Tools for In Vitro Propagation/Synchronization of the Liverwort Marchantia polymorpha and Application of a Validated HPLC-ESI-MS-MS Method for Glutathione and Phytochelatin Analysis
Abstract
:1. Introduction
2. Results and Discussion
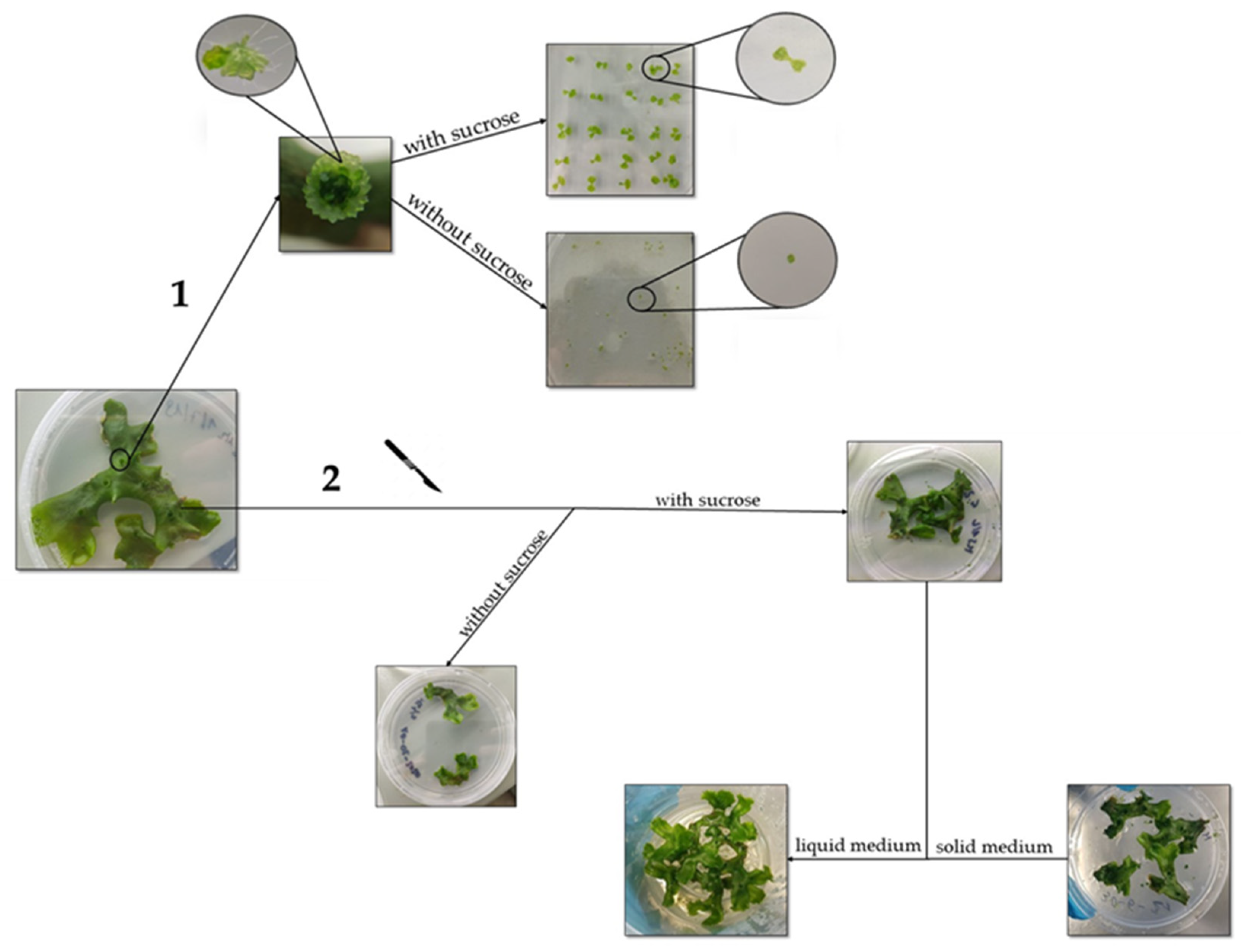
2.1. The In Vitro Propagation Protocol Employed Enables Rapid Synchronization of Gametophyte Growth
2.2. Gametophytes of M. polymorpha Lead to Rapid Medium Acidification
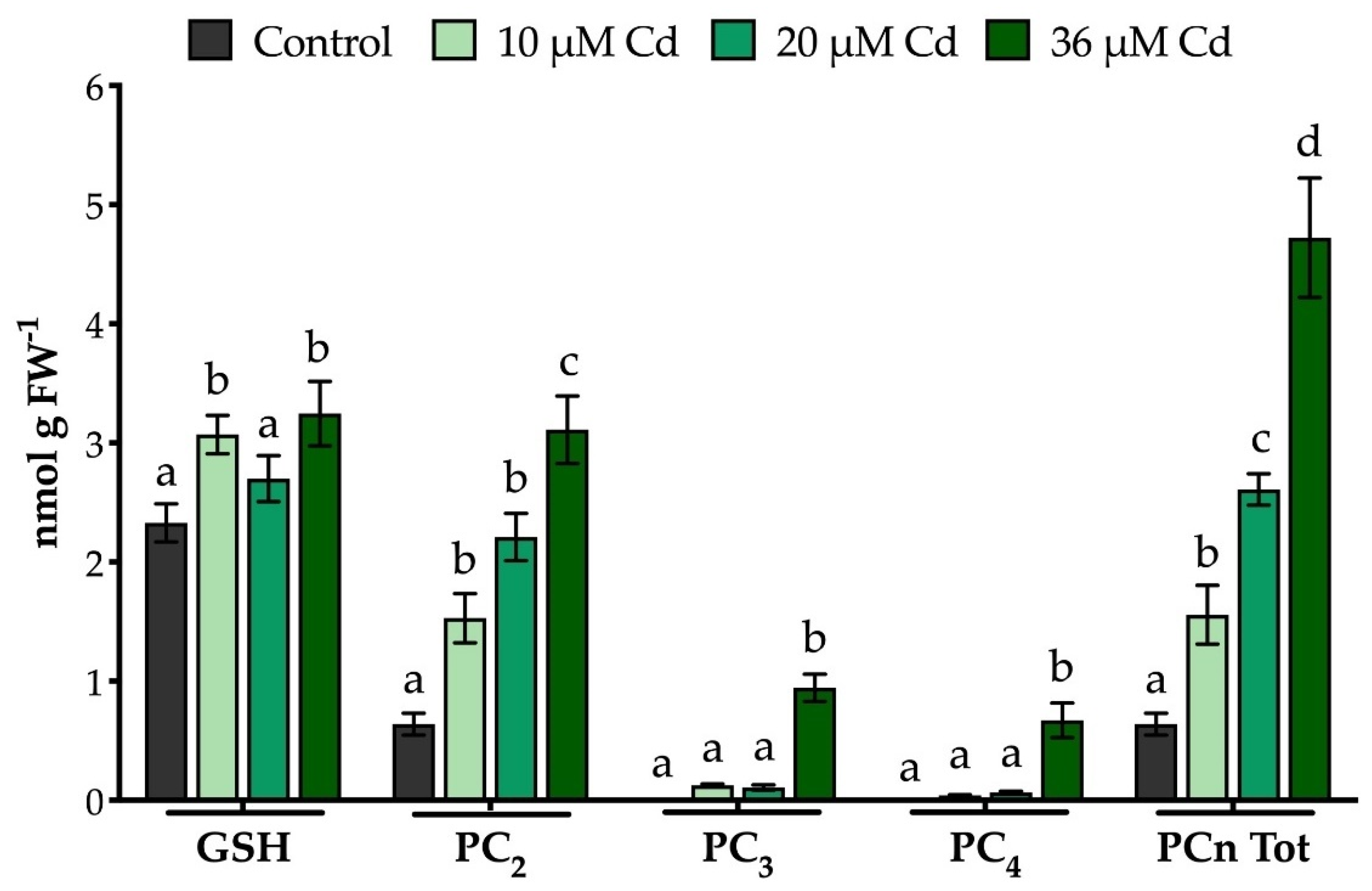
2.3. The Extraction Procedure and the Analytical Method Used Allow an Effective Quali-Quantitative Analysis of Thiol-Peptides in M. polymorpha Gametophytes
3. Materials and Methods
3.1. Set-Up of the In Vitro Cultures, Growth Conditions and Vegetative Propagation of M. polymorpha Gametophytes
3.2. Metal Treatments and pH Measurements
3.3. Extraction, Characterization and Quantification of Thiol-Peptides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frausto da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life; OUP Oxford: Oxford, UK, 2001; ISBN 978-0-19-850848-9. [Google Scholar]
- Sanità di Toppi, L.; Gabbrielli, R. Response to Cadmium in Higher Plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Perrino, E.V.; Brunetti, G.; Farrag, K. Plant Communities in Multi-Metal Contaminated Soils: A Case Study in the National Park of Alta Murgia (Apulia Region - Southern Italy). Int. J. Phytoremed. 2014, 16, 871–888. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Swizterland, 2012; pp. 133–164. ISBN 978-3-7643-8340-4. [Google Scholar]
- Stanković, J.D.; Sabovljević, A.D.; Sabovljević, M.S. Bryophytes and Heavy Metals: A Review. Acta Bot. Croat. 2018, 77, 109–118. [Google Scholar] [CrossRef]
- Tennstedt, P.; Peisker, D.; Böttcher, C.; Trampczynska, A.; Clemens, S. Phytochelatin Synthesis Is Essential for the Detoxification of Excess Zinc and Contributes Significantly to the Accumulation of Zinc. Plant Physiol. 2009, 149, 938–948. [Google Scholar] [CrossRef] [Green Version]
- Bellini, E.; Betti, C.; Sanità di Toppi, L. Responses to Cadmium in Early-Diverging Streptophytes (Charophytes and Bryophytes): Current Views and Potential Applications. Plants 2021, 10, 770. [Google Scholar] [CrossRef]
- Harmens, H.; Ilyin, I.; Mills, G.; Aboal, J.R.; Alber, R.; Blum, O.; Coşkun, M.; De Temmerman, L.; Fernández, J.Á.; Figueira, R.; et al. Country-Specific Correlations across Europe between Modelled Atmospheric Cadmium and Lead Deposition and Concentrations in Mosses. Environ. Pollut. 2012, 166, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J. Evolution of Heavy Metal Tolerance in Bryophytes Ii. an Ecological and Experimental Investigation of the “Copper Moss,” Scopelophila cataractae (Pottiaceae). Am. J. Bot. 1987, 74, 813–821. [Google Scholar] [CrossRef]
- Shimamura, M. Marchantia polymorpha: Taxonomy, Phylogeny and Morphology of a Model System. Plant Cell Physiol. 2016, 57, 230–256. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into Land Plant Evolution Garnered from the Marchantia polymorpha Genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef] [Green Version]
- Berger, F.; Bowman, J.L.; Kohchi, T. Marchantia. Curr. Biol. 2016, 26, R186–R187. [CrossRef] [Green Version]
- Nakazato, T.; Kadota, A.; Wada, M. Photoinduction of Spore Germination in Marchantia polymorpha L. Is Mediated by Photosynthesis. Plant Cell Physiol. 1999, 40, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Ishizaki, K.; Chiyoda, S.; Yamato, K.T.; Kohchi, T. Agrobacterium-Mediated Transformation of the Haploid Liverwort Marchantia polymorpha L., an Emerging Model for Plant Biology. Plant Cell Physiol. 2008, 49, 1084–1091. [Google Scholar] [CrossRef] [Green Version]
- Ishizaki, K.; Johzuka-Hisatomi, Y.; Ishida, S.; Iida, S.; Kohchi, T. Homologous Recombination-Mediated Gene Targeting in the Liverwort Marchantia polymorpha L. Sci. Rep. 2013, 3, 1532. [Google Scholar] [CrossRef]
- Kubota, A.; Ishizaki, K.; Hosaka, M.; Kohchi, T. Efficient Agrobacterium -Mediated Transformation of the Liverwort Marchantia polymorpha Using Regenerating Thalli. Biosci. Biotechnol. Biochem. 2013, 77, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Sugano, S.S.; Shirakawa, M.; Takagi, J.; Matsuda, Y.; Shimada, T.; Hara-Nishimura, I.; Kohchi, T. CRISPR/Cas9-Mediated Targeted Mutagenesis in the Liverwort Marchantia polymorpha L. Plant Cell Physiol. 2014, 55, 475–481. [Google Scholar] [CrossRef]
- Bräutigam, A.; Schaumlöffel, D.; Krauss, G.-J.; Wesenberg, D. Analytical Approach for Characterization of Cadmium-Induced Thiol Peptides—a Case Study Using Chlamydomonas reinhardtii. Anal. Bioanal. Chem. 2009, 395, 1737–1747. [Google Scholar] [CrossRef]
- Grill, E.; Löffler, S.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins, the Heavy-Metal-Binding Peptides of Plants, Are Synthesized from Glutathione by a Specific γ-Glutamylcysteine Dipeptidyl Transpeptidase (Phytochelatin Synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef] [Green Version]
- Rea, P.A. Phytochelatin Synthase: Of a Protease a Peptide Polymerase Made. Physiol. Plant. 2012, 145, 154–164. [Google Scholar] [CrossRef]
- Romanyuk, N.D.; Rigden, D.J.; Vatamaniuk, O.K.; Lang, A.; Cahoon, R.E.; Jez, J.M.; Rea, P.A. Mutagenic Definition of a Papain-Like Catalytic Triad, Sufficiency of the N-Terminal Domain for Single-Site Core Catalytic Enzyme Acylation, and C-Terminal Domain for Augmentative Metal Activation of a Eukaryotic Phytochelatin Synthase. Plant Physiol. 2006, 141, 858–869. [Google Scholar] [CrossRef] [Green Version]
- Vivares, D.; Arnoux, P.; Pignol, D. A Papain-like Enzyme at Work: Native and Acyl-Enzyme Intermediate Structures in Phytochelatin Synthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 18848–18853. [Google Scholar] [CrossRef] [Green Version]
- Rea, P.A.; Vatamaniuk, O.K.; Rigden, D.J. Weeds, Worms, and More. Papain’s Long-Lost Cousin, Phytochelatin Synthase. Plant Physiol. 2004, 136, 2463–2474. [Google Scholar] [CrossRef] [Green Version]
- Clemens, S. Toxic Metal Accumulation, Responses to Exposure and Mechanisms of Tolerance in Plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Vatamaniuk, O.K.; Mari, S.; Lu, Y.-P.; Rea, P.A. Mechanism of Heavy Metal Ion Activation of Phytochelatin (PC) Synthase. J. Biol. Chem. 2000, 275, 31451–31459. [Google Scholar] [CrossRef] [Green Version]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; Sanità di Toppi, L. A Cd/Fe/Zn-Responsive Phytochelatin Synthase Is Constitutively Present in the Ancient Liverwort Lunularia cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef] [Green Version]
- Petraglia, A.; De Benedictis, M.; Degola, F.; Pastore, G.; Calcagno, M.; Ruotolo, R.; Mengoni, A.; Sanità di Toppi, L. The Capability to Synthesize Phytochelatins and the Presence of Constitutive and Functional Phytochelatin Synthases Are Ancestral (Plesiomorphic) Characters for Basal Land Plants. J. Exp. Bot. 2014, 65, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Fontanini, D.; Andreucci, A.; Ruffini Castiglione, M.; Basile, A.; Sorbo, S.; Petraglia, A.; Degola, F.; Bellini, E.; Bruno, L.; Varotto, C.; et al. The Phytochelatin Synthase from Nitella mucronata (Charophyta) Plays a Role in the Homeostatic Control of Iron(II)/(III). Plant Physiol. Biochem. 2018, 127, 88–96. [Google Scholar] [CrossRef]
- Maresca, V.; Sorbo, S.; Loppi, S.; Funaro, F.; Del Prete, D.; Basile, A. Biological Effects from Environmental Pollution by Toxic Metals in the “Land of Fires” (Italy) Assessed Using the Biomonitor Species Lunularia cruciata L. (Dum). Environ. Pollut. 2020, 265, 115000. [Google Scholar] [CrossRef]
- Ishizaki, K.; Nishihama, R.; Yamato, K.T.; Kohchi, T. Molecular Genetic Tools and Techniques for Marchantia Polymorpha Research. Plant Cell Physiol. 2016, 57, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Riou-Khamlichi, C.; Menges, M.; Healy, J.M.S.; Murray, J.A.H. Sugar Control of the Plant Cell Cycle: Differential Regulation of Arabidopsis D-Type Cyclin Gene Expression. Mol. Cell. Biol. 2000, 20, 4513–4521. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; McCormack, M.; Li, L.; Hall, Q.; Xiang, C.; Sheen, J. Glucose–TOR Signalling Reprograms the Transcriptome and Activates Meristems. Nature 2013, 496, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Nishihama, R.; Ishizaki, K.; Hosaka, M.; Matsuda, Y.; Kubota, A.; Kohchi, T. Phytochrome-Mediated Regulation of Cell Division and Growth during Regeneration and Sporeling Development in the Liverwort Marchantia polymorpha. J. Plant Res. 2015, 128, 407–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011; ISBN 978-0-12-384906-9. [Google Scholar]
- Rico-Reséndiz, F.; Cervantes-Pérez, S.A.; Espinal-Centeno, A.; Dipp-Álvarez, M.; Oropeza-Aburto, A.; Hurtado-Bautista, E.; Cruz-Hernández, A.; Bowman, J.L.; Ishizaki, K.; Arteaga-Vázquez, M.A.; et al. Transcriptional and Morpho-Physiological Responses of Marchantia polymorpha upon Phosphate Starvation. Int. J. Mol. Sci. 2020, 21, 8354. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Chemical Constituents of Bryophyta. In Chemical Constituents of Bryophytes: Bio- and Chemical Diversity, Biological Activity, and Chemosystematics; Asakawa, Y., Ludwiczuk, A., Nagashima, F., Eds.; Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 2013; pp. 563–605. ISBN 978-3-7091-1084-3. [Google Scholar]
- Tyler, G. Bryophytes and Heavy Metals: A Literature Review. Bot. J. Linn. Soc. 1990, 104, 231–253. [Google Scholar] [CrossRef]
- Bellini, E.; Maresca, V.; Betti, C.; Castiglione, M.R.; Fontanini, D.; Capocchi, A.; Sorce, C.; Borsò, M.; Bruno, L.; Sorbo, S.; et al. The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins. Int. J. Mol. Sci. 2020, 21, 1583. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.-F.; Lou, H.-X. Secondary Metabolites in Bryophytes: An Ecological Aspect. Chem. Biodivers. 2009, 6, 303–312. [Google Scholar] [CrossRef]
- Peters; Treutler; Döll; Kindt; Hankemeier; Neumann Chemical Diversity and Classification of Secondary Metabolites in Nine Bryophyte Species. Metabolites 2019, 9, 222. [CrossRef] [Green Version]
- El-Zohri, M.H.A.; Cabala, R.; Frank, H. Quantification of Phytochelatins in Plants by Reversed-Phase HPLC–ESI–MS–MS. Anal. Bioanal. Chem. 2005, 382, 1871–1876. [Google Scholar] [CrossRef]
- Bellini, E.; Borsò, M.; Betti, C.; Bruno, L.; Andreucci, A.; Ruffini Castiglione, M.; Saba, A.; Sanità di Toppi, L. Characterization and Quantification of Thiol-Peptides in Arabidopsis thaliana Using Combined Dilution and High Sensitivity HPLC-ESI-MS-MS. Phytochemistry 2019, 164, 215–222. [Google Scholar] [CrossRef]
- Bellini, E.; Varotto, C.; Borsò, M.; Rugnini, L.; Bruno, L.; Sanità di Toppi, L. Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407. Plants 2020, 9, 914. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox Environment of the Cell as Viewed through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox Signal. 2008, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Kneer, R.; Zenk, M.H. The Formation of Cd-Phytochelatin Complexes in Plant Cell Cultures. Phytochemistry 1997, 44, 69–74. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giardini, S.; Bellini, E.; Bandoni, E.; Saba, A.; Sanità di Toppi, L. Tools for In Vitro Propagation/Synchronization of the Liverwort Marchantia polymorpha and Application of a Validated HPLC-ESI-MS-MS Method for Glutathione and Phytochelatin Analysis. Stresses 2022, 2, 136-145. https://doi.org/10.3390/stresses2010010
Giardini S, Bellini E, Bandoni E, Saba A, Sanità di Toppi L. Tools for In Vitro Propagation/Synchronization of the Liverwort Marchantia polymorpha and Application of a Validated HPLC-ESI-MS-MS Method for Glutathione and Phytochelatin Analysis. Stresses. 2022; 2(1):136-145. https://doi.org/10.3390/stresses2010010
Chicago/Turabian StyleGiardini, Silvia, Erika Bellini, Elena Bandoni, Alessandro Saba, and Luigi Sanità di Toppi. 2022. "Tools for In Vitro Propagation/Synchronization of the Liverwort Marchantia polymorpha and Application of a Validated HPLC-ESI-MS-MS Method for Glutathione and Phytochelatin Analysis" Stresses 2, no. 1: 136-145. https://doi.org/10.3390/stresses2010010
APA StyleGiardini, S., Bellini, E., Bandoni, E., Saba, A., & Sanità di Toppi, L. (2022). Tools for In Vitro Propagation/Synchronization of the Liverwort Marchantia polymorpha and Application of a Validated HPLC-ESI-MS-MS Method for Glutathione and Phytochelatin Analysis. Stresses, 2(1), 136-145. https://doi.org/10.3390/stresses2010010






