Des. 2020, 185, 108285. [Google Scholar] [CrossRef]
- Yin, J.; Li, M.; Yi, F.; Zhao, X.; Guan, D.; Wang, K.; Gao, Y.; Liu, C. Effects of micro-alloying Ag on microstructure, mechanical properties and corrosion behavior of extruded Mg-2Zn-0.2Ca-xAg alloys. J. Alloys Compd. 2024, 989, 174376. [Google Scholar] [CrossRef]
- Du, Y.; Zheng, M.; Xu, C.; Qiao, X.; Wu, K.; Liu, X.; Wang, G.; Lv, X. Microstructures and mechanical properties of as-cast and as-extruded Mg-4.50Zn-1.13Ca (wt.%) alloys. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2013, 576, 6–13. [Google Scholar] [CrossRef]
- Zhang, B.P.; Geng, L.; Huang, L.; Zhang, X.; Dong, C. Enhanced mechanical properties in fine-grained Mg-1.0Zn-0.5Ca alloys prepared by extrusion at different temperatures. Scr. Mater. 2010, 63, 1024–1027. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation and characterization of a new biomedical Mg-Zn-Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Hofstetter, J.; Rüedi, S.; Baumgartner, I.; Kilian, H.; Mingler, B.; Povoden-Karadeniz, E.; Pogatscher, S.; Uggowitzer, P.; Löffler, J. Processing and microstructure–property relations of high-strength low-alloy (HSLA) Mg-Zn-Ca alloys. Acta Mater. 2015, 98, 423–432. [Google Scholar] [CrossRef]
- Ma, Y.-Z.; Yang, C.-L.; Liu, Y.-J.; Yuan, F.-S.; Liang, S.-S.; Li, H.-X.; Zhang, J.-S. Microstructure, mechanical, and corrosion properties of extruded low-alloyed Mg-xZn-0.2Ca alloys. Int. J. Miner. Met. Mater. 2019, 26, 1274–1284. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, K.; Li, X.; Qiao, Y. Microstructure and properties of biomedical Mg-Zn-Ca alloy at different extrusion temperatures. Mater. Today Commun. 2023, 35, 105578. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg-Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Ibrahim, H.; Moghaddam, N.S.; Elahinia, M. Mechanical and In Vitro Corrosion Properties of a Heat-Treated Mg-Zn-Ca-Mn Alloy as a Potential Bioresorbable Material. Adv. Metall. Mater. Eng. 2017, 1, 1. [Google Scholar] [CrossRef]
- Goel, V.; Montiel, D.; Thornton, K. Understanding the Effect of Electrochemical Properties and Microstructure on the Microgalvanic Corrosion of Mg Alloys via Phase-Field Simulations. J. Electrochem. Soc. 2023, 170, 101502. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhao, C.; Li, H.; Liang, H.; Wang, S.; Zhai, C.; Du, W. Boosting the performance of primary Mg-air battery by regulating the second phase in the Mg-2Zn-0.2Ca anode. J. Energy Storage 2024, 94, 112332. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Ikeo, N.; Nishioka, M.; Mukai, T. Fabrication of biodegradable materials with high strength by grain refinement of Mg-0.3 at.%Ca alloys. Mater. Lett. 2018, 223, 65–68. [Google Scholar] [CrossRef]
- Zhang, A.; Kang, R.; Wu, L.; Pan, H.; Xie, H.; Huang, Q.; Liu, Y.; Ai, Z.; Ma, L.; Ren, Y.; et al. A new rare-earth-free Mg-Sn-Ca-Mn wrought alloy with ultra-high strength and good ductility. Mater. Sci. Eng. A 2019, 754, 269–274. [Google Scholar] [CrossRef]
- Li, S.; Tang, B.; Zeng, D. Effects and mechanism of Ca on refinement of AZ91D alloy. J. Alloys Compd. 2007, 437, 317–321. [Google Scholar] [CrossRef]
- Nie, K.; Zhu, Z.; Munroe, P.; Deng, K.; Han, J. The effect of Zn/Ca ratio on the microstructure, texture and mechanical properties of dilute Mg-Zn-Ca–Mn alloys that exhibit superior strength. J. Mater. Sci. 2020, 55, 3588–3604. [Google Scholar] [CrossRef]
- Tian, Y.; Shao, Y.; Yu, Q.; Guo, S.; Dang, R.; Du, Y.; Zhu, G.; Lyu, S.; Chen, M. Investigating the corrosion behavior of as-cast Mg-Zn-Ca alloys with the same Zn/Ca atomic ratio by in situ observation. Mater. Corros. 2024, 75, 1472–1483. [Google Scholar] [CrossRef]
- Le, T.; Mao, P.; Hu, W.; Le, Q. Effects of Zn/Ca Alloying on the Second Phase, Corrosion Behavior, and Mg–Air Battery Anodic Performance of Mg–1Sn-Based Alloys. Adv. Eng. Mater. 2024, 26, 2301130. [Google Scholar] [CrossRef]
- Mi, J.; Liang, S.; Luan, S.; Ren, Q.; Feng, Y.; Cui, J.; Che, X.; Jin, P. Precipitation phase conduction <a> slip improves the plasticity of Mg-Zn-Mn-Ca alloy by microalloying with Nd and La elements. Mater. Today Commun. 2025, 43, 111550. [Google Scholar] [CrossRef]
- Jing, X.; Zhou, S.; Ouyang, S.; She, J.; Peng, P.; Tang, A.; Pan, F. Unveiling synergistic strength and plasticity enhancement of Mg-Zn-Mn alloy via closed forging extrusion. J. Mater. Res. Technol. 2024, 31, 3466–3479. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, W.; Wu, G.; Wang, S.; Zhang, J.; Xiao, Y.; Mao, X. A high-performance TRIP Mg-Sc-Zn alloy enhanced by fine grain strengthening and nano-precipitate strengthening. J. Mater. Res. Technol. 2024, 33, 3874–3881. [Google Scholar] [CrossRef]
- Wang, L.; Ren, Y.; Sun, S.; Zhao, H.; Li, S.; Qin, G.-W. Microstructure, Mechanical Properties and Fracture Behavior of As-Extruded Zn–Mg Binary Alloys. Acta Met. Sin. Engl. Lett. 2017, 30, 931–940. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Ferdowsi, M.R.G.; Kada, S.R.; Dorin, T.; Barnett, M.R.; Perez, M. A coupled model for precipitation strengthening in Mg-Zn alloys. Acta Mater. 2024, 281, 120392. [Google Scholar] [CrossRef]
- Wang, J.; Ferdowsi, M.R.G.; Kada, S.R.; Lynch, P.A.; Wang, Z.; Kimpton, J.A.; Barnett, M.R. Stress relaxations during cyclic loading-unloading in precipitation hardened Mg-4.5Zn. Acta Mater. 2021, 205, 116531. [Google Scholar] [CrossRef]
- Shi, D.F.; Pérez-Prado, M.T.; Cepeda-Jiménez, C.M. Effect of solutes on strength and ductility of Mg alloys. Acta Mater. 2019, 180, 218–230. [Google Scholar] [CrossRef]
- Pande, C.S.; Cooper, K.P. Nanomechanics of Hall–Petch relationship in nanocrystalline materials. Prog. Mater. Sci. 2009, 54, 689–706. [Google Scholar] [CrossRef]
- Yu, H.; Xin, Y.; Wang, M.; Liu, Q. Hall-Petch relationship in Mg alloys: A review. J. Mater. Sci. Technol. 2018, 34, 248–256. [Google Scholar] [CrossRef]
- Ye, L.; Huang, H.; Sun, C.; Zhuo, X.; Dong, Q.; Liu, H.; Ju, J.; Xue, F.; Bai, J.; Jiang, J. Effect of grain size and volume fraction of eutectic structure on mechanical properties and corrosion behavior of as-cast Zn–Mg binary alloys. J. Mater. Res. Technol. 2022, 16, 1673–1685. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5Mg alloy. J. Alloys Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Nie, K.B.; Deng, K.K.; Han, J. Fabrication of biodegradable magnesium matrix composite with ultrafine grains and high strength by adding TiC nanoparticles to Mg-1.12Ca-0.84Zn-0.23Mn (at.%) alloy. Mater. Sci. Eng. C 2020, 107, 110360. [Google Scholar] [CrossRef] [PubMed]
- Schäublin, R.E.; Becker, M.; Cihova, M.; Gerstl, S.; Deiana, D.; Hébert, C.; Pogatscher, S.; Uggowitzer, P.; Löffler, J. Precipitation in lean Mg-Zn-Ca alloys. Acta Mater. 2022, 239, 118223. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Cubides, Y.; Zhao, D.; Nash, L.; Yadav, D.; Xie, K.; Karaman, I.; Castaneda, H. Effects of dynamic recrystallization and strain-induced dynamic precipitation on the corrosion behavior of partially recrystallized Mg–9Al–1Zn alloys. J. Magnes. Alloy. 2020, 8, 1016–1037. [Google Scholar] [CrossRef]
- Cao, X.; Cheng, X.; Wang, T.; Zhong, F.; Wu, B.; Liang, M.; Li, J. Elucidating the dependence of corrosion behavior and mechanical property on the grain structure of low-temperature as-extruded Mg-Zn-Ca-Zr alloy. J. Mater. Sci. 2024, 59, 11122–11142. [Google Scholar] [CrossRef]
- Lei, L.; Cui, Z.; Pan, H.; Pang, K.; Wang, X.; Cui, H. Effect of extrusion on the microstructure and corrosion behavior of Mg-Zn-Mn-(0, 1.5) Sr alloys in Hank’s solution. Corros. Sci. 2022, 195, 109975. [Google Scholar] [CrossRef]
- Li, H.; Qin, S.; Ma, Y.; Wang, J.; Liu, Y.-J.; Zhang, J.-S. Effects of Zn content on the microstructure and the mechanical and corrosion properties of as-cast low-alloyed Mg-Zn-Ca alloys. Int. J. Miner. Met. Mater. 2018, 25, 800–809. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, H. Illustrating the influence of anodic/cathodic second-phases on dissolution mode via Mg-Ca-Sn alloy anodes for primary Mg-air cells. J. Mater. Res. Technol. 2024, 33, 6102–6113. [Google Scholar] [CrossRef]
- Roh, H.; Park, J.; Lee, S.; Kim, D.-H.; Lee, G.-C.; Jeon, H.; Chae, M.; Lee, K.-S.; Sun, J.-Y.; Lee, D.-H.; et al. Optimization of the clinically approved Mg-Zn alloy system through the addition of Ca. Biomater. Res. 2022, 26, 41. [Google Scholar] [CrossRef]




| Sample | Mg | Zn | Ca | Al | Cu | Ni | Fe | Si |
|---|---|---|---|---|---|---|---|---|
| ZX1.0 | 98.77 | 0.99 | 0.24 | 0.0003 | 0.0003 | 0.0003 | 0.0014 | 0.0005 |
| ZX1.4 | 98.42 | 1.31 | 0.27 | 0.0003 | 0.0003 | 0.0003 | 0.0013 | 0.0005 |
| ZX1.8 | 97.86 | 1.86 | 0.28 | 0.0003 | 0.0003 | 0.0003 | 0.0014 | 0.0005 |
| ZX2.3 | 97.23 | 2.35 | 0.42 | 0.0003 | 0.0003 | 0.0003 | 0.0013 | 0.0005 |
| NaCl | MgSO4·7H2O | KCl | Na2HPO4·12H2O | MgCl2·6H2O | CaCl2 | KH2PO4 | NaHCO3 | C2H12O6 |
|---|---|---|---|---|---|---|---|---|
| 0.16 | 0.002 | 0.008 | 0.0024 | 0.002 | 0.0028 | 0.0012 | 0.007 | 0.02 |
| Alloy | Zn/Ca | α-Mg Solidification Temperature (°C) | Mg2Ca Phase Precipitation Temperature (°C) | Ca2Mg6Zn3 Phase Precipitation Temperature (°C) | Final Microstructure |
|---|---|---|---|---|---|
| ZX1.0 | 2 | 645.4 | 417.3 | 312.2 | α-Mg + Ca2Mg6Zn3 |
| ZX1.4 | 3 | 644.2 | 417.3 | 360 | α-Mg + Ca2Mg6Zn3 |
| ZX1.8 | 4 | 643.1 | 417.3 | 401.8 | α-Mg + Ca2Mg6Zn3 |
| ZX2.3 | 3 | 640.5 | 417.3 | 426.1 | α-Mg + Ca2Mg5Zn13 |
| Composition (at. %) | |||
|---|---|---|---|
| Spots | Mg | Zn | Ca |
| (b) A | 99.3 | 0.7 | - |
| (b) B | 80.93 | 9.68 | 9.4 |
| (d) C | 99.09 | 0.91 | - |
| (d) D | 82.9 | 10.32 | 6.78 |
| (d) E | 96.19 | 2.02 | 1.79 |
| (f) F | 98.67 | 1.33 | - |
| (f) G | 74.8 | 13.79 | 11.41 |
| (f) H | 74.12 | 14.29 | 11.59 |
| (h) I | 98.17 | 1.83 | - |
| (h) J | 69.05 | 16.2 | 14.74 |
| (h) K | 74.21 | 14.82 | 10.97 |
| Alloy Composition | Icorr (μA/cm2) | Ecorr (V/SCE) | Pi (mm/y) | |||
|---|---|---|---|---|---|---|
| X1 | n1 | X2 | n2 | X3 | n3 | |
| ZX1.0 | 4.27 | 0.04 | −1.312 | 0.04 | 0.95 | 0.0032 |
| ZX1.4 | 3.36 | 0.003 | −1.309 | 0.003 | 0.767 | 0.002 |
| ZX1.8 | 3.33 | 0.008 | −1.301 | 0.01 | 0.760 | 0.005 |
| ZX2.3 | 5.13 | 0.03 | −1.320 | 0.01 | 1.17 | 0.01 |
| Material Composition | R1 (Ω·cm2) | R2 (Ω·cm2) | R3 (Ω·cm2) | L (L/cm2) | ||||
|---|---|---|---|---|---|---|---|---|
| Y1 | n1 | Y2 | n2 | Y3 | n3 | Y4 | n4 | |
| ZX1.0 | 32 | 2.4 | 2768 | 301 | - | - | - | - |
| ZX1.4 | 33.8 | 3.7 | 5495 | 451 | - | - | - | - |
| ZX1.8 | 36.5 | 4.1 | 6536 | 428 | - | - | - | - |
| ZX2.3 | 34 | 4.5 | 64.11 | 6.6 | 1747 | 81 | 3093 | 208.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Lyu, S.; Yu, Q.; Chen, M. Optimizing the Mechanical Properties and Corrosion Performance of Low-Alloyed Mg-Zn-Ca Alloy by Regulating Zn/Ca Atomic Ratios. Solids 2025, 6, 17. https://doi.org/10.3390/solids6020017
Jin Y, Lyu S, Yu Q, Chen M. Optimizing the Mechanical Properties and Corrosion Performance of Low-Alloyed Mg-Zn-Ca Alloy by Regulating Zn/Ca Atomic Ratios. Solids. 2025; 6(2):17. https://doi.org/10.3390/solids6020017
Chicago/Turabian StyleJin, Yuan, Shaoyuan Lyu, Qianqian Yu, and Minfang Chen. 2025. "Optimizing the Mechanical Properties and Corrosion Performance of Low-Alloyed Mg-Zn-Ca Alloy by Regulating Zn/Ca Atomic Ratios" Solids 6, no. 2: 17. https://doi.org/10.3390/solids6020017
APA StyleJin, Y., Lyu, S., Yu, Q., & Chen, M. (2025). Optimizing the Mechanical Properties and Corrosion Performance of Low-Alloyed Mg-Zn-Ca Alloy by Regulating Zn/Ca Atomic Ratios. Solids, 6(2), 17. https://doi.org/10.3390/solids6020017







