Abstract
Aluminum hydride (AlH3) has attracted wide attention due to its high gravimetric and volumetric hydrogen capacity. AlH3 can easily release hydrogen when heated at relatively low temperature. Such high hydrogen density and low dehydrogenation temperature make it one of the most promising high-energy fuels for solid propellants. In particular, AlH3 as a component of solid propellants may greatly increase the specific impulse of rocket engines. However, AlH3 exhibits low chemical and thermal stability in an ambient atmosphere. In this paper, the research progress about the synthesis, dehydrogenation thermodynamics, and kinetics, the stabilization of AlH3 over the past decades are reviewed, with the aim of exploring more a economical synthesis and suitable stabilization methods for large-scale use in solid propellants. Finally, some suggestions regarding future research directions in this filed are proposed.
1. Introduction
Solid propellants act as the power source of the rocket motor and play an important role in the fields of military and aerospace. The pursuit of higher energy solid propellants has always been an inevitable requirement for the development of solid rockets. Metal hydrides possess high combustion heats due to the introduction of “hydrogen energy”, thus making them promising candidates for the solid fuels of propellants. In addition, considering that a decrease in the average molecular weight of gas combustion products will lead to an increase specific impulses of propellants, the low-molecular-weight H2 produced by hydride decomposition makes them more attractive.
In recent years, considerable efforts have been made to evaluate the potential of hydrides as energy fuels for solid propellants, including aluminum hydride (AlH3) [1,2,3], lithium hydride (LiH) [4], magnesium hydride (MgH2) [5,6], titanium hydride (TiH2) [7,8], and ammonia borane (NH3BH3) [9]. Table 1 summarizes the hydrogen storage properties of common metal hydrides. Among the above hydrides, AlH3 is regarded as an important promising candidate due to its high heat of combustion (about 40 MJ/kg), volumetric hydrogen storage densities (148 kgH2/km3) and hydrogen content (10.1 wt%). For example, when replacing 18% of aluminum (Al), a widely used metallic fuel in solid propellants, in a conventional ammonium perchlorate/hydroxyl-terminated polybutadiene (AP/HTPB)-based rocket propellant with aluminum hydride (AlH3), the theoretical specific impulse would increase by 10%, and the flame temperature would decrease by 5%.

Table 1.
The hydrogen storage properties of common metal hydrides.
AlH3 is a polyphasic substance, with no less than seven non-solvated crystal forms, namely α, α’, β, γ, δ, ε, and ζ-AlH3, due to different synthesis routes and reaction conditions. It is generally believed that the α-AlH3 phase is the dominant phase, while the other phases are usually considered as impurity products of the α-AlH3 phase, and they are not as stable as α-AlH3. Some of them directly decompose into elements, while the other ones convert to α-AlH3 before further decomposition. Therefore, α-AlH3 deserves much more investigation [10,11]. However, α-AlH3 is very sensitive to oxidants and could easily decompose into aluminum in an atmospheric environment [12]. Due to the poor stability of AlH3, during long-term storage process, the slight decomposition of AlH3 at room temperature promotes the generation and development of cracks in the solid propellant grain and reduces the propellant performance. In addition, the poor compatibility of α-AlH3 with certain propellant components, such as nitrates, further limits its application in solid propellants [13].
A large quantity of endeavors have been tried on the synthesis and stabilization of α-AlH3 [14,15,16,17,18,19,20], aiming at producing products with better stability, higher quality, and more energy as additive fuel for solid propellants. This review focuses on the relevant research about the above-mentioned aspects; meanwhile, thermal decomposition properties and stabilization methods were also proposed. The paper is organized as follows. Firstly, Section 2 presents the synthesis methods of aluminum hydride. Next, the thermodynamics and kinetics of AlH3 are described in Section 3. Thirdly, Section 4 summarizes the physical and chemical modification methods of AlH3 to achieve the purpose of stabilizing aluminum hydride. Finally, we propose potential future research directions and aim to offer a valuable reference for subsequent studies on AlH3 in related fields.
2. Synthesis of AlH3
2.1. Wet Chemical Methods
Synthesis of AlH3 has been studied since the 1940s, when Stecher and Wiberg first synthesized AlH3·2N(CH3)3 in a low and impure form. Later, wet chemistry method was first reported by Finholt et al. [21], but the ether cannot be completely removed at that time, so the products were mainly solvated α-AlH3.
Brower et al. [22] synthesized seven non-solvated AlH3 polymorphs, which are now commonly referenced when preparing AlH3. The process consists of two steps. Firstly, LiAlH4 (or LiH) and AlCl3 react in ether solution to form AlH3–ether complex and LiCl precipitate, seen in Reaction (1). Secondly, the ether ligand was removed through heating the product, which is also called post-crystallization, as shown in Reaction (2). Finally, the products need to undergo an ether solvent wash to eliminate any surplus LiAlH4 or LiBH4, followed by vacuum drying to produce the final AlH3.
According to Refs. [23,24,25,26], the synthesis process of the AlH3 polymorphs depends on multiple conditions, such as temperature, time, the presence of LiAlH4/LiBH4 or not, and the atmosphere. Generally, the γ-AlH3 can be obtained from the desolvation reaction of etherate AlH3 in the presence of only LiAlH4 at lower temperature (60∼70 °C), whereas the α-AlH3 can be obtained from the desolvation reaction of etherate AlH3 in the presence of excess LiBH4 and LiAlH4. Pure β-AlH3 or α’-AlH3 are difficult to prepare and usually form accompanying with γ -AlH3. However, no reproducible synthesis methods of the δ-, ε-, and ζ-AlH3 were established.
Table 2 displays the preparation conditions and properties of various AlH3 polymorphs. Additionally, a more efficient method for synthesizing high-purity α-AlH3 crystals involves directly dissolving the reactants in ether and toluene, respectively.

Table 2.
The preparation conditions and characteristics of different AlH3 polymorphs.
For wet synthesis methods, the formation of AlH3⋅Et2O requires a large amount of environmentally harmful organic solvents, like ethers or amines. Furthermore, the costly removal and recycling of these organic solvents, coupled with the challenge of controlling the preparation conditions due to the reaction’s high sensitivity to temperature and time, make achieving the desired polymorph difficult [27]. Conditions control, solvent reuse, and other factors make it relatively difficult to scale up the synthesis of AlH3 using wet methods.
2.2. Dry Synthesis Methods
As mentioned above, wet synthesis of AlH3 requires a large amount of solvents, often being expensive and toxic. Therefore, numerous methods for the synthesis of AlH3 without solvents, generally termed dry synthesis methods, have been proposed [28,29,30,31,32,33,34].
2.2.1. Mechano-Chemical Method
The mechano-chemical method is considered more environmentally friendly and cost-effective than traditional wet chemical methods, and has been used to prepare numerous aluminum hydrides, such as Mg(AlH4)2 [35,36], Ca(AlH4)2 [37,38], and LiMg(AlH4)3 [39]. Many studies have demonstrated that this solvent-free method has lower energy consumption than traditional wet synthesis process [30,31], and only necessitates inexpensive metal hydrides (such as LiH, NaH, CaH2, and MgH2) instead of LiAlH4, making it an increasingly popular alternative method for AlH3 preparation.
Duan et al. [29,40] investigated the synthesis of AlH3 by reactive milling using aluminum chloride and cheap metal hydrides as starting materials. XRD and NMR analyses indicated that nano-sized materials AlH3 were prepared using commercial AlCl3 and nanocrystalline MgH2 as reagents.
Duan et al. [30,41] also reported that α⁃AlH3 nanocomposites can be obtained by adding TiF3 to LiH and AlCl3 systems through ball milling in a short period of time, where suitable gas and pressure conditions can completely suppress the formation of metallic Al. The results indicate that TiF3 plays a synergistic role in the solid-state reaction between LiH and AlCl3, and has significantly affect dehydriding properties of AlH3, compared to the α-AlH3/LiCl nano-composite without TiF3. The as-milled α-AlH3/LiCl-TiF3 composite has a hydrogen desorption of 9.92 wt% at 160 °C within 750 s, which is very close to the theoretical hydrogen capacity of AlH3.
However, this method is not suitable for large-scale commercial applications due to prohibitively high energy consumption. In addition, it’s hard to get a pure product since slight decomposition of AlH3 is almost inevitable during process.
2.2.2. Organoaluminum Decomposition Method
Organoaluminum decomposition is a method for directly preparing AlH3 without the need for ether solvents [42]. The presence of surfactants enables the obtainment of AlH3 through the decomposition of triethylaluminum under a hydrogen pressure of 10 MPa. The following illustrates the decomposition pathways of Et3Al: Et3Al → Et2AlH → AlH3 → Al. In order to inhibit the further decomposition of generated AlH3 into Al, the second step of decomposition reaction, i.e., Et2AlH → AlH3, needs to be carried out at low temperature. In addition, Tetroctyl ammonium bromide (TOAB) and tetrabylammonium bromide (TBAB) are used as surfactants to stabilize Al/AlH3 particles.
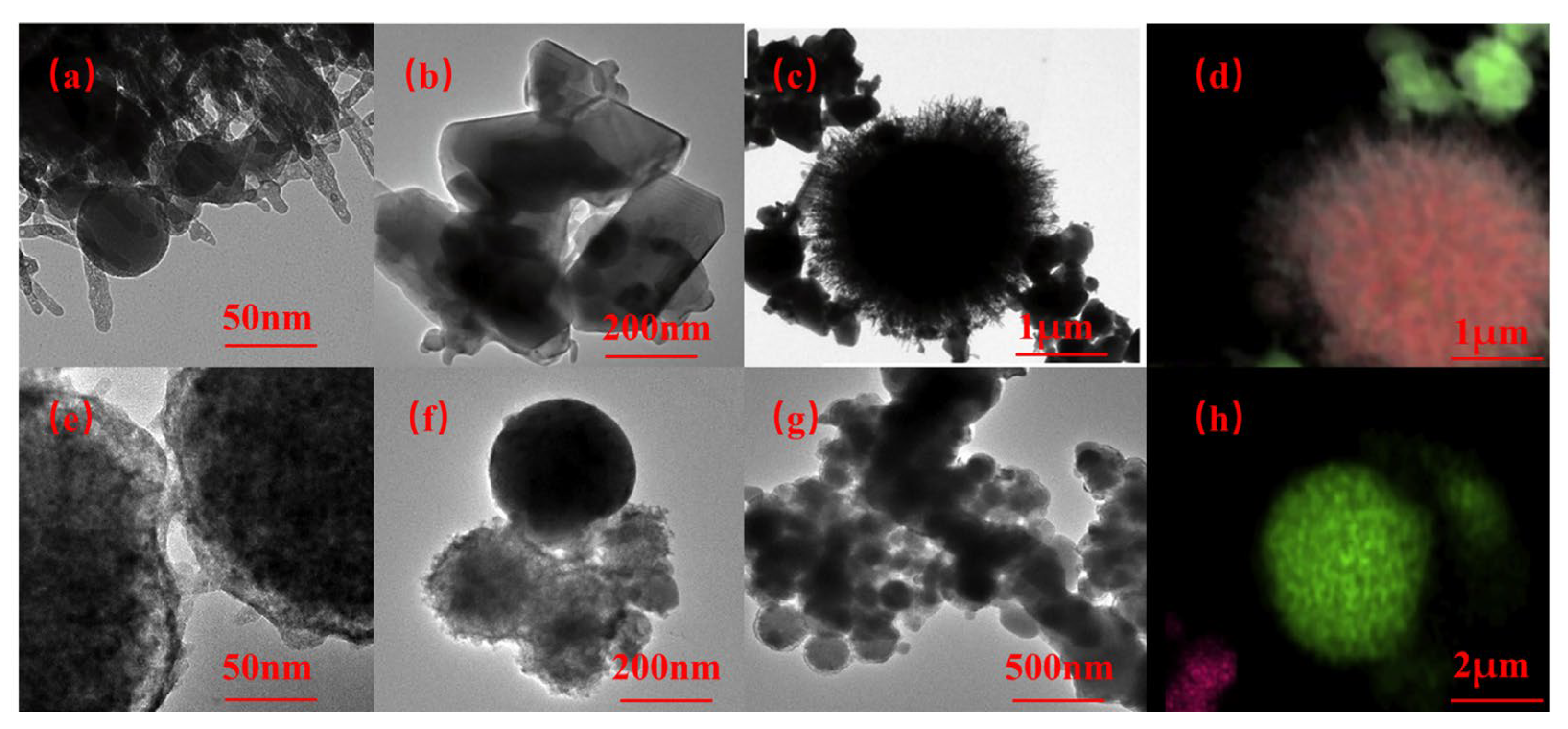
Figure 1 shows the TEM and EDS of as-prepared organo-AlH3 (TOAB and TBAB as the surfactants). When TBAB is used as a surfactant, the particles obtained are relatively large, exceeding 100 nm in size and exhibit a regular spherical shape. In contrast, AlH3 with an irregular morphology and with sizes ranging from 1 to 30 nm can be observed around the periphery of MgH2 particles when TOAB is employed as the surfactant.
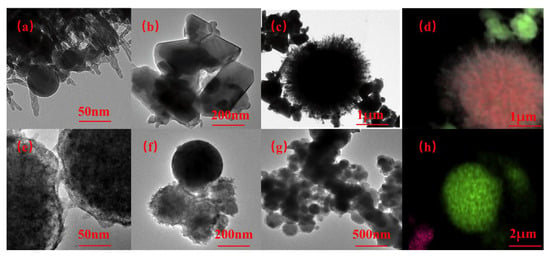
Figure 1.
TEM and EDS images of AlH3 with TOTB (a–d) and TBAB (e–h) synthesized via Et3Al thermal decomposition. Reproduced with permission from Ref. [42]. Copyright 2018, Elsevier.
It is challenging to utilize organoaluminum decomposition to achieve high-purity AlH3 products because of the presence of surfactants. Particularly, when TBAB is used as a surfactant, the synthesized particles exhibit poor stability and low decomposition temperature, limiting its application to propellants.
2.3. Other Emerging Methods
2.3.1. Supercritical Synthesis Method
Supercritical synthesis involves the dissolution and reaction of raw materials in supercritical media. Take AlH3 as an example: it can be prepared by reacting activated aluminum with hydrogen in a supercritical liquid at 60 °C for 1 h. [43,44]. The supercritical medium consists of supercritical liquid CO2 in combination with an ether co-solvent. However, due to the use of liquid CO2, the reaction temperature cannot be further increased, and hence limits the reaction activity. The synthetic method is currently in the laboratory stage and still has a long way to go before achieving industrial-scale production.
2.3.2. Organoaluminum Decomposition Method
In the electrolyte solution, active aluminum powder and ionic state hydrogen encounter to trigger an electrochemical reaction, generating AlH3 in the form of precipitation at the bottom of the electrolyte solution.
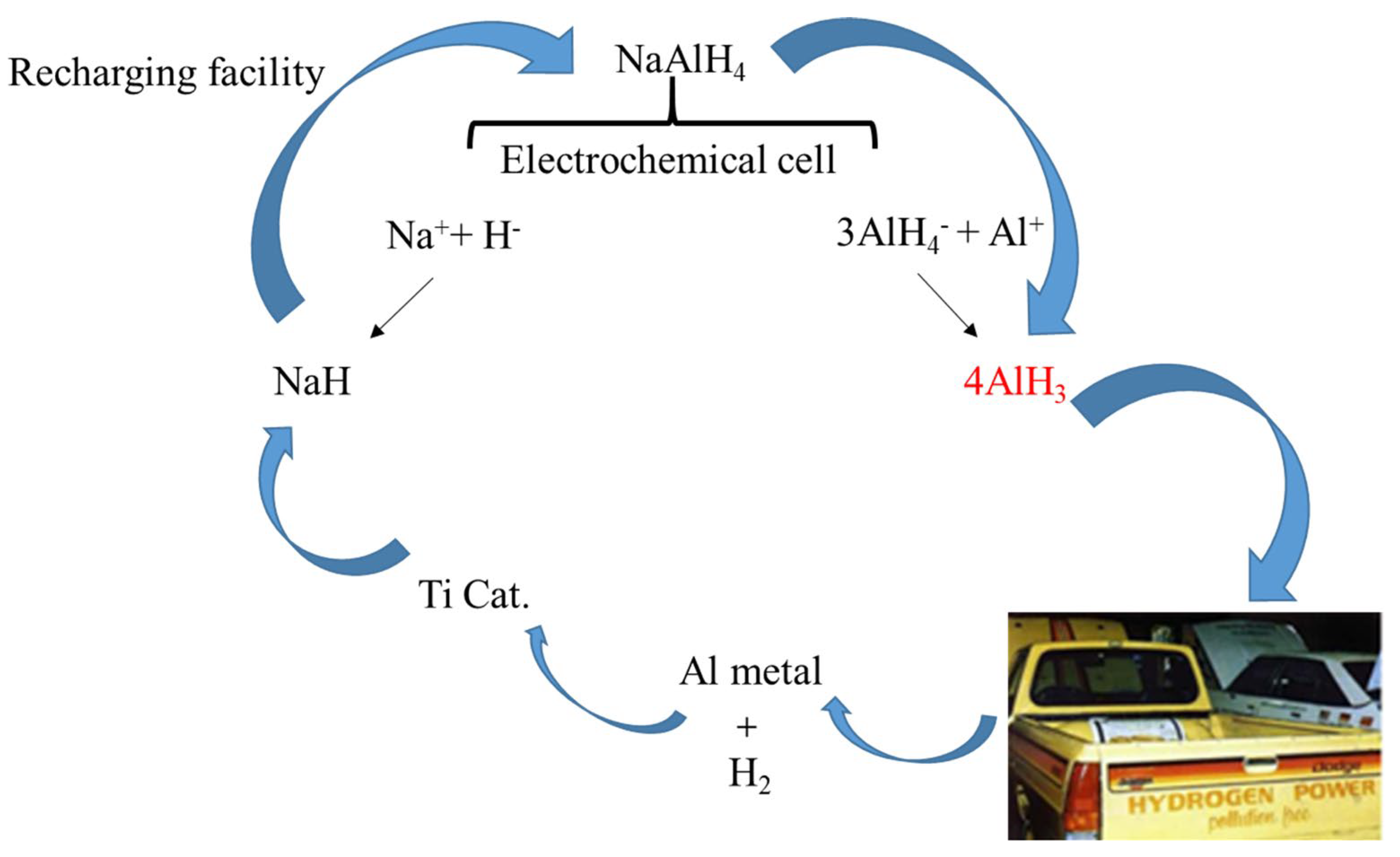
Zidanet et al. [45] used the Al as the anode and Pt as cathode, respectively, and the whole electrochemical process was realized in the NaAlH4-THF electrolyte. As shown in the reaction paths (Reactions (5) and (6), AlH3 can be produced via two distinct reaction mechanisms at the Al electrode, involving the oxidation of ions and the reaction of AlH4- with the Al anode. Based on this, a cycle using electrolysis and catalytic hydrogenation to treat waste aluminum was proposed [46] (see in Figure 2), in which the problem of the high hydrogen pressure and the formation of stable by-products such as LiCl could be avoided. It is noteworthy that the addition of LiCl is notable for its ability to enhance the yield of AlH3; meanwhile, alkali hydride (e.g., LiH, NaH and KH) could be reformed through electrochemical potential.
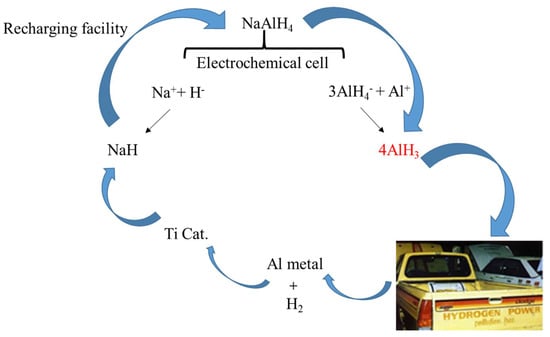
Figure 2.
Proposed reversible fuel cycle for AlH3.
2.3.3. High Pressure Hydrogenation Method
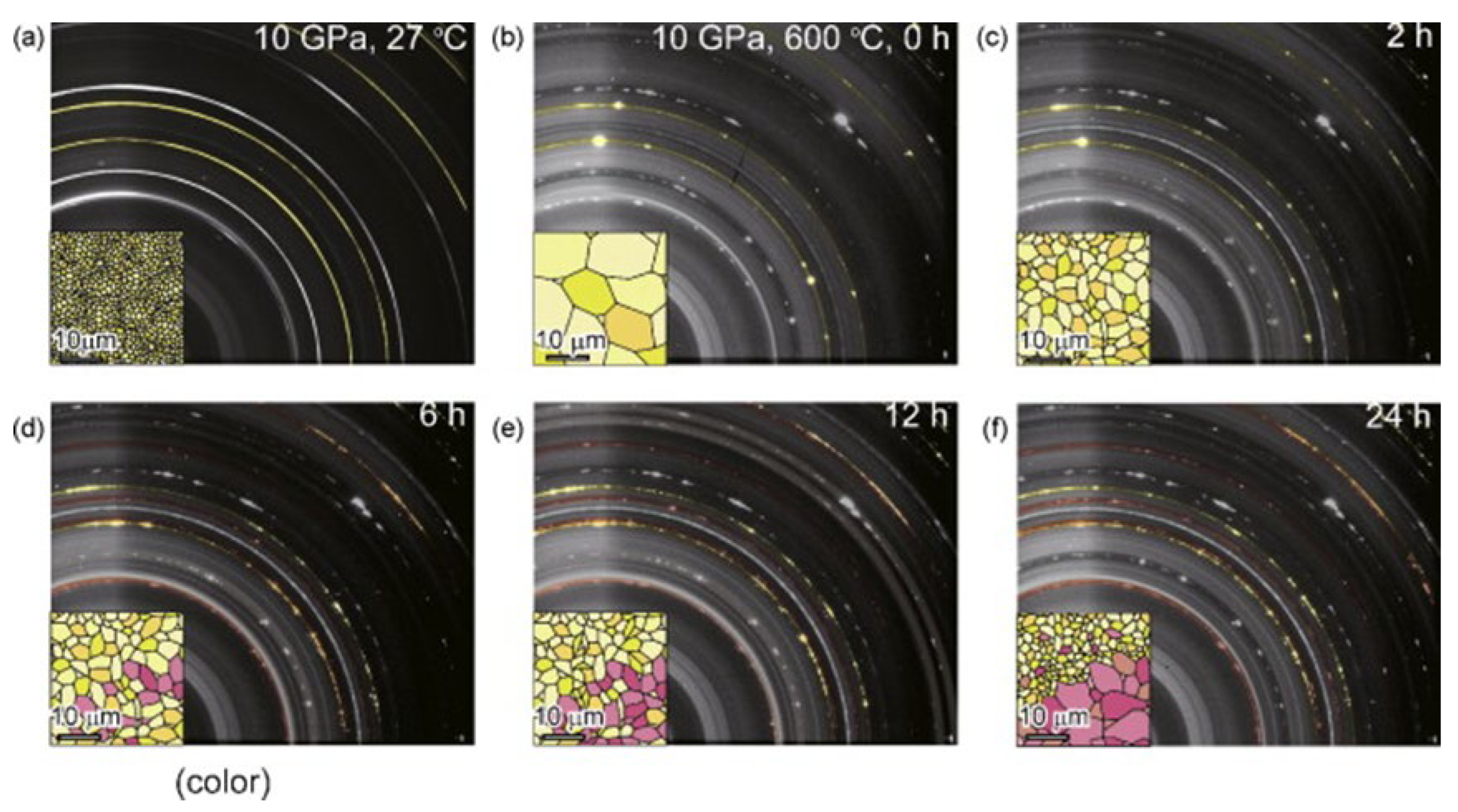
The absorption of hydrogen by aluminum occurs under high pressure without modification, and could be utilized to prepare AlH3. However, the process necessitates a hydrogen pressure exceeding 700 MPa at ambient temperature. Saitoh et al. [47] studied the reaction process of aluminum and hydrogen at 10 GPa and 600 °C using in situ X-ray diffraction. As shown in Figure 3, The yellow and red particles represent Al and AlH3 particles, respectively. After 24 h growth, the grain size of AlH3 was about 20 µm. The author also found that the growth process of an AlH3 single crystal will go through three stages: self-pulverization of aluminum (with the increase of reaction time, it is found that the size of aluminum Bragg lattice decreases), hydrogenation of pulverized aluminum (X-ray diffraction image shows a new Bragg lattice), and solid-state grain growth of AlH3.
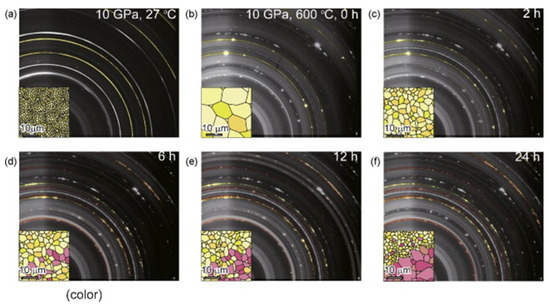
Figure 3.
X-ray diffraction patterns and crystal growth process of Al/AlH3 during high-pressure hydrogenation. (a) Complete Debye rings from aluminum at 10 GPa, 27 °C; (b) Bragg spots from aggregated aluminum (colored in yellow). This image was captured immediately after the sample was heated to 600 °C at 10 GPa; (c–f) Diffraction images taken after 2 h, 6 h, 12 h, and 24 h of heat treatment at 10 GPa and 600 °C. Reprinted with permission from Ref. Reproduced with permission from Ref. [47]. Copyright 2010, Elsevier.
The formation of AlH3 through Al hydrogenation is elucidated, wherein AlH3 particles grow into a single crystal through solid-state grain growth. In addition, even at elevated pressures and temperatures, the hydrogenation reaction can be restricted by the chemically stable oxide layer on the surface of Al. The thickness of the surface oxide layer is crucial in altering the growth of single crystals. Given the demanding reaction conditions and low yield, the direct reaction of aluminum and hydrogen under high pressure to produce AlH3 is not deemed a viable option.
2.4. Comparison of Synthetic Methods and Its Development Suggestion
To summarize, each method has its own advantages and disadvantages. Table 3 summarizes the common synthesis methods of aluminum hydride and some suggestions for development.

Table 3.
Comparison of AlH3 synthetic methods and its development proposal.
3. Thermodynamics and Kinetics of AlH3
3.1. Thermodynamics
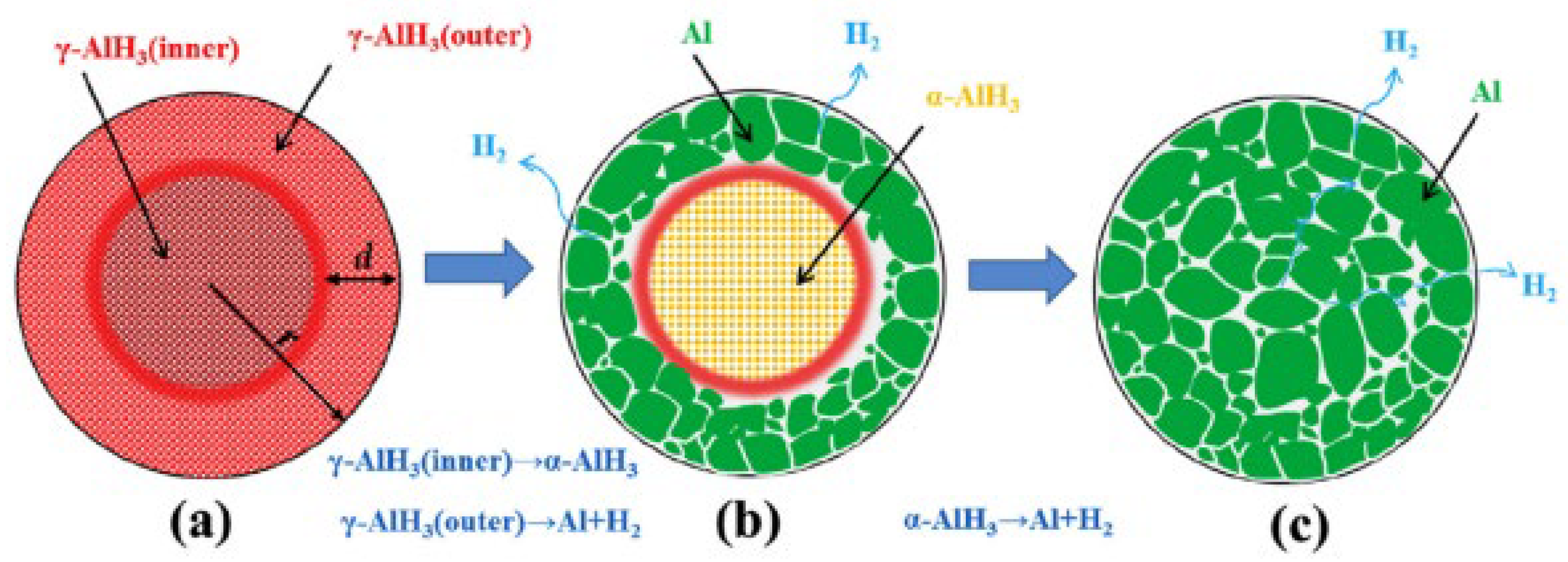
Aluminum hydride is known to degrade over time (after three days). This degradation can impact the stability and effectiveness of AlH3 as a hydrogen storage material. α-AlH3 can decomposes directly into Al and H2 through a single endothermic step (7). Sinke et al. [48] first investigated the thermodynamic properties of α-AlH3, and the formation enthalpy was measured to be −11.4 kJ mol−1 at 25 °C. The less stable polymorphs α’, β, and γ-AlH3 will undergo an exothermic transition to α-AlH3 (8) at around 100 °C [49,50,51], whereas δ, ε, and ζ-AlH3 would direct decomposition into Al without undergoing any crystal transformation. However, the direct decomposition of α’-AlH3 and γ-AlH3 into Al and H2 was also observed (9) [50,52,53,54]. Liu et al. [53] studied the decomposition mechanisms of γ-AlH3 and suggested that the outer layer of the γ-AlH3 particle is more likely to undergo direct decomposition, while the inner part tends to become the more stable α-AlH3 before decomposition (Figure 4). The enthalpies of the transition from α’, β, and γ-AlH3 to α-AlH3 were measured to be 1.6, 1.5, and 2.8 kJ/mol, respectively [50,55]. The formation enthalpy of approximately −10 kJ/mol was estimated for α-AlH3 [55], and the value ranges from −9.5 to −9.9 kJ/mol for β or γ-AlH3, while a low formation enthalpy of −2.5 kJ/mol was reported for α’-AlH3 [50].
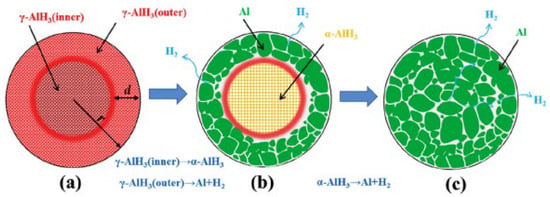
Figure 4.
The dehydrogenation mechanism of γ-AlH3. (a) as-synthesized γ-AlH3; (b) after partial decomposition; (c) after full decomposition. reproduced with permission [53]. Copyright 2017, Elsevier.
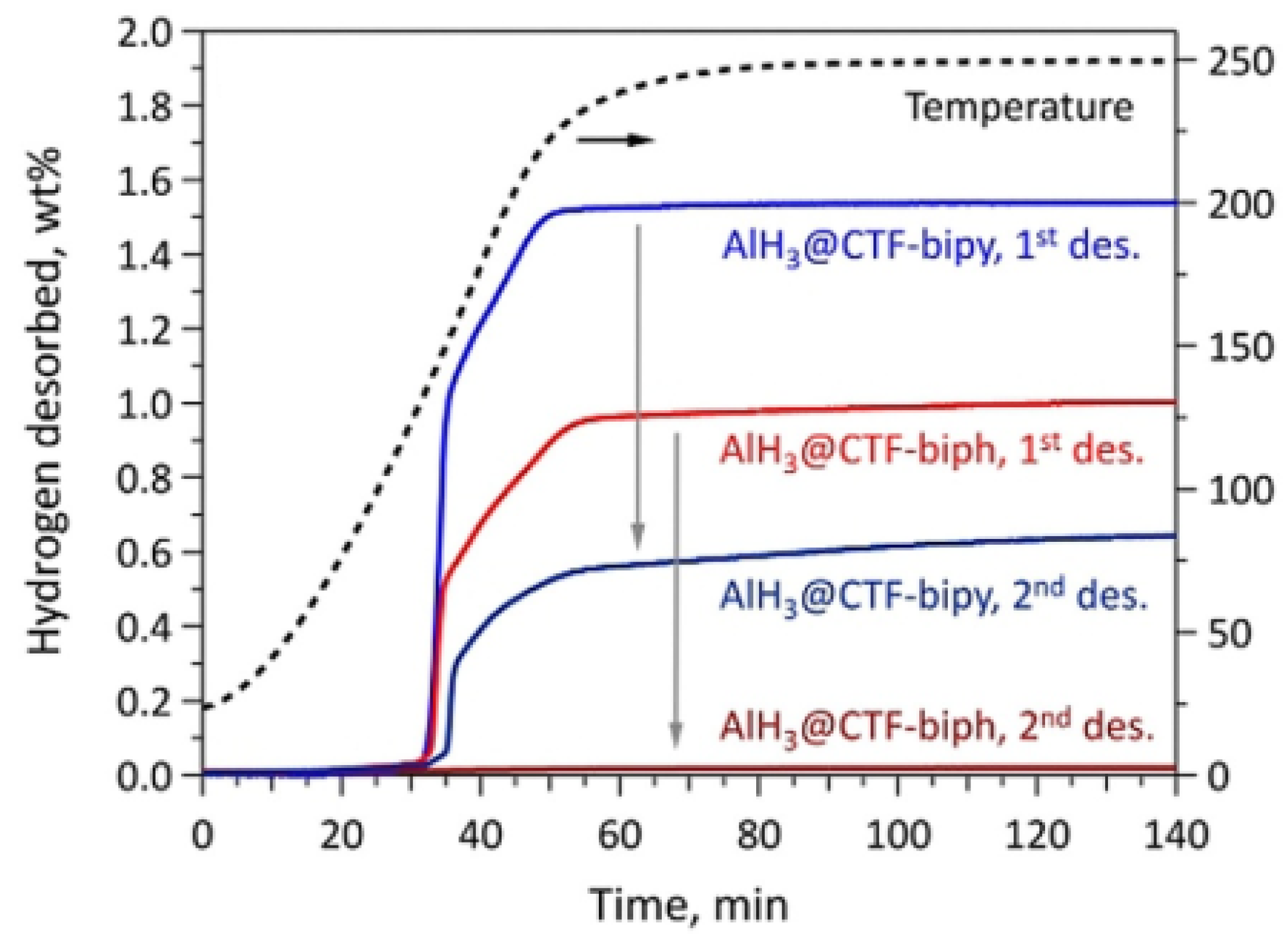
Bulk AlH3 is thermodynamically unstable (metastable) and is only kinetically stable under ambient conditions. Nanoscaling is a well-known strategy for destabilizing bulk metal hydrides, yielding faster rates of H2 desorption, reduced formation of parasitic intermediates, and greater reversibility. Stavila et al. [56] developed a new strategy for thermodynamic stabilization of metal hydrides by incorporating alane clusters within the pores of covalent triazine frameworks (CTF). The highly unfavorable thermodynamics of direct aluminum hydrogenation can be overcome by stabilizing alane within a nanoporous bipyridine-functionalized covalent triazine framework (AlH3@CTF-bipyridine). The material desorb hydrogen at temperatures as low as 95 °C, with ca. 2/3 of the total hydrogen released within less than 10 min from the onset of dehydrogenation and can be partially rehydrogenated at 70 MPa (700 bar) H2 (Figure 5). The results demonstrate that the strategy of thermodynamic stabilization through concomitant nanoconfinement could enable reversibility in high-capacity metastable metal hydrides.
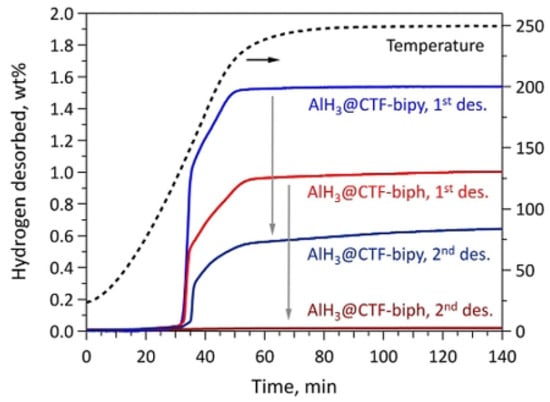
Figure 5.
Sieverts data for AlH3@CTF-biph and AlH3@CTF-bipy samples prior and after the 700 bar (70 MPa) H2 absorption cycle. Reproduced with permission [56] Copyright 2021, Wiley Online Library.
3.2. Kinetics
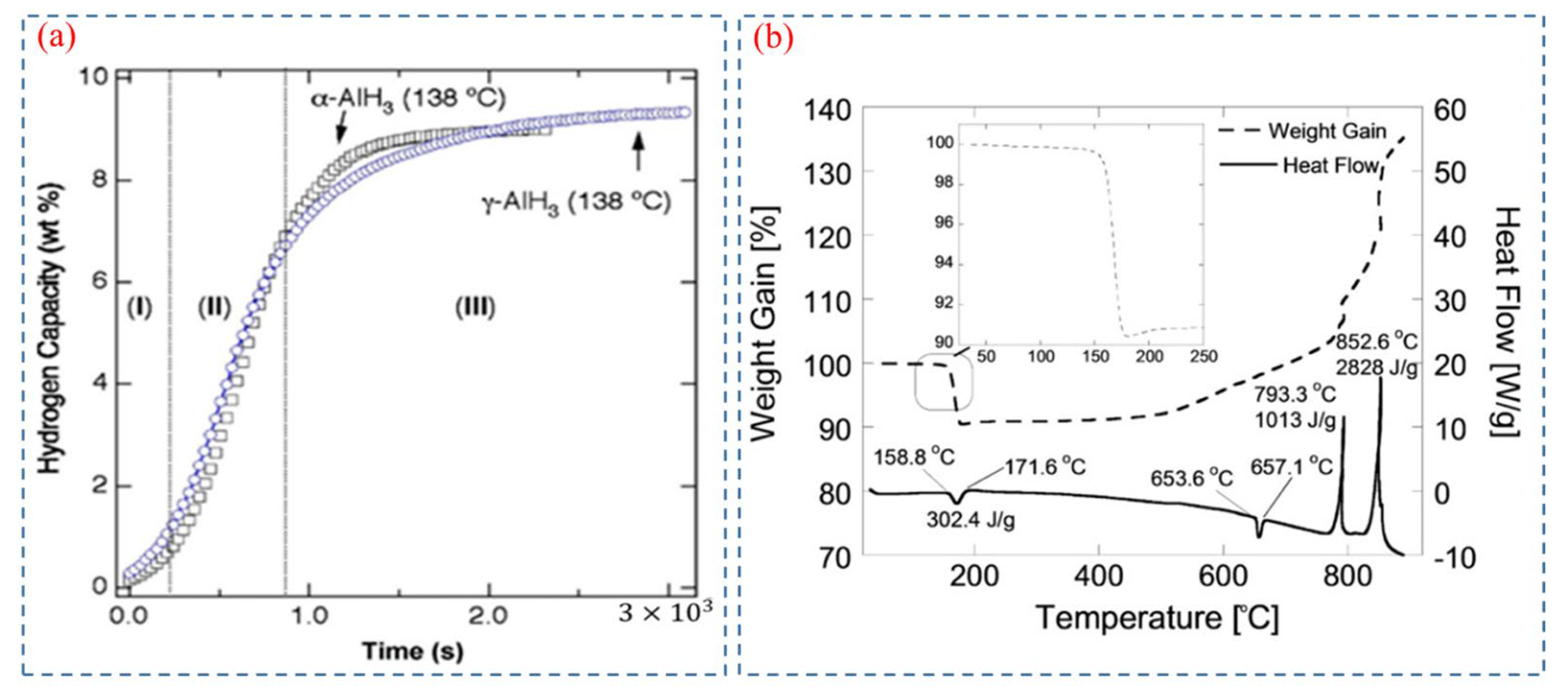
Herley et al. [57,58,59] firstly measured the thermal decomposition behavior of AlH3 provided by DOW Chemical Company. The isothermal decomposition and TG-DSC curves of AlH3 are shown in Figure 6a and Figure 6b, respectively. The decomposition process is characterized by an endothermic step, and the isothermal decomposition curves of AlH3 are usually divided into three parts: induction period, acceleratory period, and decay period. The induction period corresponds to the beginning of decomposition, and could be attributed to the crack of the surface layer. The acceleratory period results from rapid hydrogen release due to the multi-dimensional growth of aluminum [27]. The decay period marks the completion of the decomposition process. [23,27]. Therefore, through managing the time of induction period, the decomposition rate can be controlled [60]. In essence, controlling the formation of Al nuclei is the crucial factor in altering the decomposition rate.
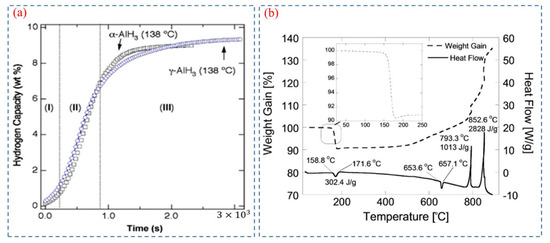
Figure 6.
(a) Isothermal decomposition curves of α-AlH3 and γ-AlH3 [51]. Copyright 2007, Elsevier. (b) TGA and DSC of neat AlH3 in an argon atmosphere [1]. Copyright 2019, Wiley Online Library.
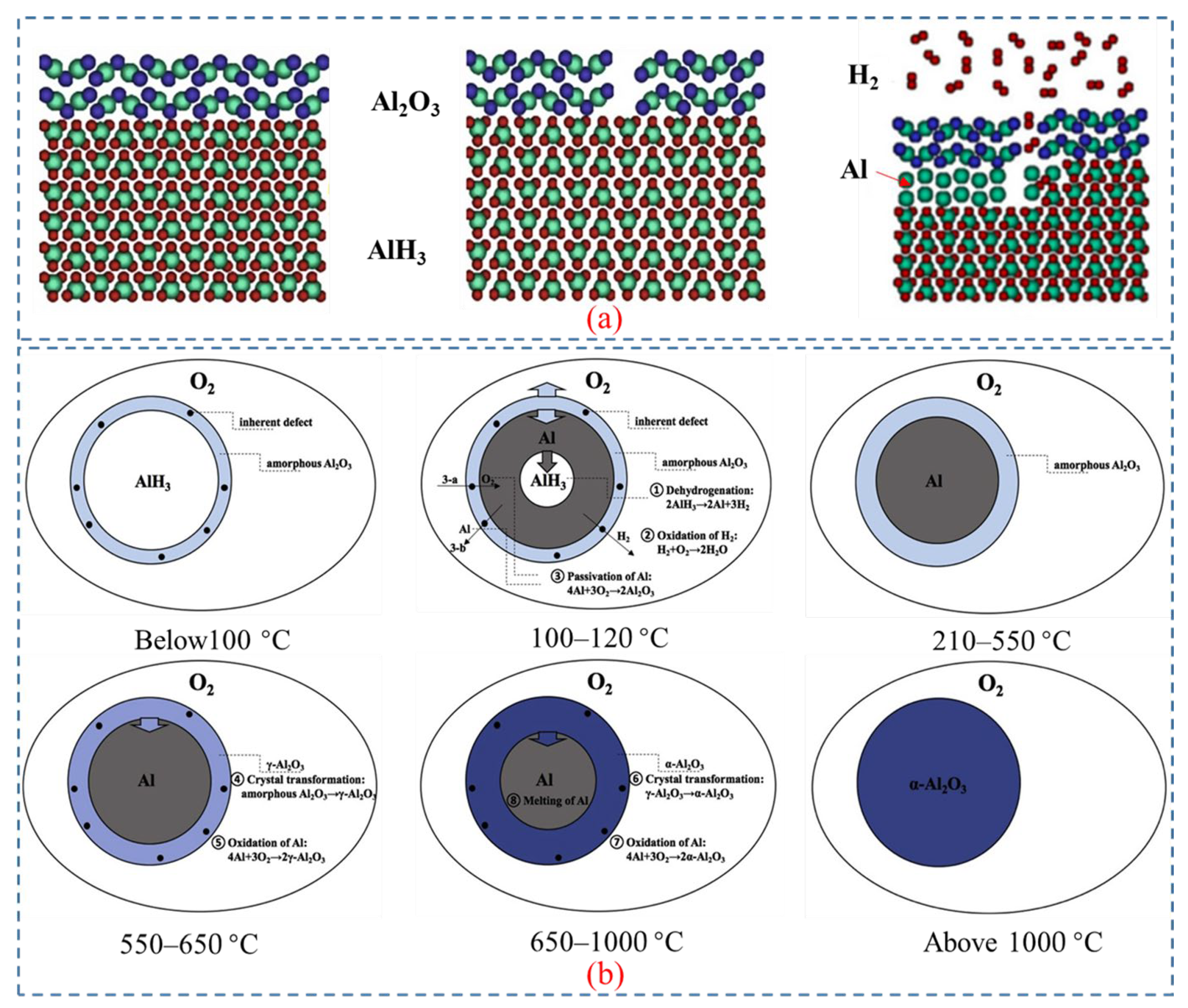
The thermal reaction of AlH3 in an air atmosphere is complex and can be categorized into three stages: dehydrogenation and passivation, primary oxidation, and secondary oxidation. It is noteworthy that the passivation process involving Al → Al2O3, occurs almost simultaneously with the dehydrogenation reactions. An amorphous Al2O3 layer formed on the particles’ surface by passivation encapsulates the contained hydrogen, and leads to incomplete dehydrogenation.
Milekhin et al. [61] employed TG-DSC, SEM, and EDS to study the dehydrogenation and oxidation kinetics and mechanisms of micron-sized α-AlH3 under various atmospheres, including nitrogen, argon, and air. The results indicated that the samples were stable below 150 °C, but can be decomposed between 150 and 180 °C. Figure 6 illustrates the theoretical kinetic model of AlH3 in an oxidative environment.
Figure 7a depicts a schematic diagram illustrating the hydrogen decomposition on the surface of Aluminum hydride [62]. The process includes breakage of the surface oxide layer (e.g., Al2O3), recombination of hydrogen, and growth of aluminum [11]. The oxide layer surrounding AlH3 particles is prone to cracking at elevated temperatures, as the volumetric thermal expansion of AlH3 is approximately double that of amorphous and crystalline Al2O3. The results also indicate that hydrogen desorption begins only when the surface oxide layer breaks and the free AlH3 on the surface of particles contact with the gas phase.
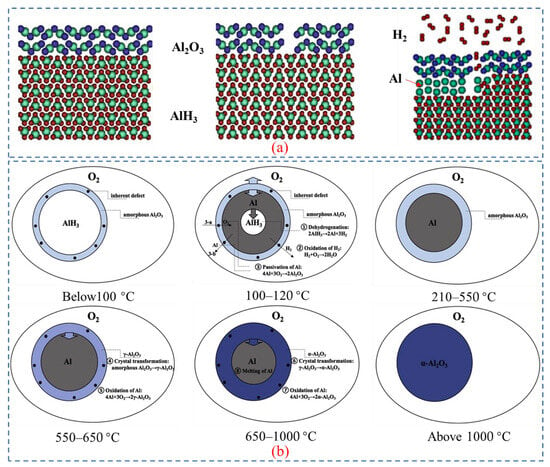
Figure 7.
(a) The mechanism underlying hydrogen release from AlH3 [62]. Copyright 2010, AIP Publishing. (b) The mechanism of dehydrogenation and oxidation of AlH3 at different temperatures [10]. Copyright 2020, Elsevier.
Liu et al. [63] investigated the thermal oxidation of AlH3 and proposed dehydrogenation/passivation mechanism. AlH3 usually has a protective layer of Al2O3 on its surface, which inhibits the dehydrogenation reaction of AlH3 at room temperature [44]. With the increase of temperature, cracks are formed in the Al2O3 layer, exposing the AlH3 surface and causing the occurrence of incomplete dehydrogenation. Since the distance between Al atoms in AlH3 is shorter than that in elemental Al, significant volume shrinkage will occur during the conversion of AlH3 to Al [11,64], resulting in the formation of pores on the particle surface and further increase the exposed AlH3.
The transformation process of Al-containing substances during dehydrogenation and oxidation at different temperatures is shown in Figure 7b. The transition follows the route: α-AlH3 → Al → γ-Al2O3 → α-Al2O3. The reaction consists of decomposing of AlH3, oxidizing of H2, passivating of Al, and the crystal transition of Al2O3 to γ-Al2O3 and γ-Al2O3 to α-Al2O3.
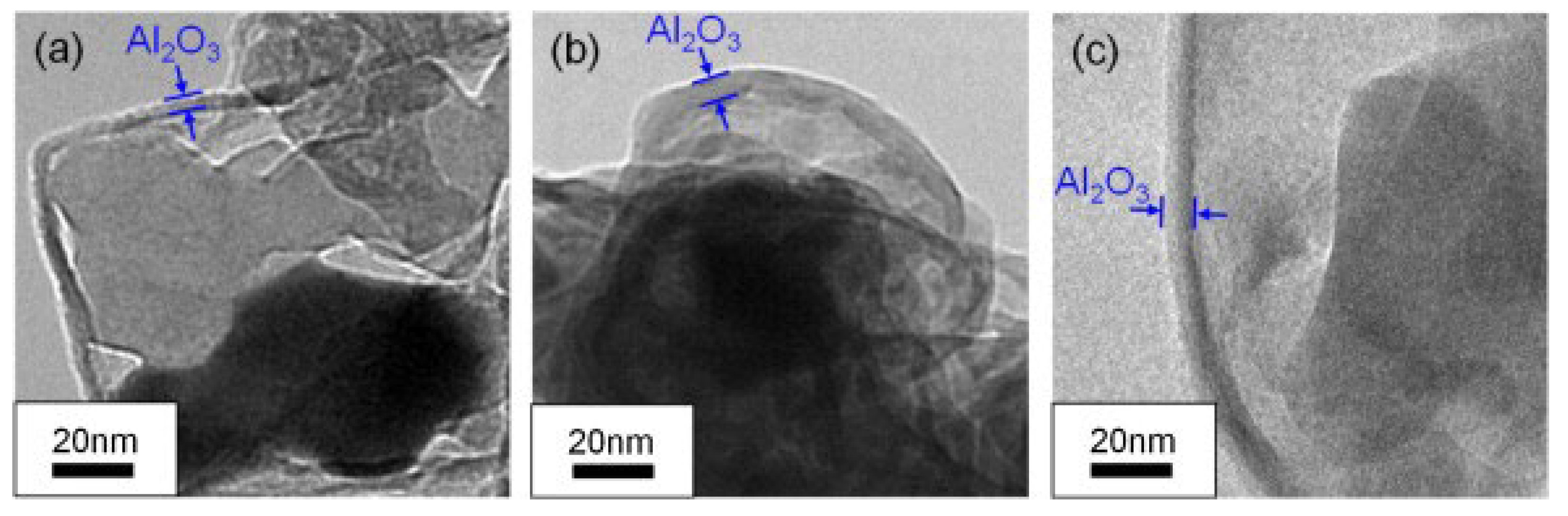
Dehydrogenation and oxidation of AlH3 are influenced by three factors: the oxide layer, the surrounding atmosphere, and temperature. As mentioned above, decomposition only takes place when the oxide layer fractures, allowing free AlH3 to come into contact with the surrounding atmosphere. Nakagawa et al. [65] investigated the effect of the thickness of the oxide layer on the dehydrogenation kinetics of α-AlH3. The TEM results (Figure 8) indicated that the thickness of Al2O3 seems to increase with exposure time through diffusion-controlled growth. Moreover, the peak temperatures during hand milling (135 °C) are significantly lower than those without milling (150 °C) under an argon atmosphere. This is believed to be due to the Al2O3 layer on the surface of AlH3, which may delay the dehydrogenation process. The Al2O3 and Al(OH)3 layer can also affect the dehydrogenation of AlH3. Yu et al. [66] prepared a novel core–shell structured α-AlH3@Al2O3@Al(OH)3 through hydrochloric acid leaching, which does not decompose under environmental conditions for several days. These findings suggest that the presence of an oxide layer can postpone the dehydrogenation process.
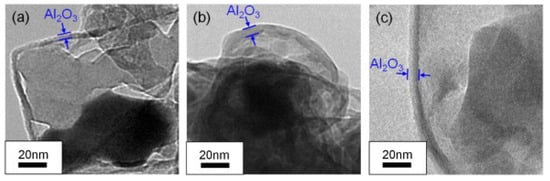
Figure 8.
The TEM images of Al2O3 films on AlH3 particles (a) without exposure to air, (b) one day after exposure to air, (c) seven days after exposure to air [65]. Copyright 2013, Elsevier.
The thermal reaction of AlH3 is significantly influenced by the surrounding atmosphere, because different products could be generated with various gaseous, such as Al2O3 and AlN. Additionally, the dehydrogenation of AlH3 is competitive with Al oxidation in an oxidative atmosphere. As illustrated in Figure 9, the surface agglomeration and roughness of particles in oxidative atmosphere are more obvious under elevating temperature.

Figure 9.
Microphotographs of the AlH3 samples: (a) initial sample (b,c) after heating to 600 °C and 1200 °C in nitrogen medium, respectively. Reproduced with permission [61]. Copyright 2015, Springer.
The dehydrogenation and oxidation properties of AlH3 are also affected by the heating rate and temperature. The impact of the heating rate was examined using a non-isothermal method. The results indicated that both the initial and final temperatures, as well as the final mass loss of the hydrothermal solution, decreased as the heating rate decreased This reason can be attributed to the slower hydrogen diffusion through particles or nucleation at aluminum sites at lower temperatures and heating rates, resulting in some hydrogen remaining trapped in the particles [23].
4. Physical and Chemical Modification of AlH3
Due to the low thermal stability of AlH3 in ambient atmosphere, dehydrogenation will occur easily. The factors contributing to the instability of AlH3 can be categorized into intrinsic and extrinsic causes. Regarding intrinsic causes, AlH3 consists of two highly reducing elements, aluminum and hydrogen, with a small enthalpy of formation and positive Gibbs free energy [26,67,68]. Therefore, AlH3 is in metastable state, and has a spontaneous tendency to decompose into aluminum and hydrogen from thermodynamic perspective.
On the other hand, impurities like LiCl, NaBH4, LiBH4, and LiAlH4, as well as unstable polymorphs formed during AlH3 synthesis can also reduce its thermal stability [69]. Apart from this, other extrinsic factors, e.g., humidity, light, oxygen also make AlH3 unstable.
When AlH3 is used as a high-energy additive in propellants, to ensure its stable storage without decomposition and reduce the potential hazards during storage, transportation and use, it is necessary to stabilize AlH3, for which multiple methods are available, including surface passivation, doping, surface coating.
4.1. Surface Passivation Methods
In this method, some solutions, e.g., aqueous organic solution, buffer solution, and various acid solutions able to react with α-AlH3, are generally used. For one thing, the solutions can remove the synthetic impurities and unstable crystal forms. For another, they can shield against external stimuli by developing passivation layers of Al2O3 or Al(OH)3 on the surface of α-AlH3.
Nile et al. [70] treated α-AlH3 with n-butylamine containing a small amount of water. It took 60 days for the decomposition rate to reach 1%, while the untreated α-AlH3 reached the same decomposition rate in 13.5 days, indicating the stabilization effect of the treatment.
Robert et al. [71] used neutral buffer solution KH2PO4 and NaOH to treat α-AlH3 at 70 °C. The contents of impurities, e.g., C, Cl, and Li, in the particles were significantly reduced after 15 min, and the decomposition rate of α-AlH3 was also decreased, it took 26 days for the decomposition rate to reach 1% compared with 8 days without treated.
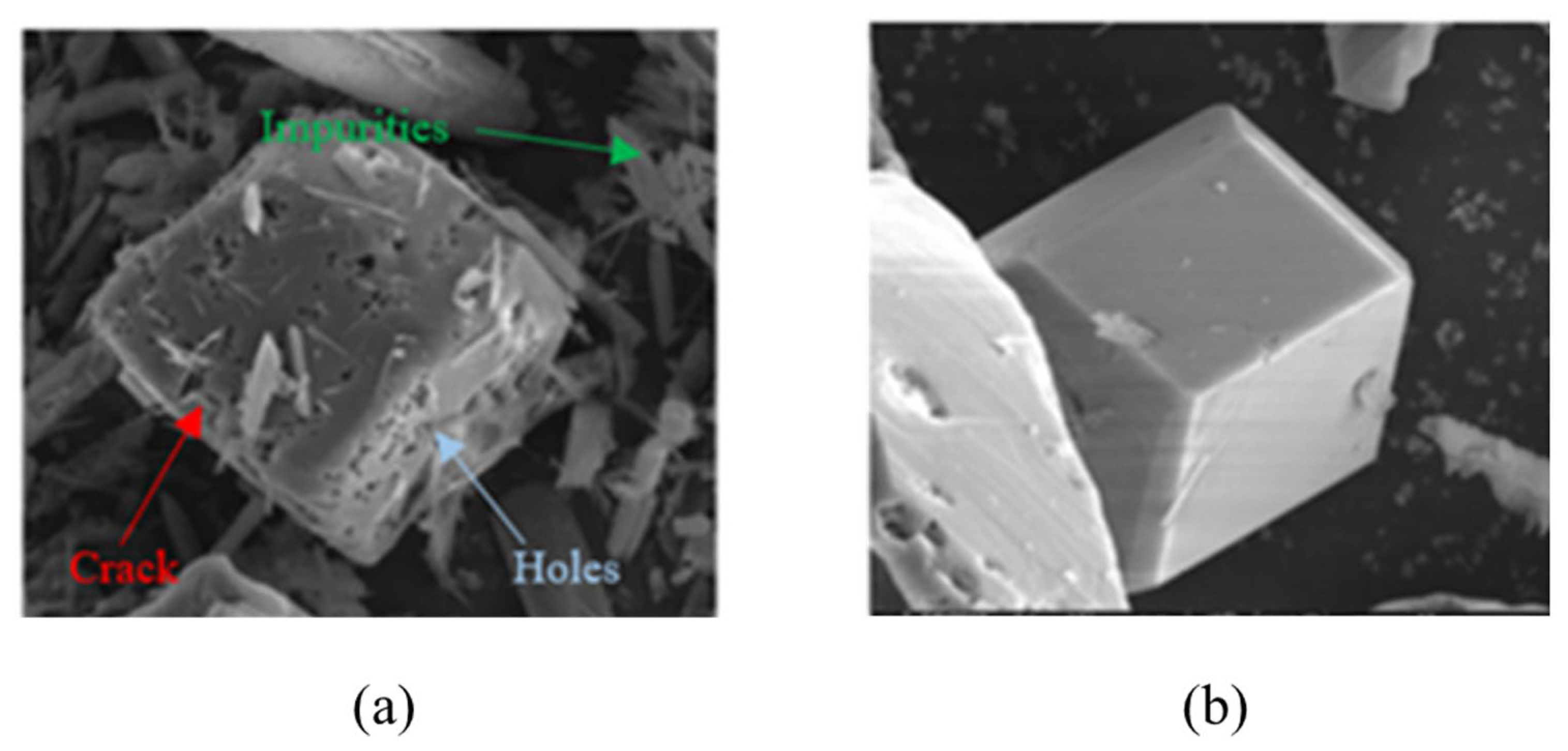
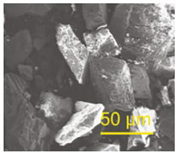
The α-AlH3 particles can also be stabilized using acid solutions. Typically, the acid choices include hydrochloric acid, hydrogen fluoride acid, hydrogen bromide acid, and others, with hydrochloric acid being the most preferable. Petrie et al. [17] used hydrochloric acid solution to clean α-AlH3. Meanwhile, the pickling process can also form Al2O3 or Al(OH)3 layer on the surface of the α-AlH3, which plays a stabilizing role. It took 12.7 days for α-AlH3 treated with hydrochloric acid solution to reach 1% decomposition at 60 °C, while the untreated α-AlH3 only takes 9.3 days. Figure 10 shows the SEM images of α-AlH3 before and after hydrochloric acid treatment. The results demonstrate that acid etching can eliminate cracks and impurities, expected to be beneficial to thermal stability of AlH3 products [24].
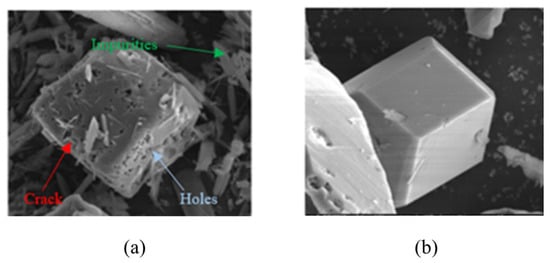
Figure 10.
SEM images of α-AlH3 (a) before and (b) after hydrochloric acid treatment.
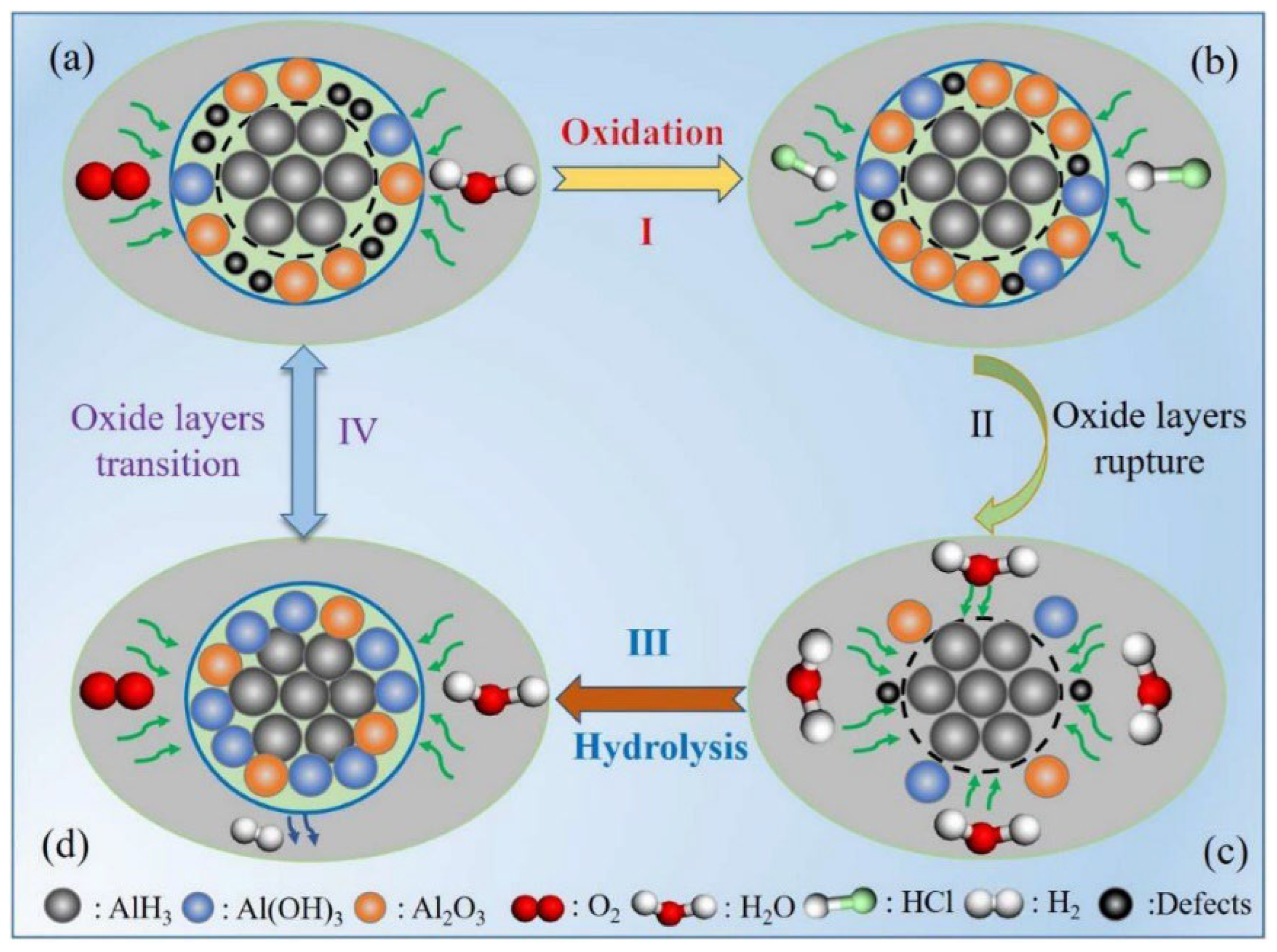
The mechanism of hydrochloric acid stabilization of α-AlH3 was investigated by Yu et al. [66]. They found that hydrochloric acid plays an important role in accelerating the hydrolysis reaction of AlH3 to generate honeycomb-like structures which can generate integrity and dense oxide layers. Moreover, the detailed acid stabilization mechanism could be divided into surface oxidation, oxide layer rupture, hydrolysis reaction, and transition of oxide layers, as depicted in Figure 11.
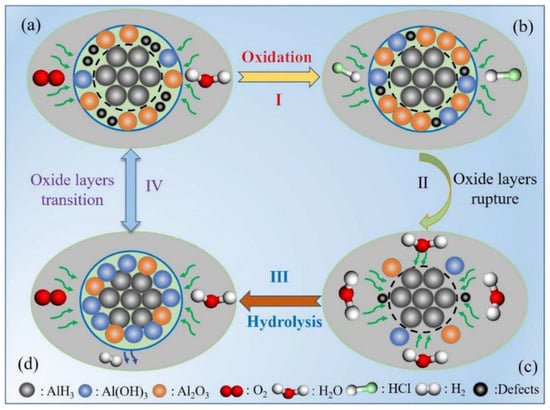
Figure 11.
The stabilization mechanisms by acid passivation for AlH3 [66]. (a) original structure; (b) surface oxidation; (c) oxide layers rupture and (d) honeycomb-like structure. Copyright 2022, Elsevier.
Additionally, surface passivation of AlH3 can be achieved by heating it in air or an oxidizing atmosphere [72]. Despite the benefits to improve the thermal stability, the presence of an oxide layer by various passivation methods will inevitably cause the energy loss of α-AlH3 and affect the combustion performance.
4.2. Doping Methods
The thermal stability of AlH3 can be improved by doping following two approaches. One approach aims to eliminate the highly active sites in α-AlH3 crystal structure, hence increasing the activation energy of decomposition reaction. Norman et al. [73] added magnesium powder to the reaction of preparing α-AlH3, and the X-ray diffraction results showed varying degrees of expansion for the crystal structure of α-AlH3. It took 26 days for α-AlH3 doped with 2% magnesium powder to reach 1% decomposition rate in an anhydrous nitrogen atmosphere at 60 °C, while the undoped one only took 5 days. Cianciolo et al. [74] prepared α-AlH3 particles by doping α-AlH3 ether complexes with Hg. The experimental results indicated that the decomposition rate of α-AlH3 particles doped with 0.02% Hg is 0.4% at 100 °C for 24 h, far lower than the value of 9.4% of undoped particles under the same conditions. Furthermore, doping with other metal elements, such as Si, can also enhance the thermal stability of AlH3 [74].
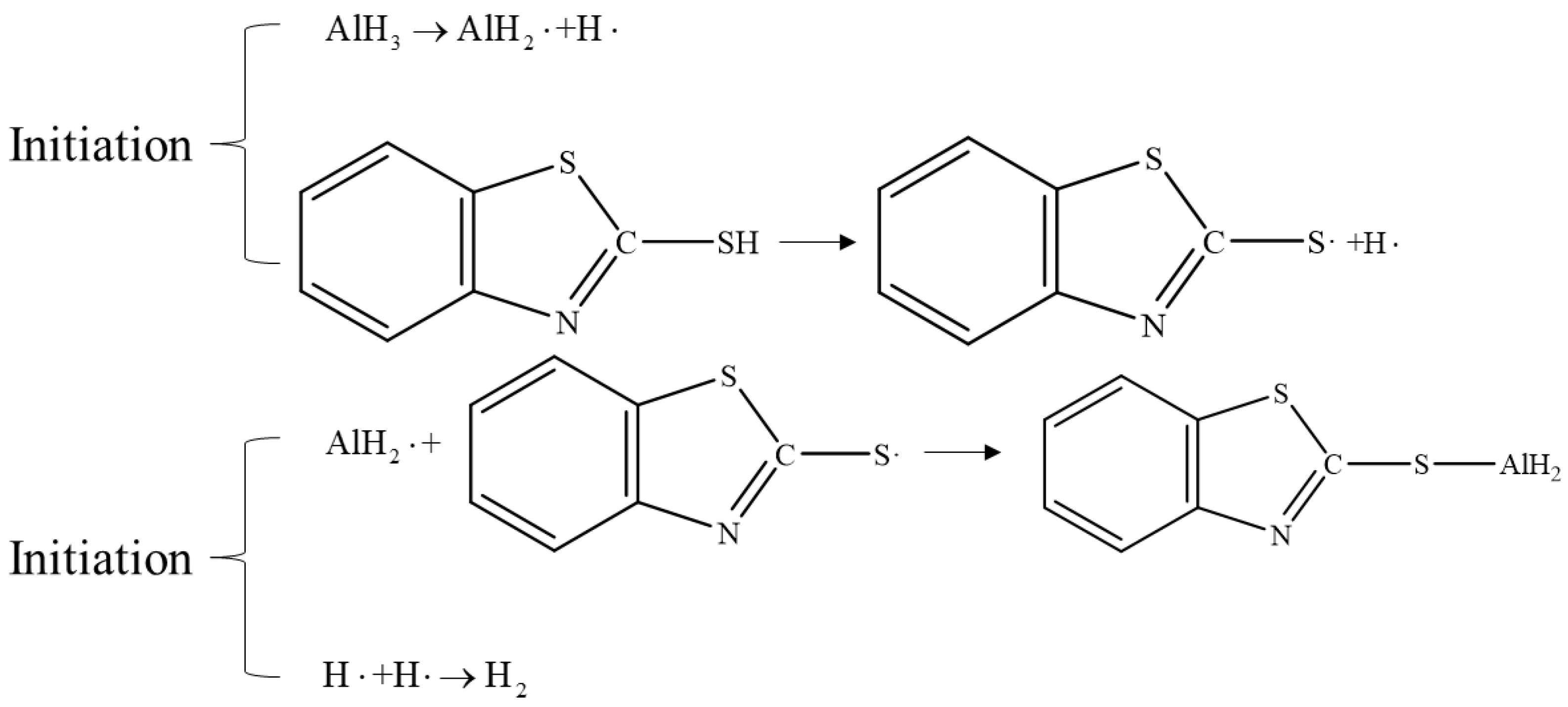
In theory, the decomposition of AlH3 generates an electron hole caused by the formation of positively charged ions, while the Al3+ ions can further catalyze the surface decomposition of the bulk sample [75]. Therefore, the other approach is inhibiting the decomposition process by increasing the Lewis acid, Lewis base, or other compounds that can coordinate with Al3+ ions. It has been reported that the addition of such free radical inhibitors can enhance the thermal stability of AlH3 by a factor of 10 to 20 [2]. For example, Alan et al. [76] doped of AlH3 using phenothiazine (PTA) or Mercaptobenzothiazole (MBT, as shown in Figure 12), making its decomposition rate only 0.97% after 27 days of storage at a constant temperature of 60 °C, far lower than that of untreated samples. Roberts et al. [77] doped aryl or alkyl-substituted silanols in the preparation process of α-AlH3. From their results, 8 and 4 days were reported for the decomposition rate to reach 1% for the doped and undoped α-AlH3, respectively.
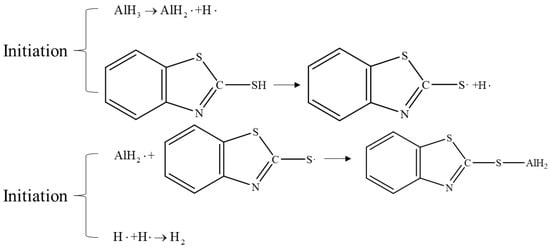
Figure 12.
Stabilization mechanism of Mercaptobenzothiazole (MBT).
Similar to surface passivation methods, doping of α-AlH3 also results in a loss of energy due to decrease in the crystal purity of α-AlH3.
4.3. Surface Coating Methods
The surface coating method has received wide attention in recent years because it does not destroy the chemical reactivity of α-AlH3 [70,77]. Typically, the surface of AlH3 is coated with inert and hydrophobic materials to create a core–shell structure, thereby altering its surface properties [78]. The structure not only prevents direct contact between the inner AlH3 and humidity or oxygen but also reduces the number of active sites on the surface, thereby increasing the activation energy of the decomposition reaction [79]. Currently, coating materials mainly consist of inorganic and organic molecules, metal oxides, organic polymers, carbon materials, and some energetic components.
4.3.1. Inorganic and Organic Molecules
Norman et al. [80] used small gaseous (such as N2F4, NO) and liquid molecules (such as Al2S3, AlCl3) to form a coating layer on the surface of α-AlH3 through an adsorption process. As a typical example, under 100 °C, the decomposition amount of the coated sample with NO molecule is reduced from 5.63% to 0.21%, for a duration of 7 h. In addition, after treatment with these small molecule stabilizers, α-AlH3 shows good compatibility with other propellant components, such as ammonium perchlorate and nitrocellulose.
Schmidt et al. [18] used organic compounds containing nitrile groups to coat α-AlH3, and the stability of AlH3 was found to be enhanced through the bond interaction of nitrile groups and free radicals on its surface. Besides, the compatibility between α-AlH3 and propellant components was also improved.
Qin et al. [14] coated α-AlH3 with stearic acid, and no ignition was observed for the coated sample even when the value of electrostatic sensitivity(E50) reached the test limit of 5390 mJ. In comparison, the E50 of α-AlH3 without coating is only 367 mJ.
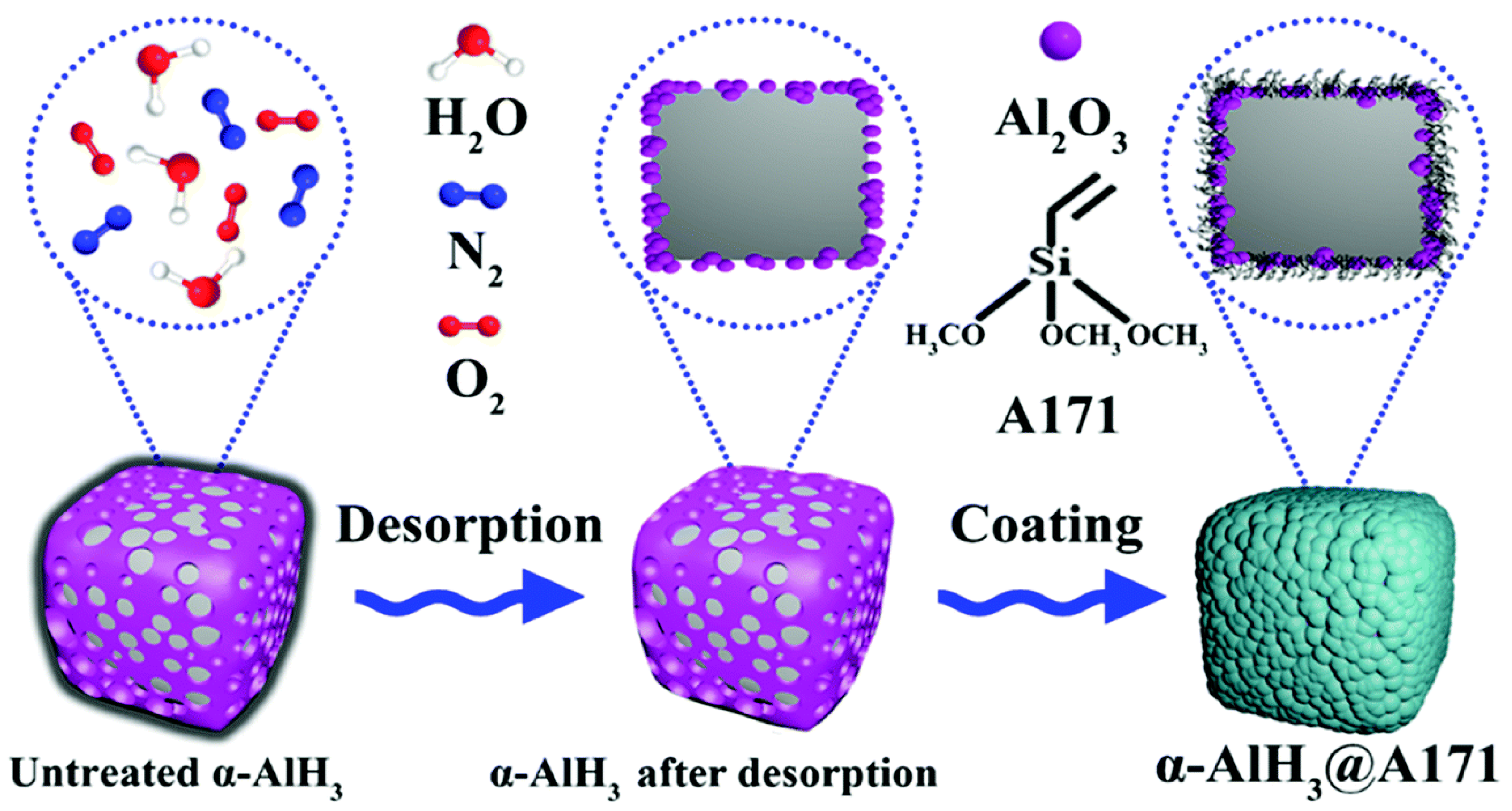
Shi et al. [81] used a silane coupling agent, A171, to modify the surface of α-AlH3. The surface was uniformly covered with a thin organic layer via the coating process shown in Figure 13. After coating, the standard 100 °C thermostatic thermal stability and activation energy of α-AlH3 increased up to 95.25 min and 4.32 kJ mol−1, respectively.
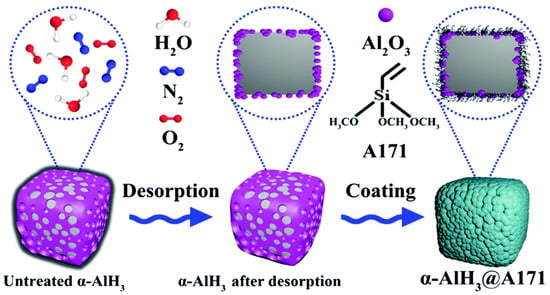
Figure 13.
The preparation process of the α-AlH3@A171 composite [81]. Copyright 2022, Royal Society Chemistry.
4.3.2. Metal Oxides
Kempa et al. [82] found that the surface aluminum oxide layer of α-AlH3 can effectively isolate water and oxygen molecules in the environment so that the crystal structure of α-AlH3 does not change significantly for a long time. In recent years, researchers have tried to control the coating structure by atomic layer deposition (ALD) and other advanced techniques to achieve accurate control of the thermal stability of AlH3 [83].
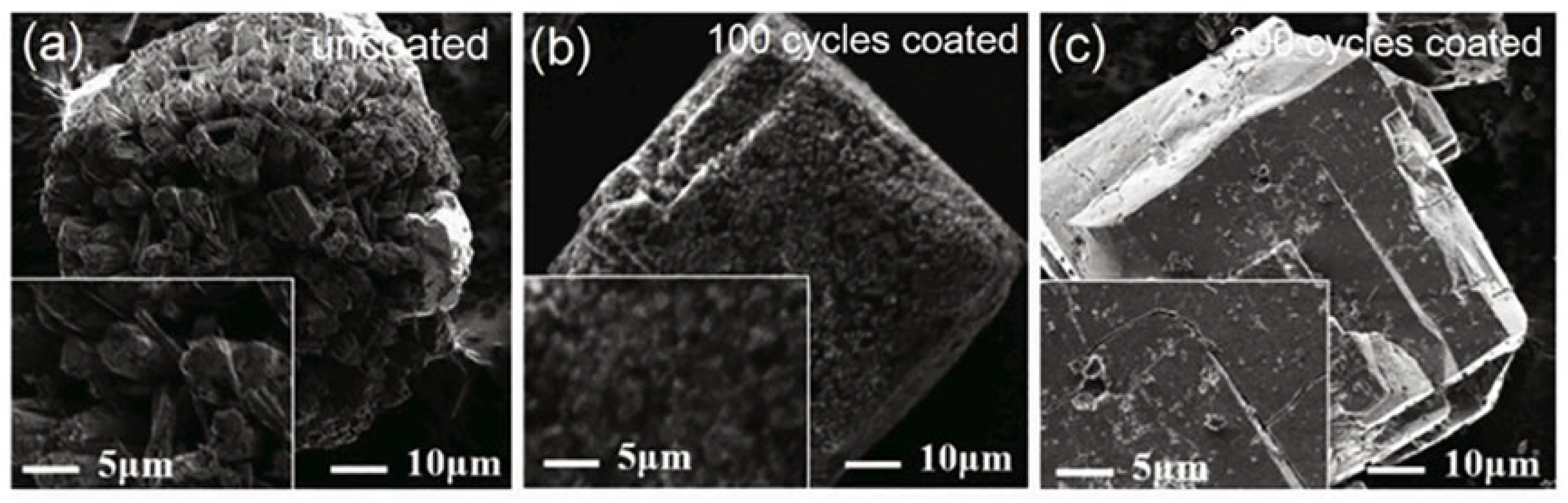
Chen et al. [12] used ALD to deposit a layer of amorphous Al2O3 on the surface of α-AlH3. The results showed that with the increase of coating cycles from 100 to 200, the decomposition temperature increased from 158.1 °C to 161.2 °C compared to 153.4 °C for the uncoated sample. Figure 14 displays the SEM images of α-AlH3@Al2O3 particles after the hydrothermal aging test. It can be clearly seen that the surface of α-AlH3 particles after 200 coating cycles becomes much smoother than the uncoated one. Moreover, its friction sensitivity decreases from 96% to 68% after the hydrothermal aging test. The Al2O3 films can effectively impede the transfer of frictional heat to the AlH3 core, thereby reducing the friction sensitivity of the core–shell structure.
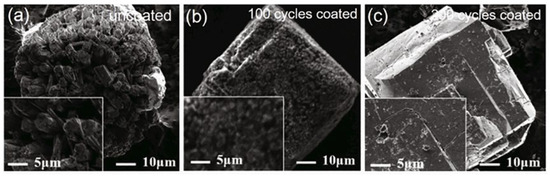
Figure 14.
SEM images of α-AlH3 stabilized by ALD method before and after. Hydrothermal aging test after ALD method (a) 0 cycles; (b,c) 100 and 200 cycles, respectively [12]. Copyright 2017, AVS.
4.3.3. Organic Polymers
Due to the non-energetic properties of small molecules as a coating layer for stabilization of AlH3, the overall energy performance of the propellant will be attenuated. Therefore, the coating layer is preferably selected from the energetic components, such as nitrocellulose (NC). Flynn et al. [20] applied α-AlH3 particles coated with nitrocellulose to a double-base solid propellant. As a result, the decomposition rate of AlH3 was only 0.63% at 50 °C for 90 days, indicating that it had a significant stabilization effect.
Cai et al. [16] used supercritical fluid technology to cover the surface of α-AlH3 with fluoroelastomer (FE26) uniformly. Total formation enthalpy (∆Hf) of alane and alane/FE26 increased from −15.7 kJ/mol to −21.0 kJ/mol at heating rate of 20 °C, while Gibbs free energy (∆Gf) decreased from 44 kJ/mol to 38.7 kJ/mol, indicating increased thermal stability. In electric sparkle sensitivity test, the E50 value of alane is 63.71 mJ, and the value increased up to 85.24 mJ after 5% FE26 is coated on alane. This result indicates that after coating the FE26, safe electrostatic discharge can be achieved.
4.3.4. Carbon Materials
Su et al. [84] reported a novel method of coating AlH3 with carbon nanotubes (CNT), where re-nucleation and growth of α-AlH3 on the surface of the coating material, rather than the formation of the coating material on the surface of α-AlH3 particles, occurred.
Xing et al. [15] attempted to improve the thermal stability of α-AlH3 by using fullerene stabilizers (C60), which allows α-AlH3 to be stored at 60 °C for 3 months with a decomposition rate of less than 1%.
Li et al. [85] used the solvent-antisolvent method to coat α-AlH3 with graphene oxide (GO). The α-AlH3-GO material obtained was successfully applied to the solid propellant slurry, for which the mechanical impact sensitivity of slurry was effectively reduced, evidenced by an increase of the I50 value from 7.3 J to 12.1 J.
4.3.5. Energetic Components
As mentioned above, increasing the content of this materials would undoubtedly reduce the energy density and detonation property of the propellants. Therefore, in order to achieve higher energy density, the coatings layer can be selected from stable energetic components.
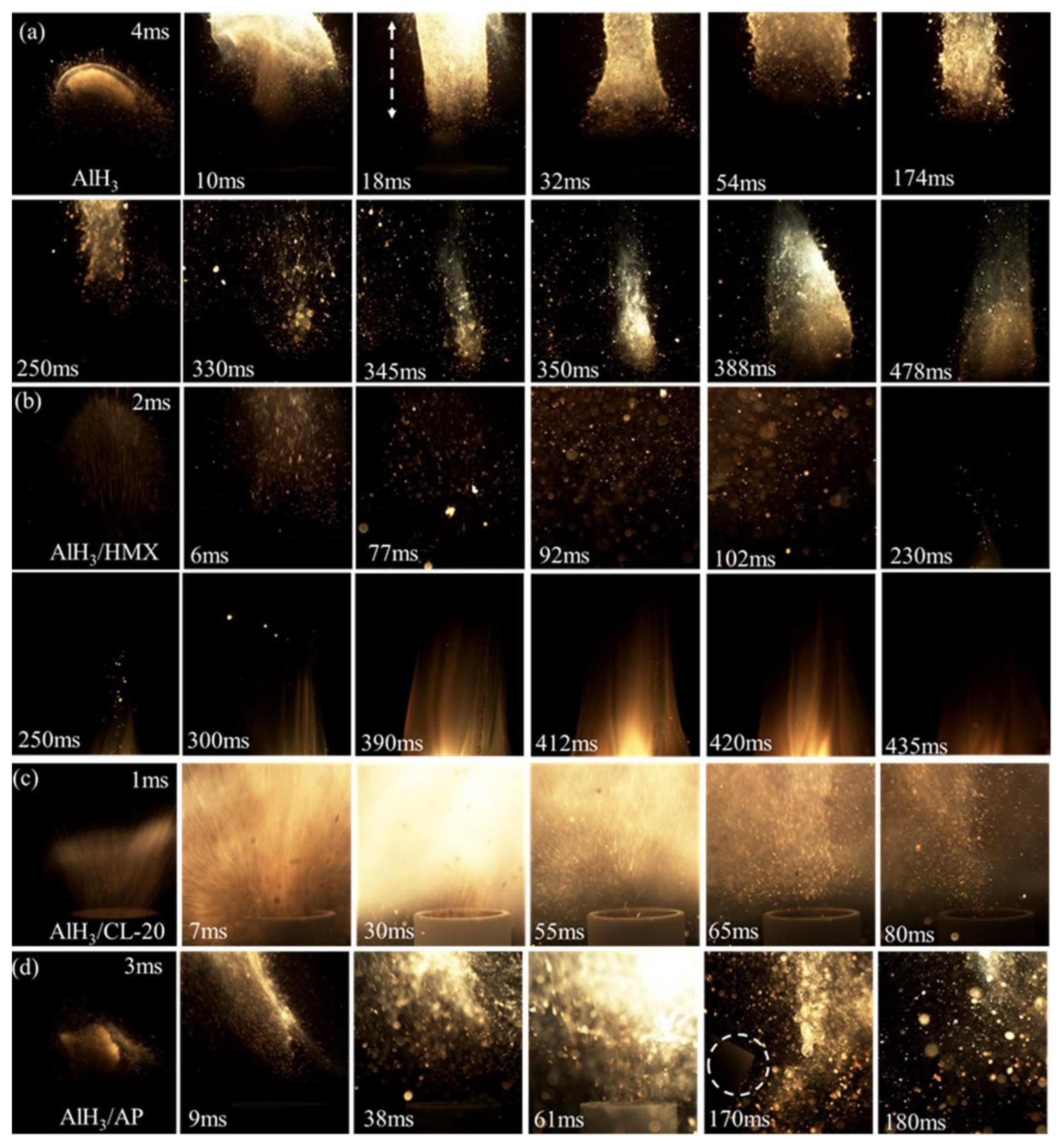
Yu et al. [86] prepared the homogenous composites of AlH3 and commonly used oxidizer (HMX, CL-20, and AP) using the in situ recrystallization method, for which thermal stability, compatibility, and ignition performance were investigated. The results showed that the initial decomposition temperatures of AlH3/oxidizer are increased by about 16 °C, and the induction time of the dehydrogenation of AlH3 is extended by 1.2 times. Such a stabilization effect of the oxidizers can be attributed to strong hydrogen bonding. Furthermore, the flame radiation intensity of each composite was enhanced, and the most intensive value of AlH3/CL-20 is 3.4 times that of pure AlH3. Moreover, the crucible is even broken (marked with a white circle) when using AlH3/AP composites as trigger for water-cooled CO2 laser ignitor, as depicted in Figure 15.
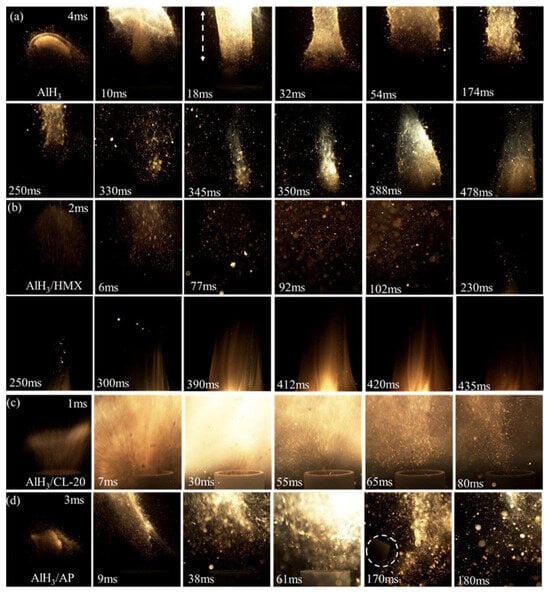
Figure 15.
Flame images of raw AlH3 and AlH3/oxidizer composites: (a) AlH3, (b) AlH3/HMX, (c) AlH3/CL-20, and (d) AlH3/AP [86]. Copyright 2023, American Chemical Society.
Yu et al. [87] prepared fluoropolymer- and AP-coated AlH3-based composites (AHFPs) via a spray-drying technology aiming to improve the stability and combustion performance of AlH3. The results showed that the initial decomposition temperatures of AHFPs were increased by 17 °C, compared with pure AlH3. Moreover, the decomposition induction time was increased by 1.82 times compared to raw AlH3, indicating improved stability by the coatings of PFPE and AP. The maximum flame radiation intensity of AHFPs-30% is 7.71 times that of pure AlH3, suggests that the combustion performance of AlH3 has also been enhanced. Table 4 summarizes the primary reported stabilization methods and properties of stabilized AlH3.

Table 4.
The methods used for stabilizing and the resulting properties of AlH3.
At present, the research of stabilizing of AlH3 mainly focus on the surface coating method, involve the screening of the coating agent, especially energetic materials. However, how to accurately control the coating thickness so as to have better compatibility with the propellant components is the key for further research.
5. High Energy Fuel for Solid Propellant
Compared with aluminum powder, a wildly metal fuel adopted to improve the energy characteristics of composite propellants [88,89], AlH3 has extraordinary potential in the application of solid propellant. NASA CEA results indicate that replacing aluminum with AlH3 will increase specific impulse by 10% [90] over AP/HTPB rocket propellant and reduce flame temperature by 5% [91]. Furthermore, AlH3 produces more H2 and H2O in the combustion products, reducing the mole fraction of combustion product. Shark et al., found the use of AlH3 hydride additives could raise the overall specific impulse of DCPD/RGHP by 4% [92]. Maggi et al., demonstrated a 6–8% gain in gravimetric specific impulse gain for the aluminum and lithium aluminum hydrides when aluminized AP/HTPB compositions were chosen as a Reference [91].
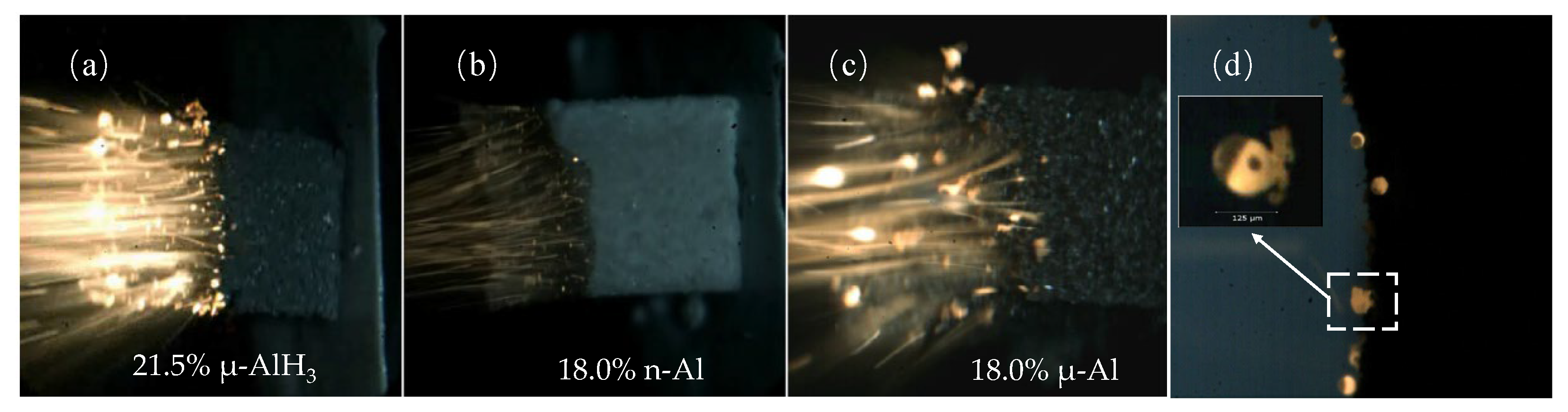
The ballistic and physical properties of propellants based on AlH3 were investigated experimentally [3]. As depicted in Figure 16a–c, the flame intensity of AlH3-based propellants is significantly lower compared to micron and nano-aluminized solid propellants, indicating a lower adiabatic temperature. Another difference lies in the aggregation/agglomeration processes on the burning surface. Figure 15d illustrates that the high burning rate of AlH3-based propellants can effectively prevent the aggregation phenomenon.

Figure 16.
(a–c) The flame structures observed during the combustion of AlH3-based propellants; (d) the burning surface [3]. Copyright 2007, Elsevier.
Bazyn et al. [93] studied the combustion characteristics of AlH3 under the condition of a solid rocket motor. The experimental results show that the decomposition time of AlH3 is exponentially correlated with temperature, which satisfies the Arrhenius type rate equation.
Although AlH3 has been used as energy fuel for solid propellant. The intrinsic metastability affects its further practical application. The easy decomposition of AlH3 causes concern for the safe storage and continuous combustion of the propellant system.
6. Conclusions and Suggestions
AlH3 is a binary metal hydride with seven known polymorphs among which α is the most stable. Due to the high volumetric energy density of AlH3, the introduction into solid propellant is helpful to provide a large amount of energy via combustion. In this paper, the synthesis methods, key properties, and tuning of AlH3 are presented, with the following conclusions drawn:
- The commonly used methods of synthesizing AlH3 are wet chemical synthesis and mechano-chemical method, both of which are faced with the difficulty in achieving high crystal purity of the product. Adding crystalline inducer helps to control the proportion of crystal types, but the presence of an inducer in the product will lead to a decrease in the thermal stability of AlH3. Therefore, highly effective inducers are easy to separate, and need to be developed.
- From a kinetics perspective, the acceleratory period is a critical stage that governs the decomposition rate of aluminum hydride, primarily due to the multi-dimensional growth of the aluminum phase. However, the acceleration period is difficult to control. Thus, how to decelerate the decomposition rate by prolonging the induction period through modifying the surface components of AlH3 is the next research direction.
- The stabilization of AlH3 involves enhancing crystal purity, isolating from external stimuli, and eliminating factors contributing to instability. Among the stabilization methods, surface passivation and the doping method will cause energy loss and negatively affect the combustion performance. As for surface coating, the ratio and thickness of the coating layer proves difficult to control, resulting in failure to accurately regulate its impact on the energy performance of α-AlH3. Efficient coating materials are crucial for advancing the application of AlH3 in solid propellants.
Author Contributions
Writing—original draft preparation, Y.L.; writing—review and editing, F.Y.; investigation and supervision, Z.W. and Z.Z.; data curation and funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author Yang Zhang was employed by the company Xi’an Modern Chemistry Research Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Young, G.; Risha, G.A.; Connell, T.L.; Yetter, R.A. Combustion of HTPB Based Solid Fuels Containing Metals and Metal Hydrides with Nitrous Oxide. Propellants Explos. Pyrotech. 2019, 44, 744–750. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Yu, H.; Bao, L.; Zhang, W.; DeLuca, L.T.; Shen, R.; Ye, Y. The Rapid H2 Release from AlH3 Dehydrogenation Forming Porous Layer in AlH3/Hydroxyl-Terminated Polybutadiene (HTPB) Fuels during Combustion. J. Hazard. Mater. 2019, 371, 53–61. [Google Scholar] [CrossRef]
- DeLuca, L.T.; Galfetti, L.; Severini, F.; Rossettini, L.; Meda, L.; Marra, G.; D’Andrea, B.; Weiser, V.; Calabro, M.; Vorozhtsov, A.B.; et al. Physical and Ballistic Characterization of AlH3-Based Space Propellants. Aerosp. Sci. Technol. 2007, 11, 18–25. [Google Scholar] [CrossRef]
- Ding, X.; Shu, Y.; Liu, N.; Wu, M.; Zhang, J.; Gou, B.; Wang, H.; Wang, C.; Dong, S.; Wang, W. Energetic Characteristics of HMX-Based Explosives Containing LiH. Propellants Explos. Pyrotech. 2016, 41, 1079–1084. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhang, L.; Tian, S. Effects of Magnesium-Based Hydrogen Storage Materials on the Thermal Decomposition, Burning Rate, and Explosive Heat of Ammonium Perchlorate-Based Composite Solid Propellant. J. Hazard. Mater. 2018, 342, 477–481. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, F.; Xu, S.; Yang, F.; Yao, E.; Ren, X.; Wu, Z.; Zhang, Z. Investigation on Adsorption and Decomposition Properties of CL-20/FOX-7 Molecules on MgH2(110) Surface by First-Principles. Molecules 2020, 25, 2726. [Google Scholar] [CrossRef]
- Pang, W.; Fan, X.; Zhao, F.; Xu, H.; Zhang, W.; Yu, H.; Li, Y.; Liu, F.; Xie, W.; Yan, N. Effects of Different Metal Fuels on the Characteristics for HTPB-based Fuel Rich Solid Propellants. Propellants Explos. Pyrotech. 2013, 38, 852–859. [Google Scholar] [CrossRef]
- Cooper, M.A.; Oliver, M.S. The Burning Regimes and Conductive Burn Rates of Titanium Subhydride Potassium Perchlorate (TiH1.65/KClO4) in Hybrid Closed Bomb-Strand Burner Experiments. Combust. Flame 2013, 160, 2619–2630. [Google Scholar] [CrossRef]
- Bi, X.; Liu, J. Detonation Properties of High Explosives Containing Ammonia Borane. Z. Anorg. Allg. Chem. 2016, 642, 773–777. [Google Scholar] [CrossRef]
- Li, H.; Liang, D.; Yu, M.; Liu, J.; Wang, Y.; Pang, A.; Tang, G.; Huang, X. Study on Dehydrogenation and Oxidation Kinetics Mechanisms of Micron α-AlH3 in an Oxidative Atmosphere. Int. J. Hydrogen Energy 2020, 45, 24958–24967. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J.; Yartys, V.A.; Maehlen, J.P.; Bulychev, B.M.; Antonov, V.E.; Tarasov, B.P.; Gabis, I.E. Aluminum Hydride as a Hydrogen and Energy Storage Material: Past, Present and Future. J. Alloys Compd. 2011, 509, S517–S528. [Google Scholar] [CrossRef]
- Chen, R.; Duan, C.L.; Liu, X.; Qu, K.; Tang, G.; Xu, X.-X.; Shan, B. Surface Passivation of Aluminum Hydride Particles via Atomic Layer Deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 03E111. [Google Scholar] [CrossRef]
- Jiang, Z.F.; Zhao, F.Q.; Zhang, M.; Li, H.; Zhang, J.-K.; Yang, Y.-J.; Li, N. Research Progress in the Stabilization of Aluminum Hydride. Huozhayao Xuebao/Chin. J. Explos. Propellants 2020, 43, 107–115. [Google Scholar] [CrossRef]
- Qin, M.-N.; Zhang, Y.; Tang, W.; Shi, Q.; Wang, W.; Qiu, S.-J. α-AlH3 Coated with Stearic Acid: Preparation and Its Electrostatic Sensitivity. Hanneng Cailiao/Chin. J. Energ. Mater. 2017, 25, 59–62. [Google Scholar] [CrossRef]
- Xing, J.H.; Xia, Y.; Wang, J.W. A Method for Improving Thermal Stability of Aluminum Hydride. CN Patent 109019507A, 5 November 2021. pp. 11–15. [Google Scholar]
- Cai, X.W.; Yang, J.H.; Zhang, J.L.; Ma, D.X.; Wang, Y.P. Liquid Carbon Dioxide as Anti-Solvent Coating Aluminum Hydride. Propellants Explos. Pyrotech. 2016, 40, 914–919. [Google Scholar] [CrossRef]
- Petrie, M.A.; Bottaro, J.C.; Schmitt, R.J.; Penwell, P.E.; Bomberger, D.C. Preparation of Aluminum Hydride Polymorphs, Particularly Stabilized α-AlH3. U.S. Patent 6228338, 8 May 2001. pp. 5–8. [Google Scholar]
- Schmidt, D. Non-Solvated Particulate Aluminum Hydride Coated with a Cyano-Coating Compound Useful in Solid Propellants. U.S. Patent 3850709, 26 November 1974. pp. 11–26. [Google Scholar]
- Roberts, C.B.; Toner, D.D. Stabilization of Light Metal Hydride (U). U.S. Patent 3850709, 26 November 1974. pp. 11–26. [Google Scholar]
- Flynn, J. Particulate Aluminum Hydride with Nitrocellulose Coating Suitable for Use in Solid Propellants. U.S. Patent 3855022, 17 December 1974. pp. 12–17. [Google Scholar]
- Finholt, A.E.; Bond, A.C.; Schlesinger, H.I. Lithium Aluminum Hydride, Aluminum Hydride and Lithium Gallium Hydride, and Some of Their Applications in Organic and Inorganic Chemistry 1. J. Am. Chem. Soc. 1947, 69, 1199–1203. [Google Scholar] [CrossRef]
- Brower, F.M.; Matzek, N.E.; Reigler, P.F.; Rinn, H.W.; Roberts, C.B.; Schmidt, D.L.; Snover, J.A.; Terada, K. Preparation and Properties of Aluminum Hydride. J. Am. Chem. Soc. 1976, 98, 2450–2453. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, Z.; Li, H.-P.; Yan, Q.-L. Advanced Preparation and Processing Techniques for High Energy Fuel AlH3. Chem. Eng. J. 2021, 421, 129753. [Google Scholar] [CrossRef]
- Park, M.; Kim, W.; Kwon, Y.; Kim, J.; Jo, Y. Wet Synthesis of Energetic Aluminum Hydride. Propellants Explos. Pyrotech. 2019, 44, 1233–1241. [Google Scholar] [CrossRef]
- Xu, B.; Liu, J.; Wang, X. Preparation and Thermal Properties of Aluminum Hydride Polymorphs. Vacuum 2014, 99, 127–134. [Google Scholar] [CrossRef]
- Xu, B.; Liu, J.P. Aluminum Hydride Polymorphs: Preparation, Characterization and Thermal Properties. Adv. Mater. Res. 2013, 712–715, 333–336. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Ma, H.; Lu, C.; Luo, H.; Wang, X.; Huang, X.; Lan, Z.; Guo, J. Aluminum Hydride for Solid-State Hydrogen Storage: Structure, Synthesis, Thermodynamics, Kinetics, and Regeneration. J. Energy Chem. 2021, 52, 428–440. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Characterisation of Mechanochemically Synthesised Alane (AlH3) Nanoparticles. J. Alloys Compd. 2009, 487, 370–376. [Google Scholar] [CrossRef]
- Duan, C.W.; Hu, L.X.; Xue, D. Solid State Synthesis of Nano-Sized AlH3 and Its Dehydriding Behaviour. Green Chem. 2015, 17, 3466–3474. [Google Scholar] [CrossRef]
- Duan, C.; Cao, Y.; Hu, L.; Fu, D.; Ma, J.; Youngblood, J. An Efficient Mechanochemical Synthesis of Alpha-Aluminum Hydride: Synergistic Effect of TiF3 on the Crystallization Rate and Selective Formation of Alpha-Aluminum Hydride Polymorph. J. Hazard. Mater. 2019, 373, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, E.-K.; Shim, J.-H.; Cho, Y.W.; Yoon, K.B. Mechanochemical Synthesis and Thermal Decomposition of Mg(AlH4)2. J. Alloys Compd. 2006, 422, 283–287. [Google Scholar] [CrossRef]
- Baranowski, B.; Tkacz, M. The Equilibrium Between Solid Aluminium Hydride and Gaseous Hydrogen. Z. Phys. Chem. 1983, 135, 27–38. [Google Scholar] [CrossRef]
- Appel, M.; Frankel, J.P. Production of Aluminum Hydride by Hydrogen-Ion Bombardment. J. Chem. Phys. 1965, 42, 3984–3988. [Google Scholar] [CrossRef]
- Saitoh, H.; Machida, A.; Katayama, Y.; Aoki, K. Formation and Decomposition of AlH3 in the Aluminum-Hydrogen System. Appl. Phys. Lett. 2008, 93, 151918. [Google Scholar] [CrossRef]
- Dymova, T.N.; Mal’tseva, N.N.; Konoplev, V.N.; Golovanova, A.I.; Aleksandrov, D.P.; Sizareva, A.S. Solid-Phase Solvate-Free Formation of Magnesium Hydroaluminates Mg(AlH4)2 and MgAlH5 upon Mechanochemical Activation or Heating of Magnesium Hydride and Aluminum Chloride Mixtures. Russ. J. Coord. Chem. Khim. 2003, 29, 385–389. [Google Scholar] [CrossRef]
- Dymova, T.N.; Konoplev, V.N.; Sizareva, A.S.; Aleksandrov, D.P. Magnesium Tetrahydroaluminate: Solid-Phase Formation with Mechanochemical Activation of a Mixture of Aluminum and Magnesium Hydrides. Russ. J. Coord. Chem. Khim. 1999, 25, 312–315. [Google Scholar]
- Fichtner, M.; Frommen, C.; Fuhr, O. Synthesis and Properties of Calcium Alanate and Two Solvent Adducts. Inorg. Chem. 2005, 44, 3479–3484. [Google Scholar] [CrossRef] [PubMed]
- Mal’tseva, N.N.; Golovanova, A.I.; Dymova, T.N.; Aleksandrov, D.P. Solid-Phase Formation of Calcium Hydridoaluminates Ca(AlH4)2 and CaHAlH4 upon Mechanochemical Activation or Heating of Mixtures of Calcium Hydride with Aluminum Chloride. Russ. J. Inorg. Chem. 2001, 46, 1793–1797. [Google Scholar]
- Mamatha, M.; Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schmidt, W.; Schüth, F.; Weidenthaler, C. Mechanochemical Preparation and Investigation of Properties of Magnesium, Calcium and Lithium–Magnesium Alanates. J. Alloys Compd. 2006, 407, 78–86. [Google Scholar] [CrossRef]
- Duan, C.; Hu, L.; Sun, Y.; Zhou, H.; Yu, H. An Insight into the Process and Mechanism of a Mechanically Activated Reaction for Synthesizing AlH3 Nano-Composites. Dalt. Trans. 2015, 44, 16251–16255. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Cao, Y.; Hu, L.; Fu, D.; Ma, J. Synergistic Effect of TiF3 on the Dehydriding Property of α-AlH3 Nano-Composite. Mater. Lett. 2019, 238, 254–257. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Formation of Aluminium Hydride (AlH3) via the Decomposition of Organoaluminium and Hydrogen Storage Properties. Int. J. Hydrogen Energy 2018, 43, 16749–16757. [Google Scholar] [CrossRef]
- Liu, H.; Bouchard, M.; Zhang, L. An Experimental Study of Hydrogen Solubility in Liquid Aluminium. J. Mater. Sci. 1995, 30, 4309–4315. [Google Scholar] [CrossRef]
- Mcgrady, G.S. Hydrogenation of Aluminum Using a Supercritical Fluid Medium. U.S. Patent 0241056, 6 December 2008. pp. 10–12. [Google Scholar]
- Liu, F.; Huang, K.; Wu, Q.; Dai, S. Solvent-Free Self-Assembly to the Synthesis of Nitrogen-Doped Ordered Mesoporous Polymers for Highly Selective Capture and Conversion of CO2. Adv. Mater. 2017, 29, 1700445. [Google Scholar] [CrossRef]
- Zidan, R.; Garcia-Diaz, B.L.; Fewox, C.S.; Stowe, A.C.; Gray, J.R.; Harter, A.G. Aluminium Hydride: A Reversible Material for Hydrogen Storage. Chem. Commun. 2009, 35, 3717. [Google Scholar] [CrossRef]
- Saitoh, H.; Okajima, Y.; Yoneda, Y.; Machida, A.; Kawana, D.; Watanuki, T.; Katayama, Y.; Aoki, K. Formation and Crystal Growth Process of AlH3 in Al–H System. J. Alloys Compd. 2010, 496, L25–L28. [Google Scholar] [CrossRef]
- Sinke, G.C.; Walker, L.C.; Oetting, F.L.; Stull, D.R. Thermodynamic Properties of Aluminum Hydride. J. Chem. Phys. 1967, 47, 2759–2761. [Google Scholar] [CrossRef]
- Chizinsky, G.; Evans, G.G.; Gibb, T.R.P.; Rice, M.J. Non-Solvated Aluminum Hydride 1. J. Am. Chem. Soc. 1955, 77, 3164–3165. [Google Scholar] [CrossRef]
- Sartori, S.; Opalka, S.M.; Løvvik, O.M.; Guzik, M.N.; Tang, X.; Hauback, B.C. Experimental Studies of α-AlD3 and α′-AlD3 versus First-Principles Modelling of the Alane Isomorphs. J. Mater. Chem. 2008, 18, 2361. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J.; Kulleck, J.G.; Bowman, R.C. Kinetics and Thermodynamics of the Aluminum Hydride Polymorphs. J. Alloys Compd. 2007, 446–447, 271–275. [Google Scholar] [CrossRef]
- Maehlen, J.P.; Yartys, V.A.; Denys, R.V.; Fichtner, M.; Frommen, C.; Bulychev, B.M.; Pattison, P.; Emerich, H.; Filinchuk, Y.E.; Chernyshov, D. Thermal Decomposition of AlH3 Studied by in Situ Synchrotron X-Ray Diffraction and Thermal Desorption Spectroscopy. J. Alloys Compd. 2007, 446–447, 280–289. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Dong, Z.; Cao, G.; Liu, Y.; Chen, L.; Yan, M. Dehydriding Properties of γ-AlH3. Int. J. Hydrogen Energy 2013, 38, 10851–10856. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Wang, X.; Xu, L.; Liu, S.; Sheng, P.; Zhao, G.; Wang, B.; Li, H.; Yan, M. Hydrogen Desorption Behaviors of γ-AlH3: Diverse Decomposition Mechanisms for the Outer Layer and the Inner Part of γ-AlH3 Particle. Int. J. Hydrogen Energy 2017, 42, 25310–25315. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J. Thermodynamics of the α, β and γ Polymorphs of AlH3. J. Alloys Compd. 2006, 424, 262–265. [Google Scholar] [CrossRef]
- Stavila, V.; Li, S.; Dun, C.; Marple, M.A.T.; Mason, H.E.; Snider, J.L.; Reynolds, J.E.; El Gabaly, F.; Sugar, J.D.; Spataru, C.D.; et al. Defying Thermodynamics: Stabilization of Alane Within Covalent Triazine Frameworks for Reversible Hydrogen Storage. Angew. Chem. 2021, 133, 26019–26028. [Google Scholar] [CrossRef]
- Herley, P.J.; Christofferson, O.; Irwin, R. Decomposition of Alpha-Aluminum Hydride Powder. 1. Thermal Decomposition. J. Phys. Chem. 1981, 85, 1874–1881. [Google Scholar] [CrossRef]
- Herley, P.J.; Christofferson, O. Decomposition of Alpha.-Aluminum Hydride Powder. 2. Photolytic Decomposition. J. Phys. Chem. 1981, 85, 1882–1886. [Google Scholar] [CrossRef]
- Herley, P.J.; Christofferson, O. Decomposition of Alpha.-Aluminum Hydride Powder. 3. Simultaneous Photolytic-Thermal Decomposition. J. Phys. Chem. 1981, 85, 1887–1892. [Google Scholar] [CrossRef]
- Tarasov, V.P.; Muravlev, Y.B.; Bakum, S.I.; Novikov, A.V. Kinetics of Formation of Metallic Aluminum upon Thermal and Photolytic Decomposition of Aluminum Trihydride and Trideuteride as Probed by NMR. Dokl. Phys. Chem. 2003, 393, 353–356. [Google Scholar] [CrossRef]
- Milekhin, Y.M.; Koptelov, A.A.; Matveev, A.A.; Baranets, Y.N.; Bakulin, D.A. Studying Aluminum Hydride by Means of Thermal Analysis. Russ. J. Phys. Chem. A 2015, 89, 1141–1145. [Google Scholar] [CrossRef]
- Kato, S.; Bielmann, M.; Ikeda, K.; Orimo, S.; Borgschulte, A.; Züttel, A. Surface Changes on AlH3 during the Hydrogen Desorption. Appl. Phys. Lett. 2010, 96, 051912. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, J.; Li, H.; Pang, A.; Xu, P.; Tang, G.; Xu, X. Thermal Oxidation and Heterogeneous Combustion of AlH3 and Al: A Comparative Study. Acta Astronaut. 2021, 179, 636–645. [Google Scholar] [CrossRef]
- Duan, C.W.; Hu, L.X.; Sun, Y.; Zhou, H.P.; Yu, H. Reaction Kinetics for the Solid State Synthesis of the AlH3/MgCl2 Nano-Composite by Mechanical Milling. Phys. Chem. Chem. Phys. 2015, 17, 22152–22159. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Isobe, S.; Wang, Y.; Hashimoto, N.; Ohnuki, S.; Zeng, L.; Liu, S.; Ichikawa, T.; Kojima, Y. Dehydrogenation Process of AlH3 Observed by TEM. J. Alloys Compd. 2013, 580, S163–S166. [Google Scholar] [CrossRef]
- Yu, M.H.; Xie, W.X.; Zhu, Z.Y.; Yan, Q.L. Stability, Reactivity and Decomposition Kinetics of Surface Passivated α-AlH3 Crystals. Int. J. Hydrogen Energy 2022, 47, 8916–8928. [Google Scholar] [CrossRef]
- Evard, E.A.; Voyt, A.P. Hydride Decomposition Characterization by Means of “Morphological Trajectory” Method—Applied to AlH3. J. Alloys Compd. 2011, 509, S667–S670. [Google Scholar] [CrossRef]
- Gabis, I.E.; Voyt, A.P.; Chernov, I.A.; Kuznetsov, V.G.; Baraban, A.P.; Elets, D.I.; Dobrotvorsky, M.A. Ultraviolet Activation of Thermal Decomposition of α-Alane. Int. J. Hydrogen Energy 2012, 37, 14405–14412. [Google Scholar] [CrossRef]
- Pang, A.; Zhu, Z.; Xu, X. Recent Progresses on Synthesis and Evaluation of AlH3. Chin. J. Energ. Mater. 2019, 27, 317–325. [Google Scholar]
- Niles, E.T.; Seaman, B.A.; Wilson, E.J. Stabilization of Aluminum Hydride. U.S. Patent 3869544, 28 June 1975. pp. 3–4. [Google Scholar]
- Roberts, C.B. Stabilization of Aluminum Hydride. U.S. Patent 3821044, 28 June 1974. [Google Scholar]
- Nylack, G.A. Method for Increasing the Thermal Stability of Aluminum Hydride Storage. RU Patent 2175637, 8 May 2001. pp. 5–8. [Google Scholar]
- Norman, M.E.; Herbert, R.C. Stabilization of Light Metal Hydride. U.S. Patent 3857922, 9 April 1974. pp. 12–31. [Google Scholar]
- Cianciolo, A.; Sabatine, S.J. Process for the Preparation of Mercury-Coating Aluminum Hydride Compositions. U.S. Patent 3785890, 15 January 1974. pp. 1–15. [Google Scholar]
- Xing, X.H.; Xia, Y.; Zhang, X.Q.; Chang, W.L.; Wang, J.W. Research Progress in Improving Thermal Stability of Aluminum Trihydride. Chem. Propellants Polym. Mater. 2018, 16, 21–25. [Google Scholar]
- Ardis, A.; Natoli, F. Thermal Stability of Aluminum Hydride through Use of Stabilizers. U.S. Patent 3801707, 2 April 1974. pp. 4–12. [Google Scholar]
- Roberts, C.B.; Toner, D.D. Stabilization of Light Metalhydride(U). U.S. Patent 3803082, 9 April 1974. pp. 4–9. [Google Scholar]
- Maycock, J.N.; Paiverneker, V.R. Crystal Lattice Doping Studies of High Energy Propellant Ingredients AD-507040; Defense Technical Information Center: Fort Belvoir, VA, USA, 1969. [Google Scholar]
- Wang, K.; Liu, Z.; Wang, X.; Cui, X. Enhancement of Hydrogen Binding Affinity with Low Ionization Energy Li2F Coating on C60 to Improve Hydrogen Storage Capacity. Int. J. Hydrogen Energy 2014, 39, 15639–15645. [Google Scholar] [CrossRef]
- Norman, M.E. Non-Solvated Aluminum Hydride Coated with Stabilizer. U.S. Patent 3844853, 29 October 1974. [Google Scholar]
- Shi, Z.; Lu, T.; Zhang, J.; Zhu, Z.; Xu, Y.; Xia, D.; Hu, Y.; Lin, K.; Yang, Y. The Enhanced Thermal Stability and Reduced Hygroscopicity of Aluminum Hydride Coated with Vinyltrimethoxysilane. New J. Chem. 2022, 46, 1643–1649. [Google Scholar] [CrossRef]
- Kempa, P.B.; Thome, V.; Herrmann, M. Structure, Chemical and Physical Behavior of Aluminum Hydride. Part. Part. Syst. Charact. 2009, 26, 132–137. [Google Scholar] [CrossRef]
- Qin, J.; Gong, T.; Yan, N.; Li, J.G.; Hui, L.F.; Hao, H.X. Research Progress on Application of Atomic Layer Deposition in Surface Fabrication of Energetic Material. Chin. J. Explos. Propellant 2019, 42, 425–431. [Google Scholar]
- Su, J.; Li, J.; Guan, H.B. A Method for Coating Aluminum Hydride with Carbon Nanotubes. CN 108163839A, 15 June 2018. [Google Scholar]
- Li, L. Desensitization of Aluminum Hydride (AlH3) by Graphene Oxide. Chin. J. Solid Rocket Technol. 2019, 42, 66–71. [Google Scholar]
- Yu, M.H.; Yang, S.L.; Xie, W.X.; Zhu, Z.Y.; Li, H.P.; Yan, Q.L. Enhanced Stability and Combustion Performance of AlH3 in Combination with Commonly Used Oxidizers. Fuel 2023, 331, 125741. [Google Scholar] [CrossRef]
- Yu, M.H.; Yang, S.L.; Xie, W.X.; Li, Y.J.; Li, H.P.; Yan, Q.L. Stabilization of AlH3 Crystals by the Coating of Hydrophobic PFPE and Resulted Reactivity with Ammonium Perchlorate. Langmuir 2023, 39, 7863–7875. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Luigi, D.T.; Fan, X.; Wang, K.; Li, J.; Zhao, F. Progress on Modification of High Active Aluminum Powder and Combustion Agglomeration in Chemical Propellants. J. Solid Rocket Technol. 2019, 42, 42–53. [Google Scholar]
- Cheng, Z.P.; He, X.X. Research Progress of Nano Aluminum Fuel. J. Solid Rocket Technol. 2017, 40, 437–447. [Google Scholar]
- Bazyn, T.; Eyer, R.; Krier, H.; Glumac, N. Combustion Characteristics of Aluminum Hydride at Elevated Pressure and Temperature. J. Propuls. Power 2004, 20, 427–431. [Google Scholar] [CrossRef]
- Maggi, F.; Gariani, G.; Galfetti, L.; DeLuca, L.T. Theoretical Analysis of Hydrides in Solid and Hybrid Rocket Propulsion. Int. J. Hydrogen Energy 2012, 37, 1760–1769. [Google Scholar] [CrossRef]
- Shark, S.; Sippel, T.; Son, S.; Heister, S.; Pourpoint, T. Theoretical Performance Analysis of Metal Hydride Fuel Additives for Rocket Propellant Applications. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar]
- Bazyn, T.; Krier, H.; Glumac, N.; Shankar, N.; Wang, X.; Jackson, T.L. Decomposition of Aluminum Hydride Under Solid Rocket Motor Conditions. J. Propuls. Power 2007, 23, 457–464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).