Feeding Mechanisms of Pathogenic Protozoa with a Focus on Endocytosis and the Digestive Vacuole
Abstract
1. Introduction
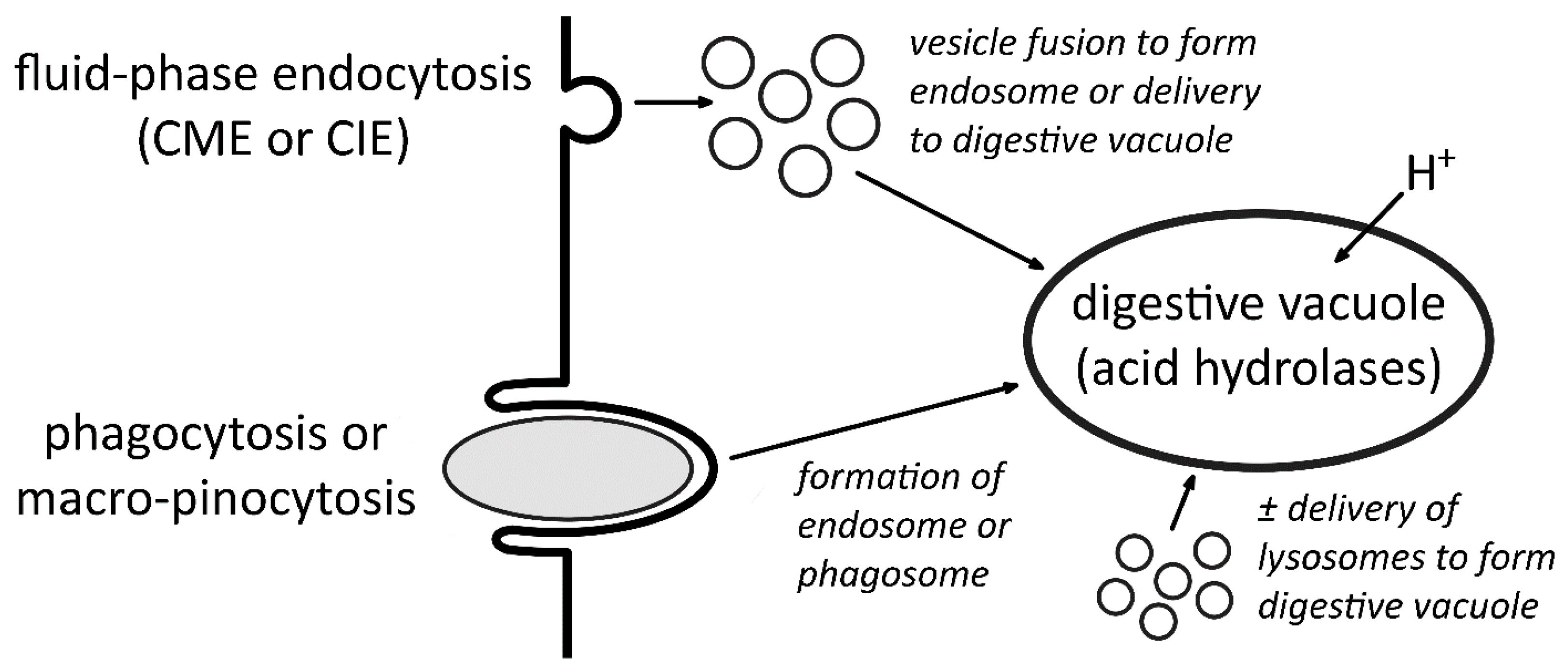
2. Endocytosis
3. The Digestive Vacuole
4. Intestinal and Other Luminal Protozoa
4.1. Entamoeba histolytica
4.2. Giardia
5. Kinetoplastids
5.1. Medically Important Trypanosomatids
5.2. Endocytosis and Trafficking to the Digestive Vacuole in Kinetoplastids
5.3. A Cytostome-Like Structure Is Found in Some Kinetoplastids
6. Apicomplexa
6.1. Myzocytosis
6.2. Plasmodium
6.3. Toxoplasma
6.4. The Parasitophorous Vacuole
6.5. The Digestive Vacuole
6.6. The Micropore of Apicomplexans
7. Summary
Funding
Conflicts of Interest
References
- Cavalier-Smith, T. Predation and eukaryote cell origins: A coevolutionary perspective. Int. J. Biochem. Cell Biol. 2009, 41, 307–322. [Google Scholar] [CrossRef]
- Miaczynska, M.; Stenmark, H. Mechanisms and functions of endocytosis. J. Cell Biol. 2008, 180, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Tielens, A.G.M.; Mentel, M.; Garg, S.G.; Gould, S.B. The physiology of phagocytosis in the context of mitochondrial origin. Microbiol. Mol. Biol. Rev. 2017, 81, e00008-17. [Google Scholar] [CrossRef]
- Matz, J.M. Plasmodium’s bottomless pit: Properties and functions of the malaria parasite’s digestive vacuole. Trends Parasitol. 2022, 38, 525–543. [Google Scholar] [CrossRef]
- Edgar, R.C.S.; Counihan, N.A.; McGowan, S.; de Koning-Ward, T.F. Methods used to investigate the Plasmodium falciparum digestive vacuole. Front. Cell. Infect. Microbiol. 2021, 11, 829823. [Google Scholar] [CrossRef]
- Jackson, A.P.; Otto, T.D.; Aslett, M.; Armstrong, S.D.; Bringaud, F.; Schlacht, A.; Hartley, C.; Sanders, M.; Wastling, J.M.; Dacks, J.B.; et al. Kinetoplastid phylogenomics reveals the evolutionary innovations associated with the origins of parasitism. Curr. Biol. 2016, 26, 161–172. [Google Scholar] [CrossRef]
- Skalický, T.; Dobáková, E.; Wheeler, R.J.; Tesařová, M.; Flegontov, P.; Jirsová, D.; Votýpka, J.; Yurchenko, V.; Ayala, F.J.; Lukeš, J. Extensive flagellar remodeling during the complex life cycle of Paratrypanosoma, an early-branching trypanosomatid. Proc. Natl. Acad. Sci. USA 2017, 114, 11757–11762. [Google Scholar] [CrossRef]
- Woo, Y.H.; Ansari, H.; Otto, T.D.; Klinger, C.M.; Kolisko, M.; Michálek, J.; Saxena, A.; Shanmugam, D.; Tayyrov, A.; Veluchamy, A.; et al. Chromerid genomes reveal the evolutionary path from photosynthetic algae to obligate intracellular parasites. eLife 2015, 4, e06974. [Google Scholar] [CrossRef] [PubMed]
- Haas, A. The phagosome: Compartment with a license to kill. Traffic 2007, 8, 311–330. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our current understanding of a universal biological process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Jeger, J.L. Endosomes, lysosomes, and the role of endosomal and lysosomal biogenesis in cancer development. Mol. Biol. Rep. 2020, 47, 9801–9810. [Google Scholar] [CrossRef]
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic pathways and endosomal trafficking: A primer. Wien. Med. Wochenschr. 2016, 166, 196–204. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Bol. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Ferreira, A.P.A.; Boucrot, E. Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol. 2018, 28, 188–200. [Google Scholar] [CrossRef]
- Mayor, S.; Parton, R.G.; Donaldson, J.G. Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol. 2014, 6, a016758. [Google Scholar] [CrossRef]
- Lajoie, P.; Nabi, I.R. Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Mol. Biol. 2010, 282, 135–163. [Google Scholar] [CrossRef]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 2014, 31, 2–10. [Google Scholar] [CrossRef]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef]
- de Marcos Lousa, C.; Denecke, J. Lysosomal and vacuolar sorting: Not so different after all! Biochem. Soc. Trans. 2016, 44, 891–897. [Google Scholar] [CrossRef]
- Wiser, M.F. Protozoa and Human Disease; Garland Science: New York, NY, USA, 2011; ISBN 9780429258282. [Google Scholar]
- Blum, H.E. The human microbiome. Adv. Med. Sci. 2017, 62, 414–420. [Google Scholar] [CrossRef]
- Skotarczak, B. The formation of primary and secondary lysosomes in Balantidium coli, Ciliata. Folia Histochem. Cytobiol. 1999, 37, 261–265. [Google Scholar]
- Pereira-Neves, A.; Benchimol, M. Phagocytosis by Trichomonas vaginalis: New insights. Biol. Cell 2007, 99, 87–101. [Google Scholar] [CrossRef]
- Zimmann, N.; Rada, P.; Žárský, V.; Smutná, T.; Záhonová, K.; Dacks, J.; Harant, K.; Hrdý, I.; Tachezy, J. Proteomic analysis of Trichomonas vaginalis phagolysosome, lysosomal targeting, and unconventional secretion of cysteine peptidases. Mol. Cell. Proteomics 2022, 21, 100174. [Google Scholar] [CrossRef]
- Stanley, S.L.J. Amoebiasis. Lancet 2003, 361, 1025–1034. [Google Scholar] [CrossRef]
- Wiser, M.F. Protozoa. In Encyclopedia of Biodiversity (Volume 2); Scheiner, S.M.B.T., Ed.; Academic Press: Oxford, UK, 2024; pp. 802–817. ISBN 978-0-323-98434-8. [Google Scholar]
- Iyer, L.R.; Verma, A.K.; Paul, J.; Bhattacharya, A. Phagocytosis of gut bacteria by Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2019, 9, 34. [Google Scholar] [CrossRef]
- Orozco, E.; Guarneros, G.; Martinez-Palomo, A.; Sánchez, T. Entamoeba histolytica. Phagocytosis as a virulence factor. J. Exp. Med. 1983, 158, 1511–1521. [Google Scholar] [CrossRef]
- Boettner, D.R.; Huston, C.D.; Sullivan, J.A.; Petri, W.A.J. Entamoeba histolytica and Entamoeba dispar utilize externalized phosphatidylserine for recognition and phagocytosis of erythrocytes. Infect. Immun. 2005, 73, 3422–3430. [Google Scholar] [CrossRef]
- Talamás-Lara, D.; Chávez-Munguía, B.; González-Robles, A.; Talamás-Rohana, P.; Salazar-Villatoro, L.; Durán-Díaz, Á.; Martínez-Palomo, A. Erythrophagocytosis in Entamoeba histolytica and Entamoeba dispar: A comparative study. Biomed Res. Int. 2014, 2014, 626259. [Google Scholar] [CrossRef]
- Watanabe, N.; Nakada-Tsukui, K.; Nozaki, T. Molecular dissection of phagocytosis by proteomic analysis in Entamoeba histolytica. Genes 2023, 14, 379. [Google Scholar] [CrossRef]
- Nakada-Tsukui, K.; Tsuboi, K.; Furukawa, A.; Yamada, Y.; Nozaki, T. A novel class of cysteine protease receptors that mediate lysosomal transport. Cell. Microbiol. 2012, 14, 1299–1317. [Google Scholar] [CrossRef]
- Bañuelos, C.; Betanzos, A.; Javier-Reyna, R.; Galindo, A.; Orozco, E. Molecular interplays of the Entamoeba histolytica endosomal sorting complexes required for transport during phagocytosis. Front. Cell. Infect. Microbiol. 2022, 12, 855797. [Google Scholar] [CrossRef]
- Verma, K.; Nozaki, T.; Datta, S. Role of EhRab7A in phagocytosis of type 1 fimbriated E. coli by Entamoeba histolytica. Mol. Microbiol. 2016, 102, 1043–1061. [Google Scholar] [CrossRef]
- Verma, K.; Srivastava, V.K.; Datta, S. Rab GTPases take centre stage in understanding Entamoeba histolytica biology. Small GTPases 2020, 11, 320–333. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Bettadapur, A.; Miller, H.W.; Ralston, K.S. Biting off what can be chewed: Trogocytosis in health, infection, and disease. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef]
- Ralston, K.S.; Solga, M.D.; Mackey-Lawrence, N.M.; Somlata; Bhattacharya, A.; Petri, W.A. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 2014, 508, 526–530. [Google Scholar] [CrossRef]
- Miller, H.W.; Suleiman, R.L.; Ralston, K.S. Trogocytosis by Entamoeba histolytica mediates acquisition and display of human cell membrane proteins and evasion of lysis by human serum. MBio 2019, 10. [Google Scholar] [CrossRef]
- Somlata; Nakada-Tsukui, K.; Nozaki, T. AGC family kinase 1 participates in trogocytosis but not in phagocytosis in Entamoeba histolytica. Nat. Commun. 2017, 8, 101. [Google Scholar] [CrossRef]
- Kinnear, F.B. Cytopathogenicity of Acanthamoeba, Vahlkampfia and Hartmannella: Quantative & qualitative in vitro studies on keratocytes. J. Infect. 2003, 46, 228–237. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Zoghby, K.L.; Bradley, S.G. Cytopathic action of Naegleria fowleri amoebae on rat neuroblastoma target cells. J. Protozool. 1990, 37, 138–144. [Google Scholar] [CrossRef]
- Xiang, C.; Li, Y.; Jing, S.; Han, S.; He, H. Trichomonas gallinae kills host cells using trogocytosis. Pathogens 2023, 12, 1008. [Google Scholar] [CrossRef]
- Holberton, D. V Fine structure of the ventral disk apparatus and the mechanism of attachment in the flagellate Giardia muris. J. Cell Sci. 1973, 13, 11–41. [Google Scholar] [CrossRef]
- Lanfredi-Rangel, A.; Attias, M.; de Carvalho, T.M.; Kattenbach, W.M.; De Souza, W. The peripheral vesicles of trophozoites of the primitive protozoan Giardia lamblia may correspond to early and late endosomes and to lysosomes. J. Struct. Biol. 1998, 123, 225–235. [Google Scholar] [CrossRef]
- Touz, M.C.; Rivero, M.R.; Miras, S.L.; Bonifacino, J.S. Lysosomal protein trafficking in Giardia lamblia: Common and distinct features. Front. Biosci. 2012, 4, 1898–1909. [Google Scholar] [CrossRef]
- Rivero, M.R.; Jausoro, I.; Bisbal, M.; Feliziani, C.; Lanfredi-Rangel, A.; Touz, M.C. Receptor-mediated endocytosis and trafficking between endosomal-lysosomal vacuoles in Giardia lamblia. Parasitol. Res. 2013, 112, 1813–1818. [Google Scholar] [CrossRef]
- Zumthor, J.P.; Cernikova, L.; Rout, S.; Kaech, A.; Faso, C.; Hehl, A.B. Static clathrin assemblies at the peripheral vacuole-plasma membrane interface of the parasitic protozoan Giardia lamblia. PLoS Pathog. 2016, 12, e1005756. [Google Scholar] [CrossRef]
- Abodeely, M.; DuBois, K.N.; Hehl, A.; Stefanic, S.; Sajid, M.; DeSouza, W.; Attias, M.; Engel, J.C.; Hsieh, I.; Fetter, R.D.; et al. A contiguous compartment functions as endoplasmic reticulum and endosome/lysosome in Giardia lamblia. Eukaryot. Cell 2009, 8, 1665–1676. [Google Scholar] [CrossRef]
- Benchimol, M. Giardia intestinalis can interact, change its shape and internalize large particles and microorganisms. Parasitology 2021, 148, 500–510. [Google Scholar] [CrossRef]
- Deschamps, P.; Lara, E.; Marande, W.; López-García, P.; Ekelund, F.; Moreira, D. Phylogenomic analysis of kinetoplastids supports that trypanosomatids arose from within bodonids. Mol. Biol. Evol. 2011, 28, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.A.; Kieft, R.; Macleod, A.; Hajduk, S.L. Trypanosome resistance to human innate immunity: Targeting Achilles’ heel. Trends Parasitol. 2012, 28, 539–545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Field, M.C.; Carrington, M. The trypanosome flagellar pocket. Nat. Rev. Microbiol. 2009, 7, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Webster, P.; Russell, D.G. The flagellar pocket of trypanosomatids. Parasitol. Today 1993, 9, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Coppens, I.; Courtoy, P.J. The adaptative mechanisms of Trypanosoma brucei for sterol homeostasis in its different life-cycle environments. Annu. Rev. Microbiol. 2000, 54, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Engstler, M.; Pfohl, T.; Herminghaus, S.; Boshart, M.; Wiegertjes, G.; Heddergott, N.; Overath, P. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell 2007, 131, 505–515. [Google Scholar] [CrossRef]
- Reyes-López, M.; Piña-Vázquez, C.; Serrano-Luna, J. Transferrin: Endocytosis and cell signaling in parasitic protozoa. BioMed Res. Int. 2015, 2015, 641392. [Google Scholar] [CrossRef]
- Allen, C.L.; Goulding, D.; Field, M.C. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J. 2003, 22, 4991–5002. [Google Scholar] [CrossRef]
- Manna, P.T.; Obado, S.O.; Boehm, C.; Gadelha, C.; Sali, A.; Chait, B.T.; Rout, M.P.; Field, M.C. Lineage-specific proteins essential for endocytosis in trypanosomes. J. Cell Sci. 2017, 130, 1379–1392. [Google Scholar] [CrossRef]
- Adung’a, V.O.; Gadelha, C.; Field, M.C. Proteomic analysis of clathrin interactions in trypanosomes reveals dynamic evolution of endocytosis. Traffic 2013, 14, 440–457. [Google Scholar] [CrossRef]
- Kalb, L.C.; Antunes Frederico, Y.C.; Batista, C.M.; Eger, I.; Fragoso, S.P.; Soares, M.J. Clathrin expression in Trypanosoma cruzi. BMC Cell Biol. 2014, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Ansari, I.; Basak, R.; Mukhopadhyay, A. Hemoglobin endocytosis and intracellular trafficking: A novel way of heme acquisition by Leishmania. Pathogens 2022, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; de Souza, W. Endocytosis of gold-labeled proteins and LDL by Trypanosoma cruzi. Parasitol. Res. 1991, 77, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Neto, V.V.; Cicco, N.N.T.; Cunha-Junior, E.F.; Canto-Cavalheiro, M.M.; Atella, G.C.; Torres-Santos, E.C. The pharmacological inhibition of sterol biosynthesis in Leishmania is counteracted by enhancement of LDL endocytosis. Acta Trop. 2011, 119, 194–198. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, N.N.T.; Pereira, M.G.; Corrêa, J.R.; Andrade-Neto, V.V.; Saraiva, F.B.; Chagas-Lima, A.C.; Gondim, K.C.; Torres-Santos, E.C.; Folly, E.; Saraiva, E.M.; et al. LDL uptake by Leishmania amazonensis: Involvement of membrane lipid microdomains. Exp. Parasitol. 2012, 130, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Engstler, M.; Thilo, L.; Weise, F.; Grünfelder, C.G.; Schwarz, H.; Boshart, M.; Overath, P. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J. Cell Sci. 2004, 117, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Langreth, S.G.; Balber, A.E. Protein uptake and digestion in bloodstream and culture forms of Trypanosoma brucei. J. Protozool. 1975, 22, 40–53. [Google Scholar] [CrossRef]
- Morgan, G.W.; Hall, B.S.; Denny, P.W.; Field, M.C.; Carrington, M. The endocytic apparatus of the kinetoplastida. Part II: Machinery and components of the system. Trends Parasitol. 2002, 18, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Link, F.; Borges, A.R.; Jones, N.G.; Engstler, M. To the surface and back: Exo- and endocytic pathways in Trypanosoma brucei. Front. Cell Dev. Biol. 2021, 9, 720521. [Google Scholar] [CrossRef]
- Porto-Carreiro, I.; Attias, M.; Miranda, K.; De Souza, W.; Cunha-e-Silva, N. Trypanosoma cruzi epimastigote endocytic pathway: Cargo enters the cytostome and passes through an early endosomal network before storage in reservosomes. Eur. J. Cell Biol. 2000, 79, 858–869. [Google Scholar] [CrossRef]
- Cunha-e-Silva, N.; Sant’Anna, C.; Pereira, M.G.; Porto-Carreiro, I.; Jeovanio, A.L.; de Souza, W. Reservosomes: Multipurpose organelles? Parasitol. Res. 2006, 99, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, C.; Parussini, F.; Lourenço, D.; de Souza, W.; Cazzulo, J.J.; Cunha-e-Silva, N.L. All Trypanosoma cruzi developmental forms present lysosome-related organelles. Histochem. Cell Biol. 2008, 130, 1187–1198. [Google Scholar] [CrossRef]
- Wang, Z.; Wheeler, R.J.; Sunter, J.D. Lysosome assembly and disassembly changes endocytosis rate through the Leishmania cell cycle. Microbiol. Open 2020, 9, e969. [Google Scholar] [CrossRef] [PubMed]
- Mullin, K.A.; Foth, B.J.; Ilgoutz, S.C.; Callaghan, J.M.; Zawadzki, J.L.; McFadden, G.I.; McConville, M.J. Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana. Mol. Biol. Cell 2001, 12, 2364–2377. [Google Scholar] [CrossRef] [PubMed]
- Ueda-Nakamura, T.; Attias, M.; de Souza, W. Megasome biogenesis in Leishmania amazonensis: A morphometric and cytochemical study. Parasitol. Res. 2001, 87, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Waller, R.F.; McConville, M.J. Developmental changes in lysosome morphology and function Leishmania parasites. Int. J. Parasitol. 2002, 32, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, R.D. Protozoan phagotrophy from predators to parasites: An overview of the enigmatic cytostome-cytopharynx complex of Trypanosoma cruzi. J. Eukaryot. Microbiol. 2022, 69, e12896. [Google Scholar] [CrossRef]
- Flegontov, P.; Votýpka, J.; Skalický, T.; Logacheva, M.D.; Penin, A.A.; Tanifuji, G.; Onodera, N.T.; Kondrashov, A.S.; Volf, P.; Archibald, J.M.; et al. Paratrypanosoma is a novel early-branching trypanosomatid. Curr. Biol. 2013, 23, 1787–1793. [Google Scholar] [CrossRef]
- Alcantara, C.L.; de Souza, W.; Cunha, E.; Silva, N.L. The cytostome-cytopharynx complex of intracellular and extracellular amastigotes of Trypanosoma cruzi exhibit structural and functional differences. Cell. Microbiol. 2021, 23, e13346. [Google Scholar] [CrossRef]
- Votýpka, J.; Modrý, D.; Oborník, M.; Šlapeta, J.; Lukeš, J. Apicomplexa. In Handbook of the Protists; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Margulis, L., Melkonian, M., Chapman, D.J., Corliss, J.O., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–58. ISBN 978-3-319-32669-6. [Google Scholar]
- Janouškovec, J.; Tikhonenkov, D.V.; Burki, F.; Howe, A.T.; Kolísko, M.; Mylnikov, A.P.; Keeling, P.J. Factors mediating plastid dependency and the origins of parasitism in apicomplexans and their close relatives. Proc. Natl. Acad. Sci. USA 2015, 112, 10200–10207. [Google Scholar] [CrossRef]
- Cumbo, V.R.; Baird, A.H.; Moore, R.B.; Negri, A.P.; Neilan, B.A.; Salih, A.; van Oppen, M.J.H.; Wang, Y.; Marquis, C.P. Chromera velia is endosymbiotic in larvae of the reef corals Acropora digitifera and A. tenuis. Protist 2013, 164, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Getty, T.A.; Peterson, J.W.; Fujioka, H.; Walsh, A.M.; Sam-Yellowe, T.Y. Colpodella sp. (ATCC 50594) life cycle: Myzocytosis and possible links to the origin of intracellular parasitism. Trop. Med. Infect. Dis. 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Frénal, K.; Dubremetz, J.-F.; Lebrun, M.; Soldati-Favre, D. Gliding motility powers invasion and egress in Apicomplexa. Nat. Rev. Microbiol. 2017, 15, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Besteiro, S.; Dubremetz, J.-F.; Lebrun, M. The moving junction of apicomplexan parasites: A key structure for invasion. Cell. Microbiol. 2011, 13, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.H.; Duraisingh, M.T. The molecular basis of erythrocyte invasion by malaria parasites. Cell Host Microbe 2017, 22, 232–245. [Google Scholar] [CrossRef]
- Wiser, M.F. Unique endomembrane systems and virulence in pathogenic protozoa. Life 2021, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Valigurová, A.; Florent, I. Nutrient acquisition and attachment strategies in basal lineages: A tough nut to crack in the evolutionary puzzle of Apicomplexa. Microorganisms 2021, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, M.-J.; Duraisingh, M.T. Evolution of apicomplexan secretory organelles. Int. J. Parasitol. 2012, 42, 1071–1081. [Google Scholar] [CrossRef]
- Cavalier-Smith, T.; Chao, E.E. Protalveolate phylogeny and systematics and the origins of sporozoa and dinoflagellates (phylum Myzozoa nom. nov.). Eur. J. Protistol. 2004, 40, 185–212. [Google Scholar] [CrossRef]
- Hansen, P.J.; Calado, A.J. Phagotrophic mechanisms and prey selection in free-living dinoflagellates. J. Eukaryot. Microbiol. 1999, 46, 382–389. [Google Scholar] [CrossRef]
- Brugerolle, G. Colpodella vorax: Ultrastructure, predation, life-cycle, mitosis, and phylogenetic relationships. Eur. J. Protistol. 2002, 38, 113–125. [Google Scholar] [CrossRef]
- Sam-Yellowe, T.Y.; Asraf, M.M.; Peterson, J.W.; Fujioka, H. Fluorescent nanoparticle uptake by myzocytosis and endocytosis in Colpodella sp. ATCC 50594. Microorganisms 2023, 11, 1945. [Google Scholar] [CrossRef] [PubMed]
- Simdyanov, T.G.; Kuvardina, O.N. Fine structure and putative feeding mechanism of the archigregarine Selenidium orientale (Apicomplexa: Gregarinomorpha). Eur. J. Protistol. 2007, 43, 17–25. [Google Scholar] [CrossRef]
- Schrével, J.; Valigurová, A.; Prensier, G.; Chambouvet, A.; Florent, I.; Guillou, L. Ultrastructure of Selenidium pendula, the type species of archigregarines, and phylogenetic relations to other marine apicomplexa. Protist 2016, 167, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Gerace, E.; Lo Presti, V.D.M.; Biondo, C. Cryptosporidium infection: Epidemiology, pathogenesis, and differential diagnosis. Eur. J. Microbiol. Immunol. 2019, 9, 119–123. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.P.; Small, A.J.; Chen, X.-M.; LaRusso, N.F. Host cell actin remodeling in response to Cryptosporidium. Subcell. Biochem. 2008, 47, 92–100. [Google Scholar] [CrossRef]
- Lendner, M.; Daugschies, A. Cryptosporidium infections: Molecular advances. Parasitology 2014, 141, 1511–1532. [Google Scholar] [CrossRef]
- Borowski, H.; Clode, P.L.; Thompson, R.C.A. Active invasion and/or encapsulation? A reappraisal of host-cell parasitism by Cryptosporidium. Trends Parasitol. 2008, 24, 509–516. [Google Scholar] [CrossRef]
- Perkins, M.E.; Riojas, Y.A.; Wu, T.W.; Le Blancq, S.M. CpABC, a Cryptosporidium parvum ATP-binding cassette protein at the host–parasite boundary in intracellular stages. Proc. Natl. Acad. Sci. USA 1999, 96, 5734–5739. [Google Scholar] [CrossRef]
- Ehrenman, K.; Wanyiri, J.W.; Bhat, N.; Ward, H.D.; Coppens, I. Cryptosporidium parvum scavenges LDL-derived cholesterol and micellar cholesterol internalized into enterocytes. Cell. Microbiol. 2013, 15, 1182–1197. [Google Scholar] [CrossRef]
- Beĭer, T.V.; Sidorenko, N.V.; Lakovnikova, E. V [Electron microscopic research on Cryptosporidium. I. The asexual stages in the development of Cryptosporidium parvum]. Tsitologiia 1990, 32, 462–468. [Google Scholar] [PubMed]
- Wiser, M.F. The digestive vacuole of the malaria parasite: A specialized lysosome. Pathogens 2024, 13, 182. [Google Scholar] [CrossRef]
- Wunderlich, J.; Rohrbach, P.; Dalton, J.P. The malaria digestive vacuole. Front. Biosci. 2012, 4, 1424–1448. [Google Scholar] [CrossRef]
- Ngwa, C.J.; Rosa, T.F.; Pradel, G. The biology of malaria gametocytes. In Current Topics in Malaria; Rodriguez-Morales, A.J., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 7; pp. 117–144. Available online: https://www.intechopen.com/chapters/52526 (accessed on 17 June 2024).
- Slomianny, C. Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells 1990, 16, 369–378. [Google Scholar] [PubMed]
- Spielmann, T.; Gras, S.; Sabitzki, R.; Meissner, M. Endocytosis in Plasmodium and Toxoplasma parasites. Trends Parasitol. 2020, 36, 520–532. [Google Scholar] [CrossRef]
- Henrici, R.C.; Edwards, R.L.; Zoltner, M.; van Schalkwyk, D.A.; Hart, M.N.; Mohring, F.; Moon, R.W.; Nofal, S.D.; Patel, A.; Flueck, C.; et al. The Plasmodium falciparum artemisinin susceptibility-associated AP-2 adaptin μ subunit is clathrin independent and essential for schizont maturation. MBio 2020, 11, e02918-19. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, J.; Scharf, S.; Schmidt, S.; Jonscher, E.; Hoeijmakers, W.A.M.; Flemming, S.; Toenhake, C.G.; Schmitt, M.; Sabitzki, R.; Bergmann, B.; et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 2020, 367, 51–59. [Google Scholar] [CrossRef]
- Pan, M.; Ge, C.-C.; Fan, Y.-M.; Jin, Q.-W.; Shen, B.; Huang, S.-Y. The determinants regulating Toxoplasma gondii bradyzoite development. Front. Microbiol. 2022, 13, 1027073. [Google Scholar] [CrossRef] [PubMed]
- Frölich, S.; Wallach, M. Use of fluorescent nanoparticles to investigate nutrient acquisition by developing Eimeria maxima macrogametocytes. Sci. Rep. 2016, 6, 29030. [Google Scholar] [CrossRef]
- Kannan, G.; Thaprawat, P.; Schultz, T.L.; Carruthers, V.B. Acquisition of host cytosolic protein by Toxoplasma gondii bradyzoites. mSphere 2021, 6, e00934-20. [Google Scholar] [CrossRef]
- McGovern, O.L.; Rivera-Cuevas, Y.; Carruthers, V.B. Emerging mechanisms of endocytosis in Toxoplasma gondii. Life 2021, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Gras, S.; Jimenez-Ruiz, E.; Klinger, C.M.; Schneider, K.; Klingl, A.; Lemgruber, L.; Meissner, M. An endocytic-secretory cycle participates in Toxoplasma gondii in motility. PLoS Biol. 2019, 17, e3000060. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Zimmerberg, J. Hardly vacuous: The parasitophorous vacuolar membrane of malaria parasites. Trends Parasitol. 2020, 36, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Matz, J.M.; Beck, J.R.; Blackman, M.J. The parasitophorous vacuole of the blood-stage malaria parasite. Nat. Rev. Microbiol. 2020, 18, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.; Kats, L.M.; Sturm, A.; Cooke, B.M. Recent insights into alteration of red blood cells by Babesia bovis: Moovin’ forward. Trends Parasitol. 2010, 26, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Repnik, U.; Gangopadhyay, P.; Bietz, S.; Przyborski, J.M.; Griffiths, G.; Lingelbach, K. The apicomplexan parasite Babesia divergens internalizes band 3, glycophorin A and spectrin during invasion of human red blood cells. Cell. Microbiol. 2015, 17, 1052–1068. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.K.; Tilney, L.G.; Musoke, A.J. The entry of Theileria parva sporozoites into bovine lymphocytes: Evidence for MHC class I involvement. J. Cell Biol. 1991, 113, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.A.; Kaplan, A.D.; Lis, A.; Bett, G.C.L.; Rosowski, E.E.; Cirelli, K.M.; Bougdour, A.; Sidik, S.M.; Beck, J.R.; Lourido, S.; et al. The Toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host Microbe 2015, 17, 642–652. [Google Scholar] [CrossRef]
- Garten, M.; Nasamu, A.S.; Niles, J.C.; Zimmerberg, J.; Goldberg, D.E.; Beck, J.R. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat. Microbiol. 2018, 3, 1090–1098. [Google Scholar] [CrossRef]
- Dluzewski, A.R.; Ling, I.T.; Hopkins, J.M.; Grainger, M.; Margos, G.; Mitchell, G.H.; Holder, A.A.; Bannister, L.H. Formation of the food vacuole in Plasmodium falciparum: A potential role for the 19 kDa fragment of merozoite surface protein 1 (MSP1(19)). PLoS ONE 2008, 3, e3085. [Google Scholar] [CrossRef]
- Romano, J.D.; Nolan, S.J.; Porter, C.; Ehrenman, K.; Hartman, E.J.; Hsia, R.-C.; Coppens, I. The parasite Toxoplasma sequesters diverse Rab host vesicles within an intravacuolar network. J. Cell Biol. 2017, 216, 4235–4254. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.J.; Romano, J.D.; Coppens, I. Host lipid droplets: An important source of lipids salvaged by the intracellular parasite Toxoplasma gondii. PLoS Pathog. 2017, 13, e1006362. [Google Scholar] [CrossRef] [PubMed]
- Coppens, I.; Dunn, J.D.; Romano, J.D.; Pypaert, M.; Zhang, H.; Boothroyd, J.C.; Joiner, K.A. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 2006, 125, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Cuevas, Y.; Mayoral, J.; Di Cristina, M.; Lawrence, A.-L.E.; Olafsson, E.B.; Patel, R.K.; Thornhill, D.; Waldman, B.S.; Ono, A.; Sexton, J.Z.; et al. Toxoplasma gondii exploits the host ESCRT machinery for parasite uptake of host cytosolic proteins. PLOS Pathog. 2021, 17, e1010138. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.E.; Sullivan, D.J.; Goldberg, D.E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 1997, 51, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.J. Haemozoin formation. Mol. Biochem. Parasitol. 2008, 157, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Stasic, A.J.; Moreno, S.N.J.; Carruthers, V.B.; Dou, Z. The Toxoplasma plant-like vacuolar compartment (PLVAC). J. Eukaryot. Microbiol. 2022, 69, e12951. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; McGovern, O.L.; Di Cristina, M.; Carruthers, V.B. Toxoplasma gondii ingests and digests host cytosolic proteins. MBio 2014, 5, e01188-14. [Google Scholar] [CrossRef] [PubMed]
- Saliba, K.J.; Allen, R.J.W.; Zissis, S.; Bray, P.G.; Ward, S.A.; Kirk, K. Acidification of the malaria parasite’s digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J. Biol. Chem. 2003, 278, 5605–5612. [Google Scholar] [CrossRef]
- Rea, P.A.; Poole, R.J. Vacuolar H+-translocating pyrophosphatase. Annu. Rev. Plant Biol. 1993, 44, 157–180. [Google Scholar] [CrossRef]
- Shafik, S.H.; Cobbold, S.A.; Barkat, K.; Richards, S.N.; Lancaster, N.S.; Llinás, M.; Hogg, S.J.; Summers, R.L.; McConville, M.J.; Martin, R.E. The natural function of the malaria parasite’s chloroquine resistance transporter. Nat. Commun. 2020, 11, 3922. [Google Scholar] [CrossRef] [PubMed]
- Warring, S.D.; Dou, Z.; Carruthers, V.B.; McFadden, G.I.; van Dooren, G.G. Characterization of the chloroquine resistance transporter homologue in Toxoplasma gondii. Eukaryot. Cell 2014, 13, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Senaud, J.; Chobotar, B.; Scholtyseck, E. Role of the micropore in nutrition of the Sporozoa. Ultrastructural study of Plasmodium cathemerium, Eimeria ferrisi, E. stiedai, Besnoitia jellisoni, and Frenkelia sp. Tropenmed. Parasitol. 1976, 27, 145–159. [Google Scholar] [PubMed]
- Kono, M.; Prusty, D.; Parkinson, J.; Gilberger, T.W. The apicomplexan inner membrane complex. Front. Biosci. 2013, 18, 982–992. [Google Scholar] [CrossRef]
- Gould, S.B.; Tham, W.-H.; Cowman, A.F.; McFadden, G.I.; Waller, R.F. Alveolins, a new family of cortical proteins that define the protist infrakingdom Alveolata. Mol. Biol. Evol. 2008, 25, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Appleton, P.L.; Vickerman, K. Presence of apicomplexan-type micropores in a parasitic dinoflagellate, Hematodinium sp. Parasitol. Res. 1996, 82, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.A.; Chiappino, M.L.; Pavesio, C.E. Endocytosis at the micropore of Toxoplasma gondii. Parasitol. Res. 1994, 80, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Dong, H.; Lai, D.-H.; Yang, J.; He, K.; Tang, X.; Liu, Q.; Hide, G.; Zhu, X.-Q.; Sibley, L.D.; et al. The Toxoplasma micropore mediates endocytosis for selective nutrient salvage from host cell compartments. Nat. Commun. 2023, 14, 977. [Google Scholar] [CrossRef] [PubMed]
- Koreny, L.; Mercado-Saavedra, B.N.; Klinger, C.M.; Barylyuk, K.; Butterworth, S.; Hirst, J.; Rivera-Cuevas, Y.; Zaccai, N.R.; Holzer, V.J.C.; Klingl, A.; et al. Stable endocytic structures navigate the complex pellicle of apicomplexan parasites. Nat. Commun. 2023, 14, 2167. [Google Scholar] [CrossRef]
- Xie, S.C.; Ralph, S.A.; Tilley, L. K13, the cytostome, and artemisinin resistance. Trends Parasitol. 2020, 36, 533–544. [Google Scholar] [CrossRef]
- Ferreira, J.L.; Heincke, D.; Wichers, J.S.; Liffner, B.; Wilson, D.W.; Gilberger, T.-W. The dynamic roles of the inner membrane complex in the multiple stages of the malaria parasite. Front. Cell. Infect. Microbiol. 2020, 10, 611801. [Google Scholar] [CrossRef] [PubMed]
| Type | Mechanism | Size |
|---|---|---|
| Phagocytosis | Particles surrounded by pseudopodia | >0.5 μm |
| Macro-pinocytosis | Fluid uptake by membrane ruffles | 0.2–10 μm |
| Clathrin-mediated endocytosis (CME) | Clathrin in conjunction with adaptor proteins forms coated vesicles | 60–120 nm |
| Clathrin-independent endocytosis (CIE) | Several distinct mechanisms involving various proteins and lipid rafts | 50–80 nm |
| Kinetoplastid | Endocytosis | Digestive Vacuole |
|---|---|---|
| African trypanosomes | Clathrin-mediated at the flagellar pocket | Single large lysosome |
| T. cruzi | Clathrin-mediated at the flagellar pocket and clathrin-independent at the cytostome | Several lysosome-like vacuoles called reservosomes |
| Leishmania | Clathrin-mediated at the flagellar pocket | Vesicle-filled tubules or vacuoles called megasomes |
| Pathogen | Primary Feeding Mechanism |
|---|---|
| Entamoeba | Extensive phagocytosis of bacteria and host cells including trogocytosis |
| Giardia | Possible clathrin-mediated process involving a unique lysosomal compartment called peripheral vacuoles |
| Kinetoplastids | Clathrin-mediated endocytosis at the flagellar pocket |
| Plasmodium | Specialized digestive vacuole for hemoglobin catabolism and hemozoin formation |
| Toxoplasma | Endocytosis from a modified parasitophorous vacuole and a plant-like digestive vacuole |
| Cryptosporidium | May lack endocytosis and digestive vacuole and take up nutrients via a feeder organelle and myzocytosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiser, M.F. Feeding Mechanisms of Pathogenic Protozoa with a Focus on Endocytosis and the Digestive Vacuole. Parasitologia 2024, 4, 222-237. https://doi.org/10.3390/parasitologia4030019
Wiser MF. Feeding Mechanisms of Pathogenic Protozoa with a Focus on Endocytosis and the Digestive Vacuole. Parasitologia. 2024; 4(3):222-237. https://doi.org/10.3390/parasitologia4030019
Chicago/Turabian StyleWiser, Mark F. 2024. "Feeding Mechanisms of Pathogenic Protozoa with a Focus on Endocytosis and the Digestive Vacuole" Parasitologia 4, no. 3: 222-237. https://doi.org/10.3390/parasitologia4030019
APA StyleWiser, M. F. (2024). Feeding Mechanisms of Pathogenic Protozoa with a Focus on Endocytosis and the Digestive Vacuole. Parasitologia, 4(3), 222-237. https://doi.org/10.3390/parasitologia4030019






