The Presence of a Pet Dog Is Associated with a More Balanced Response to a Social Stressor
Abstract
:1. Introduction
2. Results
2.1. Cohort Demographics and Baseline Characteristics
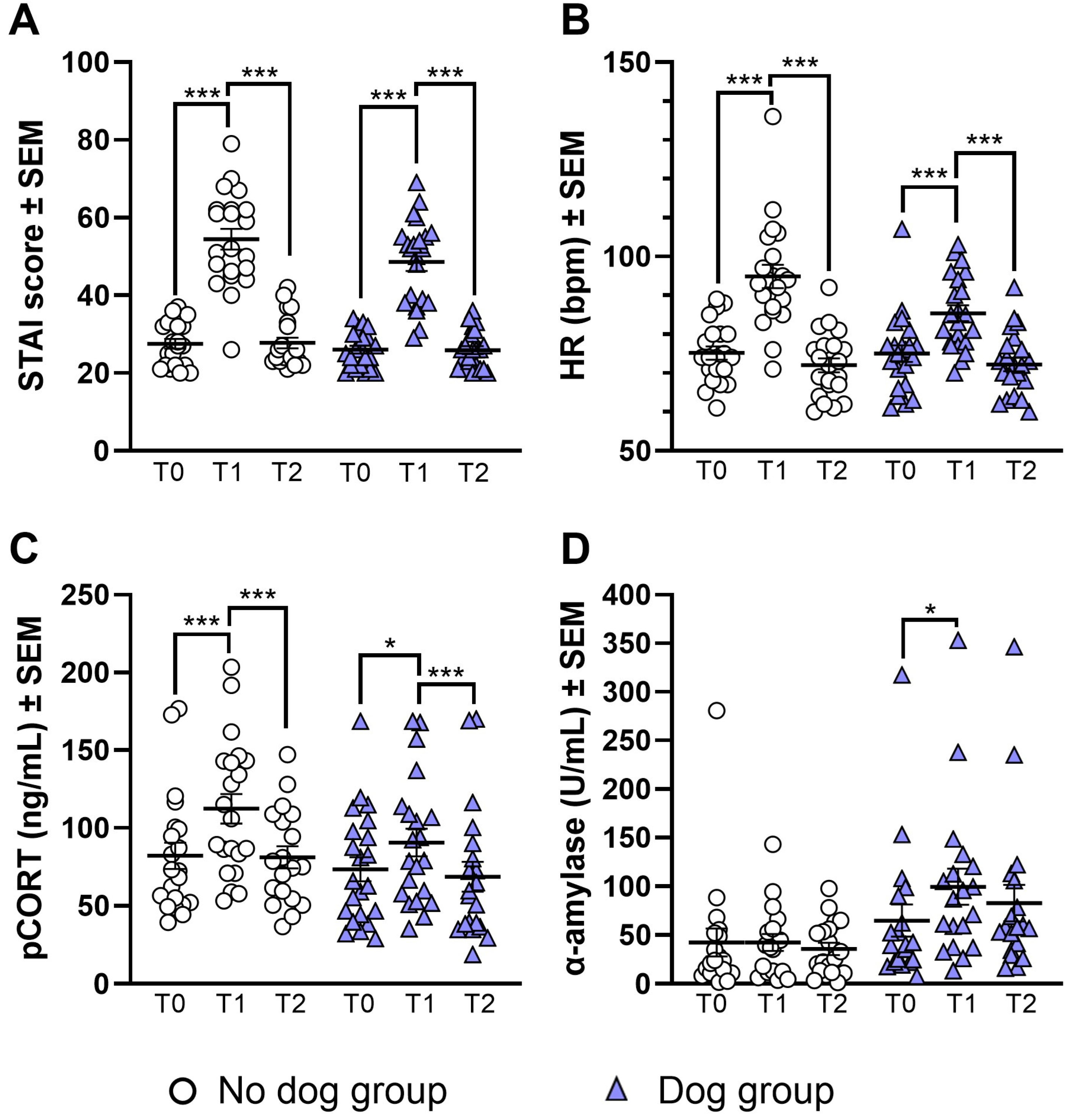
2.2. TSST Response
2.2.1. STAI-AD State
2.2.2. Heart Rate
2.2.3. Plasma Cortisol
2.2.4. Salivary Alpha-Amylase
2.3. Dog Effect
2.3.1. STAI-AD State
2.3.2. Heart Rate
2.3.3. Plasma Cortisol
2.3.4. Salivary Alpha-Amylase
3. Discussion
3.1. Limitations
3.2. Future Directions
4. Materials and Methods
4.1. Participants
4.2. Procedure
4.3. Task and Materials
4.3.1. The Trier Social Stress Test (TSST)
4.3.2. Questionnaires
4.3.3. Heart Rate
4.3.4. Blood Collection and Processing
4.3.5. Salivary Alpha-Amylase
4.3.6. Plasma Cortisol
4.4. Statistical Analysis
4.4.1. Demographics and Baseline Characteristics
4.4.2. TSST Response
4.4.3. Dog Effect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inc, G. Global Emotions Report. Available online: https://www.gallup.com/analytics/349280/gallup-global-emotions-report.aspx (accessed on 27 August 2024).
- Stress in America 2022: Concerned for the Future, Beset by Inflation. Available online: https://www.apa.org/news/press/releases/stress/2022/concerned-future-inflation (accessed on 27 August 2024).
- Mariotti, A. The Effects of Chronic Stress on Health: New Insights into the Molecular Mechanisms of Brain–Body Communication. Future Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.I.; Smyth, N.; Hall, S.J.; Torres, S.J.; Hussein, M.; Jayasinghe, S.U.; Ball, K.; Clow, A.J. Psychological Stress Reactivity and Future Health and Disease Outcomes: A Systematic Review of Prospective Evidence. Psychoneuroendocrinology 2020, 114, 104599. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Hamer, M.; Chida, Y. The Effects of Acute Psychological Stress on Circulating Inflammatory Factors in Humans: A Review and Meta-Analysis. Brain Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Steptoe, A. Greater Cardiovascular Responses to Laboratory Mental Stress Are Associated with Poor Subsequent Cardiovascular Risk Status: A Meta-Analysis of Prospective Evidence. Hypertension 2010, 55, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.C.; Hunt, K.; Der, G.; Carroll, D. Blunted Cardiac Reactions to Acute Psychological Stress Predict Symptoms of Depression Five Years Later: Evidence from a Large Community Study. Psychophysiology 2011, 48, 142–148. [Google Scholar] [CrossRef]
- Ronaldson, A.; Gazali, A.M.; Zalli, A.; Kaiser, F.; Thompson, S.J.; Henderson, B.; Steptoe, A.; Carvalho, L. Increased Percentages of Regulatory T Cells Are Associated with Inflammatory and Neuroendocrine Responses to Acute Psychological Stress and Poorer Health Status in Older Men and Women. Psychopharmacology 2016, 233, 1661–1668. [Google Scholar] [CrossRef]
- Yuenyongchaiwat, K.; Sheffield, D. Blunted Cardiovascular Reactions Are a Predictor of Negative Health Outcomes: A Prospective Cohort Study. J. Appl. Biobehav. Res. 2017, 22, e12091. [Google Scholar] [CrossRef]
- Allen, K.; Blascovich, J.; Mendes, W.B. Cardiovascular Reactivity and the Presence of Pets, Friends, and Spouses: The Truth about Cats and Dogs. Psychosom. Med. 2002, 64, 727–739. [Google Scholar] [CrossRef]
- Allen, A.P.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Biological and Psychological Markers of Stress in Humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014, 38, 94–124. [Google Scholar] [CrossRef]
- Wadsworth, M.E.; Broderick, A.V.; Loughlin-Presnal, J.E.; Bendezu, J.J.; Joos, C.M.; Ahlkvist, J.A.; Perzow, S.E.D.; McDonald, A. Co-Activation of SAM and HPA Responses to Acute Stress: A Review of the Literature and Test of Differential Associations with Preadolescents’ Internalizing and Externalizing. Dev. Psychobiol. 2019, 61, 1079–1093. [Google Scholar] [CrossRef]
- Jones, E.J.; Rohleder, N.; Schreier, H.M.C. Neuroendocrine Coordination and Youth Behavior Problems: A Review of Studies Assessing Sympathetic Nervous System and Hypothalamic-Pituitary Adrenal Axis Activity Using Salivary Alpha Amylase and Salivary Cortisol. Horm. Behav. 2020, 122, 104750. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Kennedy, P.J.; Dockray, S.; Cryan, J.F.; Dinan, T.G.; Clarke, G. The Trier Social Stress Test: Principles and Practice. Neurobiol. Stress 2017, 6, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Corazon, S.S.; Sidenius, U.; Poulsen, D.V.; Gramkow, M.C.; Stigsdotter, U.K. Psycho-Physiological Stress Recovery in Outdoor Nature-Based Interventions: A Systematic Review of the Past Eight Years of Research. Int. J. Env. Res. Public Health 2019, 16, 1711. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Rush, S.E. Mindfulness-Based Stress Reduction as a Stress Management Intervention for Healthy Individuals: A Systematic Review. J. Evid. Based Complement. Altern. Med. 2014, 19, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, K.; Kasten, N.; Fuchs, R. The Effect of Physical Activity on Sleep Quality, Well-Being, and Affect in Academic Stress Periods. Nat. Sci. Sleep 2017, 9, 117–126. [Google Scholar] [CrossRef]
- Allen, K.M.; Blascovich, J.; Tomaka, J.; Kelsey, R.M. Presence of Human Friends and Pet Dogs as Moderators of Autonomic Responses to Stress in Women. J. Pers. Soc. Psychol. 1991, 61, 582–589. [Google Scholar] [CrossRef]
- Beetz, A.; Kotrschal, K.; Turner, D.C.; Hediger, K.; Uvnäs-Moberg, K.; Julius, H. The Effect of a Real Dog, Toy Dog and Friendly Person on Insecurely Attached Children during a Stressful Task: An Exploratory Study. Anthrozoös 2011, 24, 349–368. [Google Scholar] [CrossRef]
- Beetz, A.; Julius, H.; Turner, D.; Kotrschal, K. Effects of Social Support by a Dog on Stress Modulation in Male Children with Insecure Attachment. Front. Psychol. 2012, 3, 352. [Google Scholar] [CrossRef]
- Campo, R.A.; Uchino, B.N. Humans’ Bonding with Their Companion Dogs: Cardiovascular Benefits during and after Stress. J. Sociol. Soc. Welf. 2013, 40, 237–249. [Google Scholar] [CrossRef]
- Polheber, J.P.; Matchock, R.L. The Presence of a Dog Attenuates Cortisol and Heart Rate in the Trier Social Stress Test Compared to Human Friends. J. Behav. Med. 2014, 37, 860–867. [Google Scholar] [CrossRef]
- Kertes, D.A.; Liu, J.; Hall, N.J.; Hadad, N.A.; Wynne, C.D.L.; Bhatt, S.S. Effect of Pet Dogs on Children’s Perceived Stress and Cortisol Stress Response. Soc. Dev. 2017, 26, 382–401. [Google Scholar] [CrossRef] [PubMed]
- American Veterinary Medical Association. 2022 AVMA Pet Ownership and Demographic Sourcebook; American Veterinary Medical Association: Schaumburg, IL, USA, 2022. [Google Scholar]
- Gandenberger, J.; Flynn, E.; Moratto, E.; Wendt, A.; Morris, K.N. Molecular Biomarkers of Adult Human and Dog Stress during Canine-Assisted Interventions: A Systematic Scoping Review. Animals 2022, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Ehlert, U. Acute Psychosocial Stress: Does the Emotional Stress Response Correspond with Physiological Responses? Psychoneuroendocrinology 2012, 37, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.M.; Quas, J.A.; Boyce, W.T. Associations between Physiological Reactivity and Children’s Behavior: Advantages of a Multisystem Approach. J. Dev. Behav. Pediatr. 2002, 23, 102–113. [Google Scholar] [CrossRef]
- Allwood, M.A.; Handwerger, K.; Kivlighan, K.T.; Granger, D.A.; Stroud, L.R. Direct and Moderating Links of Salivary Alpha-Amylase and Cortisol Stress-Reactivity to Youth Behavioral and Emotional Adjustment. Biol. Psychol. 2011, 88, 57–64. [Google Scholar] [CrossRef]
- Pham, H.T.; Bendezú, J.J.; Wadsworth, M.E. HPA-SAM Co-Activation among Racially Diverse, Economically Disadvantaged Early Adolescents: Secondary Analysis with a Preliminary Test of a Multisystem, Person-Centered Approach. Biol. Psychol. 2023, 179, 108546. [Google Scholar] [CrossRef]
- Jurblum, M.; Ng, C.H.; Castle, D.J. Psychological Consequences of Social Isolation and Quarantine: Issues Related to COVID-19 Restrictions. Aust. J. Gen. Prac. 2020, 49, 778–783. [Google Scholar] [CrossRef]
- Leung, C.M.C.; Ho, M.K.; Bharwani, A.A.; Cogo-Moreira, H.; Wang, Y.; Chow, M.S.C.; Fan, X.; Galea, S.; Leung, G.M.; Ni, M.Y. Mental Disorders Following COVID-19 and Other Epidemics: A Systematic Review and Meta-Analysis. Transl. Psychiatry 2022, 12, 205. [Google Scholar] [CrossRef]
- Thoma, M.V.; Kirschbaum, C.; Wolf, J.M.; Rohleder, N. Acute Stress Responses in Salivary Alpha-Amylase Predict Increases of Plasma Norepinephrine. Biol. Psychol. 2012, 91, 342–348. [Google Scholar] [CrossRef]
- Petrakova, L.; Doering, B.K.; Vits, S.; Engler, H.; Rief, W.; Schedlowski, M.; Grigoleit, J.-S. Psychosocial Stress Increases Salivary Alpha-Amylase Activity Independently from Plasma Noradrenaline Levels. PLoS ONE 2015, 10, e0134561. [Google Scholar] [CrossRef]
- Glenn, A.L.; Remmel, R.J.; Raine, A.; Schug, R.A.; Gao, Y.; Granger, D.A. Alpha-Amylase Reactivity in Relation to Psychopathic Traits in Adults. Psychoneuroendocrinology 2015, 54, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Bohus, M.; Abbruzzese, E.; Ditzen, B.; Gaab, J.; Kleindienst, N.; Ebner-Priemer, U.; Mauchnik, J.; Ehlert, U. Increased Psychological and Attenuated Cortisol and Alpha-Amylase Responses to Acute Psychosocial Stress in Female Patients with Borderline Personality Disorder. Psychoneuroendocrinology 2010, 35, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Scognamiglio, P.; Canestrelli, B.; Serino, I.; Monteleone, A.M.; Maj, M. Asymmetry of Salivary Cortisol and α-Amylase Responses to Psychosocial Stress in Anorexia Nervosa but Not in Bulimia Nervosa. Psychol. Med. 2011, 41, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- von Majewski, K.; Kraus, O.; Rhein, C.; Lieb, M.; Erim, Y.; Rohleder, N. Acute Stress Responses of Autonomous Nervous System, HPA Axis, and Inflammatory System in Posttraumatic Stress Disorder. Transl. Psychiatry 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; Díaz, M.M.; Santos, T.V.d.S.; Bernardes, J.T.M.; Peixoto, L.G.; Bocanegra, O.L.; Neto, M.B.; Espindola, F.S. Chronic Stress Induces a Hyporeactivity of the Autonomic Nervous System in Response to Acute Mental Stressor and Impairs Cognitive Performance in Business Executives. PLoS ONE 2015, 10, e0119025. [Google Scholar] [CrossRef]
- Hawes, S.M.; Hupe, T.M.; Gandenberger, J.; Saucedo, M.; Arrington, A.; Morris, K.N. Detailed Assessment of Pet Ownership Rates in Four Underserved Urban and Rural Communities in the United States. J. Appl. Anim. Welf. Sci. 2022, 25, 326–337. [Google Scholar] [CrossRef]
- Narvaez Linares, N.F.; Charron, V.; Ouimet, A.J.; Labelle, P.R.; Plamondon, H. A Systematic Review of the Trier Social Stress Test Methodology: Issues in Promoting Study Comparison and Replicable Research. Neurobiol. Stress 2020, 13, 100235. [Google Scholar] [CrossRef]
- Martos-Montes, R.; Ordóñez-Pérez, D.; Ruiz-Maatallah, J.; Martínez-Cobos, M. Psychophysiological Effects of Human-Dog Interaction in University Students Exposed to a Stress-Induced Situation Using the Trier Social Stress Test (TSST). Hum.-Anim. Interact. Bull. 2020, 8, 93–107. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Faulkner, M.E. The “Pet Effect”: Physiological Calming in the Presence of Canines. Soc. Anim. J. Hum.-Anim. Stud. 2015, 23, 425–438. [Google Scholar] [CrossRef]
- Ghiciuc, C.M.; Cozma-Dima, C.L.; Pasquali, V.; Renzi, P.; Simeoni, S.; Lupusoru, C.E.; Patacchioli, F.R. Awakening Responses and Diurnal Fluctuations of Salivary Cortisol, DHEA-S and α-Amylase in Healthy Male Subjects. Neuro. Endocrinol. Lett. 2011, 32, 475–480. [Google Scholar]
- Birkett, M.A. The Trier Social Stress Test Protocol for Inducing Psychological Stress. J. Vis. Exp. 2011, 56, e3238. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Hellhammer, D.H.; Kirschbaum, C. Ten Years of Research with the Trier Social Stress Test—Revisited. In Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior; The Guilford Press: New York, NY, USA, 2007; pp. 56–83. ISBN 978-1-59385-404-1. [Google Scholar]
- Goodman, W.K.; Janson, J.; Wolf, J.M. Meta-Analytical Assessment of the Effects of Protocol Variations on Cortisol Responses to the Trier Social Stress Test. Psychoneuroendocrinology 2017, 80, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, I.; Grace, C.; Rendell, P.; Terrett, G.; Heinrichs, M. An Introductory Guide to Conducting the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2019, 107, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Ludyga, S.; Colledge, F.; Gerber, M. Influence of Regular Physical Activity and Fitness on Stress Reactivity as Measured with the Trier Social Stress Test Protocol: A Systematic Review. Sports Med. 2018, 48, 2607–2622. [Google Scholar] [CrossRef]
- Grös, D.F.; Antony, M.M.; Simms, L.J.; McCabe, R.E. Psychometric Properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): Comparison to the State-Trait Anxiety Inventory (STAI). Psychol. Assess 2007, 19, 369–381. [Google Scholar] [CrossRef]
- Balodis, I.M.; Wynne-Edwards, K.E.; Olmstead, M.C. The Other Side of the Curve: Examining the Relationship between Pre-Stressor Physiological Responses and Stress Reactivity. Psychoneuroendocrinology 2010, 35, 1363–1373. [Google Scholar] [CrossRef]
- Gamaiunova, L.; Kreibig, S.D.; Dan-Glauser, E.; Pellerin, N.; Brandt, P.-Y.; Kliegel, M. Effects of Two Mindfulness Based Interventions on the Distinct Phases of the Stress Response across Different Physiological Systems. Biol. Psychol. 2022, 172, 108384. [Google Scholar] [CrossRef]
- Maki, P.M.; Mordecai, K.L.; Rubin, L.H.; Sundermann, E.; Savarese, A.; Eatough, E.; Drogos, L. Menstrual Cycle Effects on Cortisol Responsivity and Emotional Retrieval Following a Psychosocial Stressor. Horm. Behav. 2015, 74, 201–208. [Google Scholar] [CrossRef]
- Quade, D. Rank Analysis of Covariance. J. Am. Stat. Assoc. 1967, 62, 1187–1200. [Google Scholar] [CrossRef]
- García-Álvarez, L.; Caso, J.R.; García-Portilla, M.P.; de la Fuente-Tomás, L.; González-Blanco, L.; Sáiz Martínez, P.; Leza, J.C.; Bobes, J. Regulation of Inflammatory Pathways in Schizophrenia: A Comparative Study with Bipolar Disorder and Healthy Controls. Eur. Psychiatry 2018, 47, 50–59. [Google Scholar] [CrossRef]

| Characteristic | Dog (n = 22) | No Dog (n = 21) | Significance |
|---|---|---|---|
| Gender | 4 (18.2%) Male 18 (81.8%) Female 0 (0%) Non-binary | 1 (4.8%) Male 19 (90.5%) Female 1 (4.8%) Non-binary | χ2 = 2.72, p = 0.26 |
| Age (years) | 39.0 ± 14.3 | 35.7 ± 15.1 | t = 0.73, p = 0.47 |
| Race/ethnicity | 1 (4.5%) American Indian/Alaska Native 1 (4.5%) Asian 1 (4.5%) Black/African American 1 (4.5%) Hispanic/Latino 18 (81.8%) White/Caucasian | 1 (4.8%) Black/African American 2 (9.5%) Hispanic/Latino 18 (85.7%) White/Caucasian | χ2 = 0.08, p = 0.78 |
| Physical activity days/week | 3.41 ± 2.02 | 4.29 ± 2.26 | t = −1.34, p = 0.19 |
| Menstrual cycle phase | 13 (59.1%) N/A 4 (18.2%) Follicular 5 (22.7%) Luteal | 10 (47.6%) N/A 7 (33.3%) Follicular 4 (19.0%) Luteal | χ2 = 1.51, p = 0.47 |
| STAI-AD trait anxiety | 32.9 ± 6.73 | 34.0 ± 5.63 | t = 0.60, p = 0.55 |
| STAI-AD state anxiety, T0 | 26.1 ± 4.62 | 27.6 ± 5.71 | t = −0.97, p = 0.34 |
| Heart rate, T0 (BPM) | 75.0 ± 10.1 | 75.2 ± 7.80 | t = −0.07, p = 0.95 |
| Cortisol, T0 (ng/mL) | 73.5 ± 36.1 | 82.1 ± 38.8 | t = −0.76, p = 0.45 |
| Sal α-amylase, T0 (U/mL) | 65.0 ± 71.7 | 42.5 ± 62.3 | t = −1.0, p = 0.31 |
| Measure | Group | Maulchy’s Test of Sphericity | Greenhouse–Geisser Correction ε | RM ANOVA Results | Mean Difference (T1–T0) and Post Hoc p Value | Mean Difference (T2–T1) and Post Hoc p Value | Mean Difference (T0–T2) and Post Hoc p Value |
|---|---|---|---|---|---|---|---|
| STAI-AD state | Aggregate | χ2(2) = 34.8 p < 0.001 | 0.64 | F1.27,53.45 = 203 p < 0.001 partial η2 = 0.83 | 24.7 p < 0.001 | −24.7 p < 0.001 | −0.02 p = 1.0 |
| Dog | χ2(2) = 21.7 p < 0.001 | 0.60 | F1.20,25.27 = 109 p < 0.001 partial η2 = 0.84 | 22.6 p < 0.001 | −22.7 p < 0.001 | 0.14 p = 1.0 | |
| No dog | χ2(2) = 13.4 p = 0.001 | 0.66 | F1.33,26.57 = 98.8 p < 0.001 partial η2 = 0.83 | 26.9 p < 0.001 | −26.7 p < 0.001 | −0.19 p = 1.0 | |
| Heart rate (BPM) | Aggregate | χ2(2) = 15.7 p < 0.001 | 0.76 | F1.52,63.71 = 85.4 p < 0.001 partial η2 = 0.67 | 15.0 p < 0.001 | −17.9 p < 0.001 | 2.98 p = 0.01 |
| Dog | χ2(2) = 0.79 p = 0.675 | N/A | F2,42 = 29.6 p < 0.001 partial η2 = 0.59 | 10.4 p < 0.001 | −13.2 p < 0.001 | 2.77 p = 0.37 | |
| No dog | χ2(2) = 25.1 p < 0.001 | 0.58 | F1.15,23.08 = 73.9 p < 0.001 partial η2 = 0.79 | 19.7 p < 0.001 | −22.9 p < 0.001 | 3.19 p = 0.005 | |
| Plasma cortisol (ng/mL) | Aggregate | χ2(2) = 9.70 p = 0.008 | 0.81 | F1.62,59.86 = 42.1 p < 0.001 partial η2 = 0.53 | 29.3 p < 0.001 | −32.1 p < 0.001 | 2.79 p = 1.0 |
| Dog | χ2(2) = 8.45 p = 0.015 | 0.72 | F1.44,25.87 = 15.0 p < 0.001 partial η2 = 0.46 | 20.4 p = 0.02 | −28.5 p < 0.001 | 8.12 p = 0.15 | |
| No dog | χ2(2) = 1.68 p = 0.433 | N/A | F2,36 = 32.1 p < 0.001 partial η2 = 0.64 | 38.2 p < 0.001 | −35.6 p < 0.001 | −2.54 p = 1.0 | |
| Sal α-amylase (U/mL) | Aggregate | χ2(2) = 3.38 p = 0.19 | N/A | F2,72 = 2.12 p = 0.13 partial η2 = 0.06 | 17.1 p = 0.24 | −12.1 p = 0.32 | −5.03 p = 1.0 |
| Dog | χ2(2) = 1.63 p = 0.44 | N/A | F2,36 = 4.45 p = 0.02 partial η2 = 0.20 | 34.6 p = 0.02 | −16.7 p = 0.65 | −17.9 p = 0.27 | |
| No dog | χ2(2) = 13.8 p = 0.001 | 0.63 | F1.27,21.56 = 0.29 p = 0.65 partial η2 = 0.02 | −1.46 p = 1.0 | −7.13 p = 0.74 | 8.58 p = 1.0 |
| Measure | Time | Unadjusted Mean (±SEM) | Adjusted Mean (±SEM) | ANCOVA Results | ||
|---|---|---|---|---|---|---|
| Dog | No Dog | Dog | No Dog | |||
| STAI-state percent change | T0–T1 | 89.6 ± 9.46 | 102 ± 10.5 | 86.8 ± 9.07 | 105 ± 9.29 | F1,40 = 1.94 p = 0.17 partial η2 = 0.05 |
| T1–T2 | −45.0 ± 2.52 | −46.9 ± 3.21 | −44.7 ± 2.86 | −47.2 ± 2.93 | F1,40 = 0.35 p = 0.56 partial η2 = 0.01 | |
| T0–T2 * | 0.21 ± 2.99 | 2.36 ± 4.50 | −0.73 ± 3.54 | 3.34 ± 3.62 | F1,41 = 0.47 p = 0.50 partial η2 = 0.01 | |
| Heart rate percent change | T0–T1 * | 14.6 ± 2.28 | 26.7 ± 3.83 | 14.6 ± 2.90 | 26.8 ± 2.96 | F1,41 = 7.84 p = 0.008 partial η2 = 0.16 |
| T1–T2 * | −14.9 ± 2.11 | −23.5 ± 1.73 | −14.9 ± 1.93 | −23.5 ± 1.97 | F1,41 = 10.66 p = 0.002 partial η2 = 0.20 | |
| T0–T2 | −3.07 ± 1.99 | −4.22 ± 1.16 | −3.10 ± 1.52 | −4.19 ± 1.56 | F1,40 = 0.25 p = 0.62 partial η2 = 0.006 | |
| Cortisol percent change | T0–T1 * | 33.7 ± 13.4 | 48.7 ± 11.5 | 31.1 ± 11.2 | 51.3 ± 11.2 | F1,40 = 4.18 p = 0.047 partial η2 = 0.09 |
| T1–T2 | −31.2 ± 4.03 | −29.3 ± 3.38 | −30.8 ± 3.64 | −29.8 ± 3.64 | F1,35 = 0.04 p = 0.84 partial η2 = 0.001 | |
| T0–T2 * | 4.28 ± 15.5 | 9.16 ± 8.83 | 2.67 ± 12.5 | 10.9 ± 12.8 | F1,37 = 4.49 p = 0.043 partial η2 = 0.11 | |
| Sal α-amylase percent change | T0–T1 * | 97.6 ± 28.1 | 42.4 ± 17.6 | 103 ± 22.2 | 36.3 ± 22.8 | F1,35 = 4.55 p = 0.040 partial η2 = 0.11 |
| T1–T2 * | −11.0 ± 10.0 | −8.72 ± 9.69 | −11.4 ± 9.92 | −8.21 ± 10.2 | F1,35 = 0.08 p = 0.78 partial η2 = 0.002 | |
| T0–T2 * | 50.8 ± 14.8 | 25.0 ± 19.7 | 55.1 ± 16.8 | 20.8 ± 16.8 | F1,36 = 6.14 p = 0.018 partial η2 = 0.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandenberger, J.; Ledreux, A.; Taeckens, A.; Murphy, K.; Forkin, J.; Gilmore, A.; Morris, K.N. The Presence of a Pet Dog Is Associated with a More Balanced Response to a Social Stressor. Stresses 2024, 4, 598-613. https://doi.org/10.3390/stresses4030038
Gandenberger J, Ledreux A, Taeckens A, Murphy K, Forkin J, Gilmore A, Morris KN. The Presence of a Pet Dog Is Associated with a More Balanced Response to a Social Stressor. Stresses. 2024; 4(3):598-613. https://doi.org/10.3390/stresses4030038
Chicago/Turabian StyleGandenberger, Jaci, Aurélie Ledreux, Ashley Taeckens, Kerry Murphy, Jenni Forkin, Anah Gilmore, and Kevin N. Morris. 2024. "The Presence of a Pet Dog Is Associated with a More Balanced Response to a Social Stressor" Stresses 4, no. 3: 598-613. https://doi.org/10.3390/stresses4030038
APA StyleGandenberger, J., Ledreux, A., Taeckens, A., Murphy, K., Forkin, J., Gilmore, A., & Morris, K. N. (2024). The Presence of a Pet Dog Is Associated with a More Balanced Response to a Social Stressor. Stresses, 4(3), 598-613. https://doi.org/10.3390/stresses4030038






