Enhanced Cadmium Removal by Raw Argan Shell Adsorbent: Experimental and Theoretical Investigations for Ecological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Natural Adsorbent: Preparation of Argan Nut Shell Powder (ArS)
2.2. Adsorbate: Synthetic Solution Preparation
2.3. Characterization
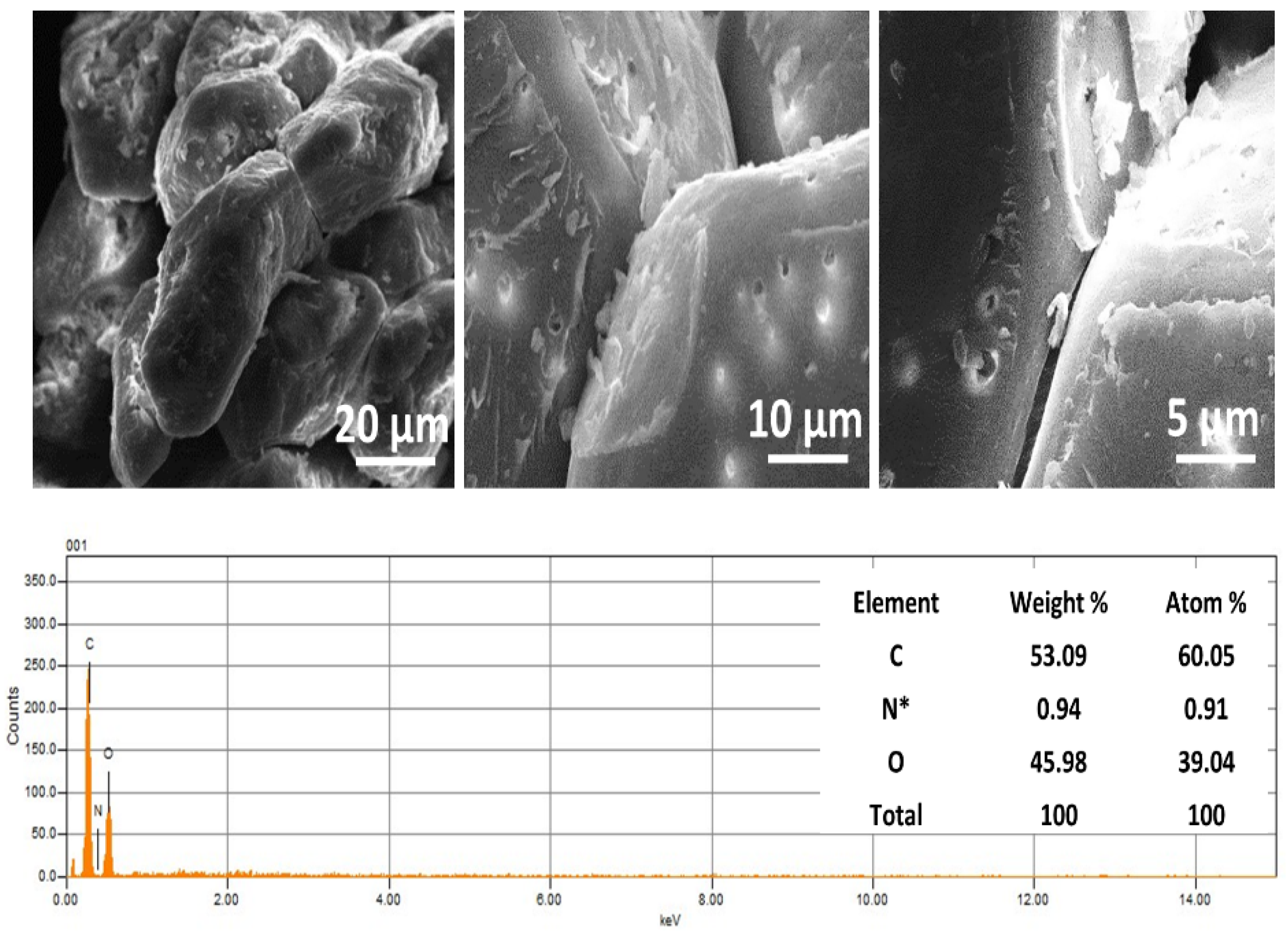
2.3.1. Scanning Electron Microscopy (SEM-EDX)
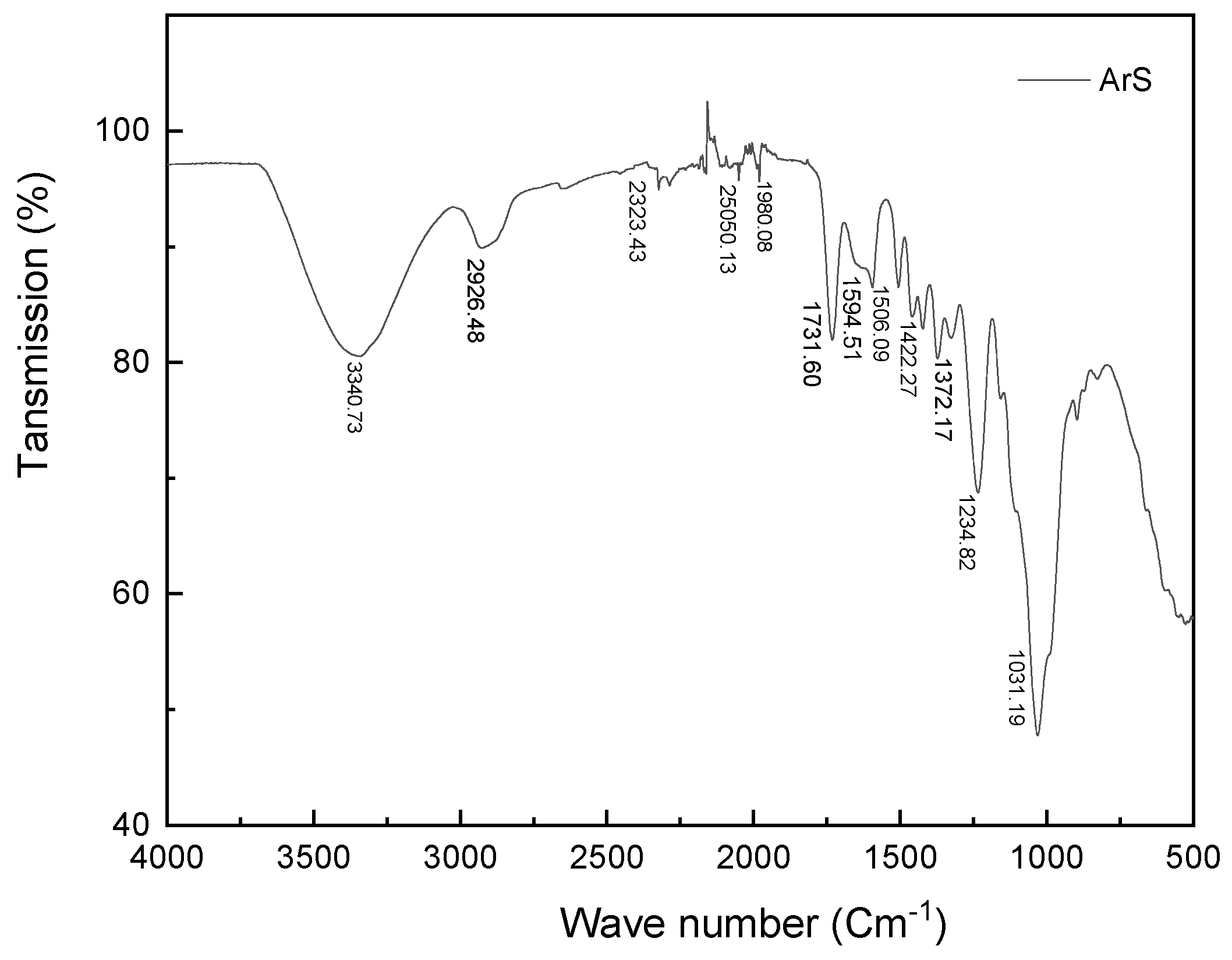
2.3.2. FTIR Analysis
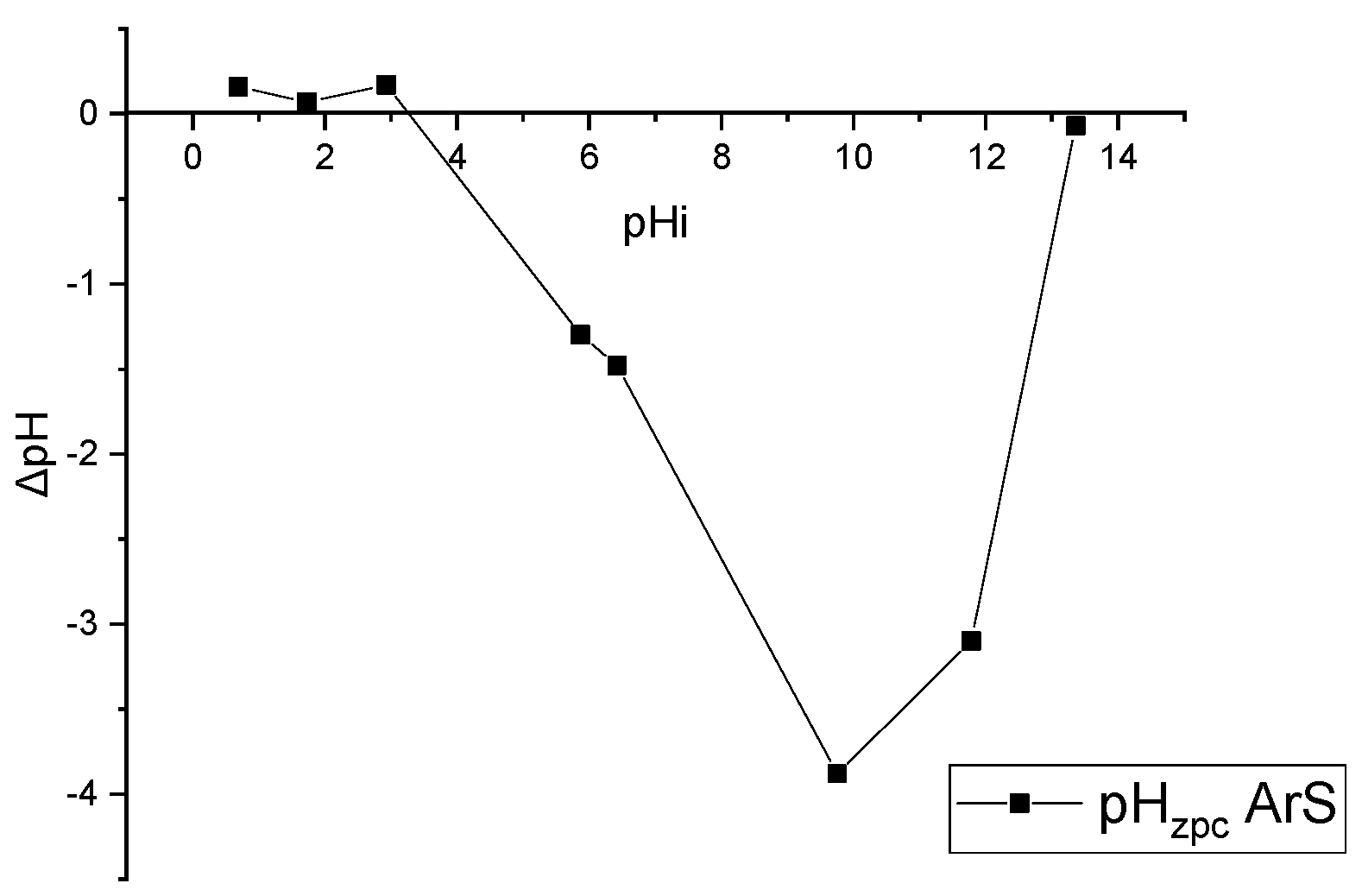
2.3.3. pH Zero Charge Point: pHzpc
2.3.4. Boehm Titration
2.3.5. Specific Area
2.3.6. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.4. Response Surface Methodology (RSM): Batch Experiments
2.5. Batch Adsorption Process
3. Results and Discussion
3.1. Characterization
3.1.1. SEM Analysis of Argan Nutshell Powder
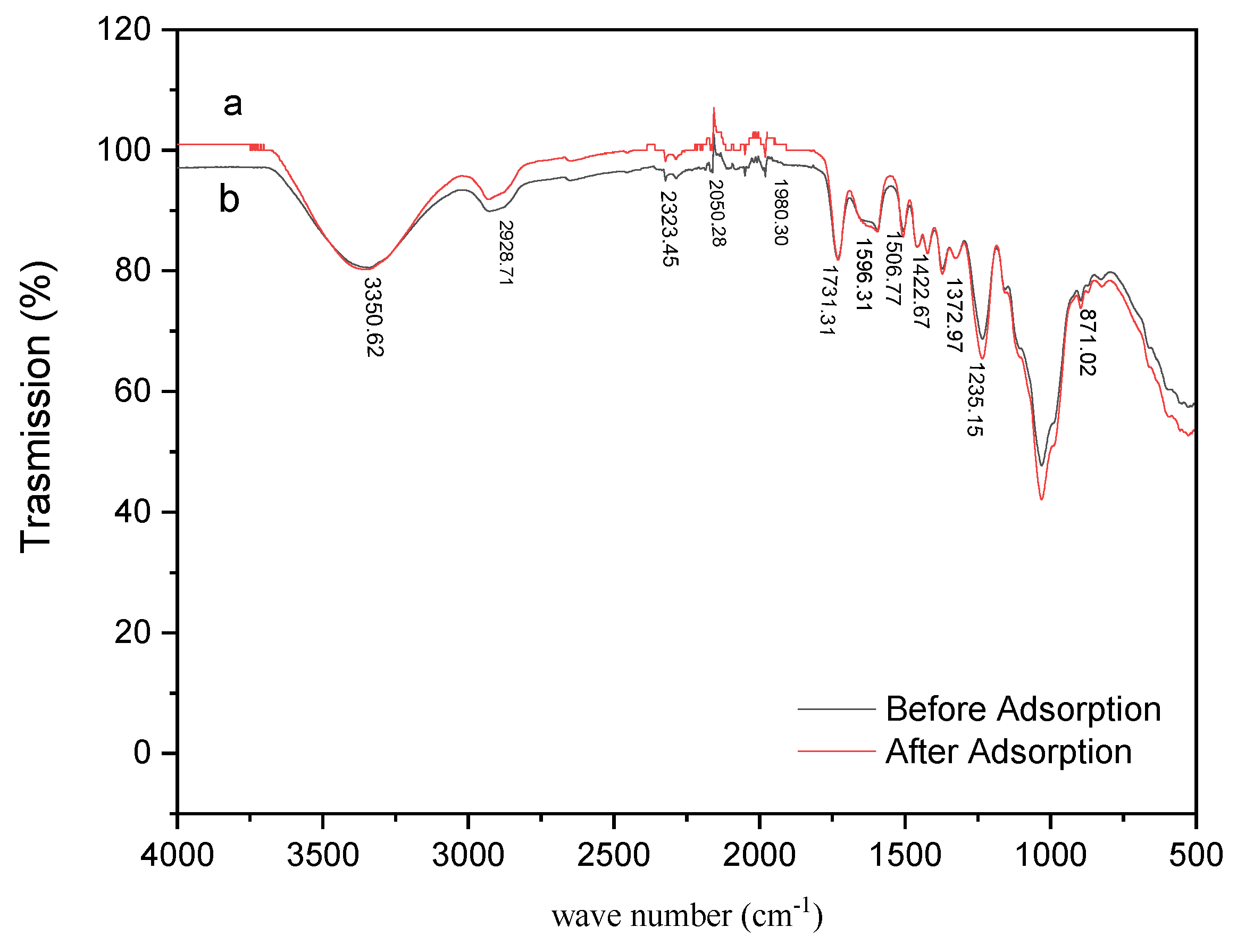
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.3. pH of Point Zero Charge: pHzpc
3.1.4. Boehm Titration
3.2. Batch Experiments: Response Surface Methodology
3.2.1. Study of the Parameters: Statistical Study
3.2.2. Correlation Coefficient
3.2.3. Analysis of Variance
3.2.4. Residue Review
3.2.5. Estimation of the Parameters
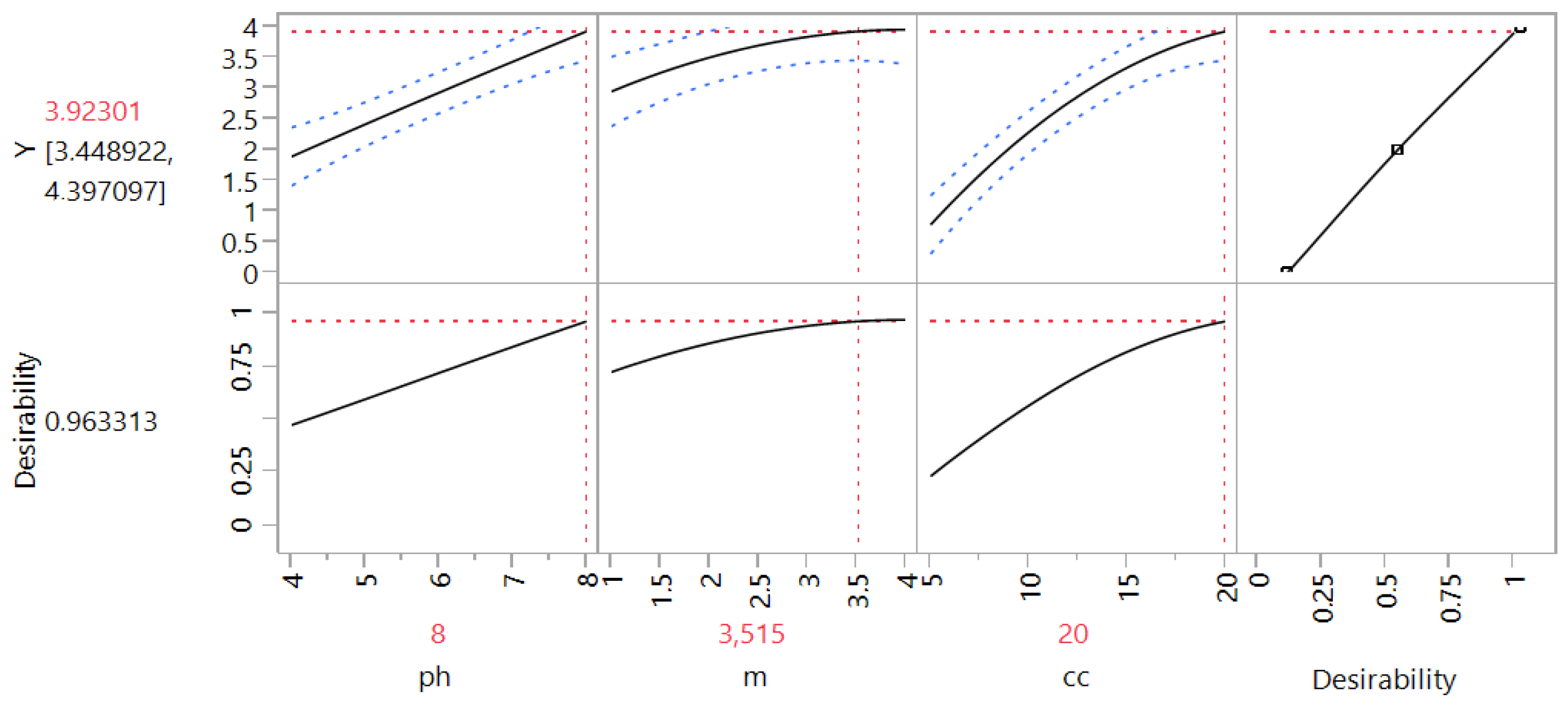
3.2.6. Optimization of the Parameters
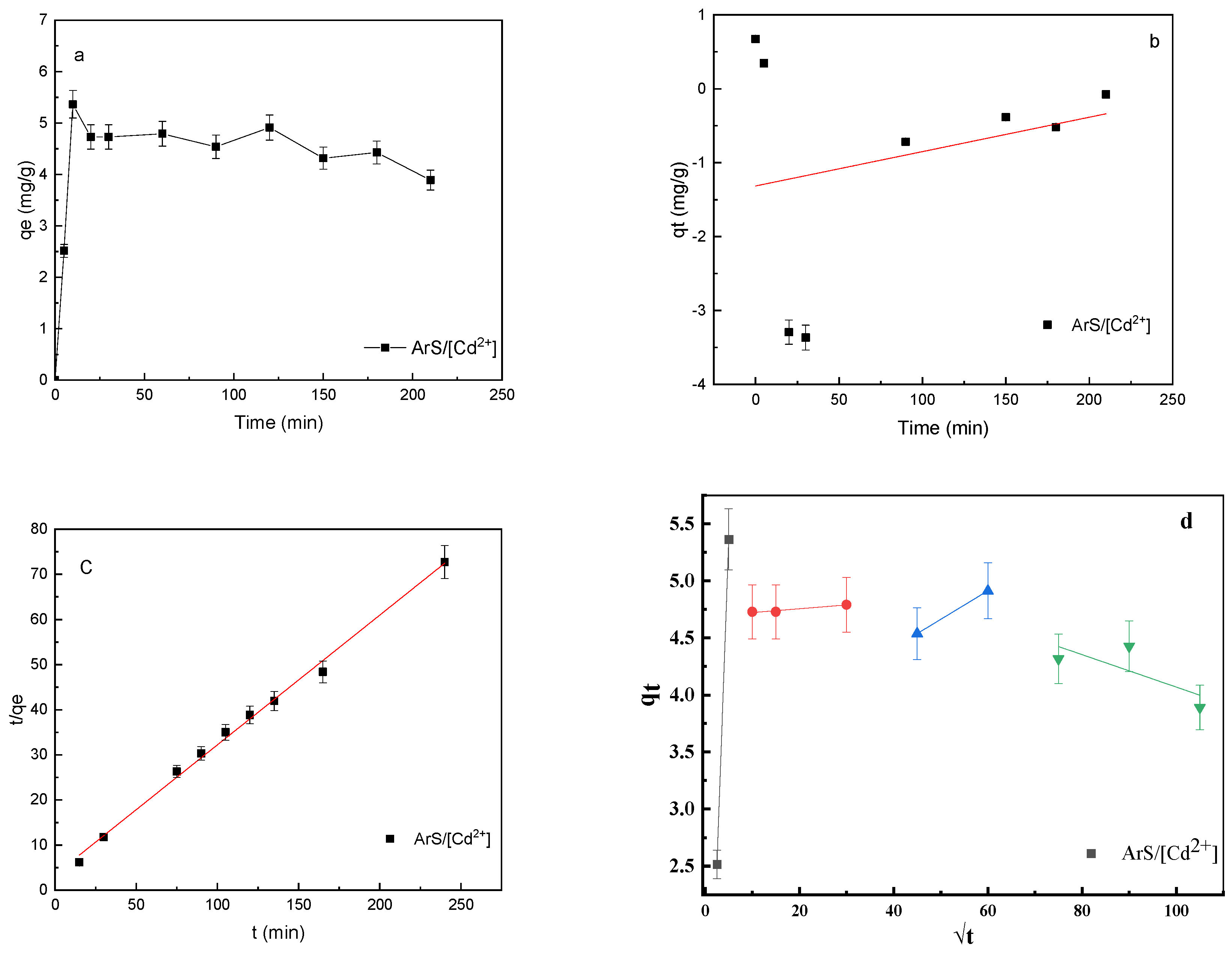
3.3. Batch Experiments: Kinetic Study
3.4. Batch Experiments: Isotherms

| Isotherms | Parameters | Values |
|---|---|---|
| Langmuir | qm (mg·g−1) | 4 |
| KL (L·g−1) | 0.91 | |
| RL | 0.0521 | |
| R2 | 0.99 | |
| Freundlich [50] | 1/nf | 0.33 |
| Kf (mg(1−n)Ln·g−1) | 1.8516 | |
| R2 | 0.859 | |
| Temkin | BT (J·mol−1) | 0.718 |
| KT (L·g−1) | 1.432 | |
| R2 | 0.902 | |
| bT (J/molK) | 1731.13 | |
| Dubinin–Radushkevich [51] | E (KJ·mol−1) | 3.488 |
| Qe (mg·g−1) | 3.525 | |
| β (J2/mol2) | 4.933 | |
| R2 | 0.984 |
| Samples | Adsorption Capacity (mg·g−1) | pH | Reference |
|---|---|---|---|
| Pine bark (P. pinaster Ait) | 3.23 | 3.4 | [14] |
| Walnut shells | 4.36 | 6 | [20] |
| Cashew activated carbon | 2.87 | 5 | [18] |
| Raw walnut shells (RWS) | 5.38 | 6 | [15] |
| Coffee beans | 3.80 | 8 | [16] |
| Ash produced from rice husks | 3.04 | 6 | [19] |
| Rice-hull-derived AC | 2.49 | 4.5 | [17] |
| Raw argan shells | 4 | 8 | This work |
3.5. Batch Experiments: Thermodynamic Parameters
3.6. Desorption Study of Cd2+ from ArS
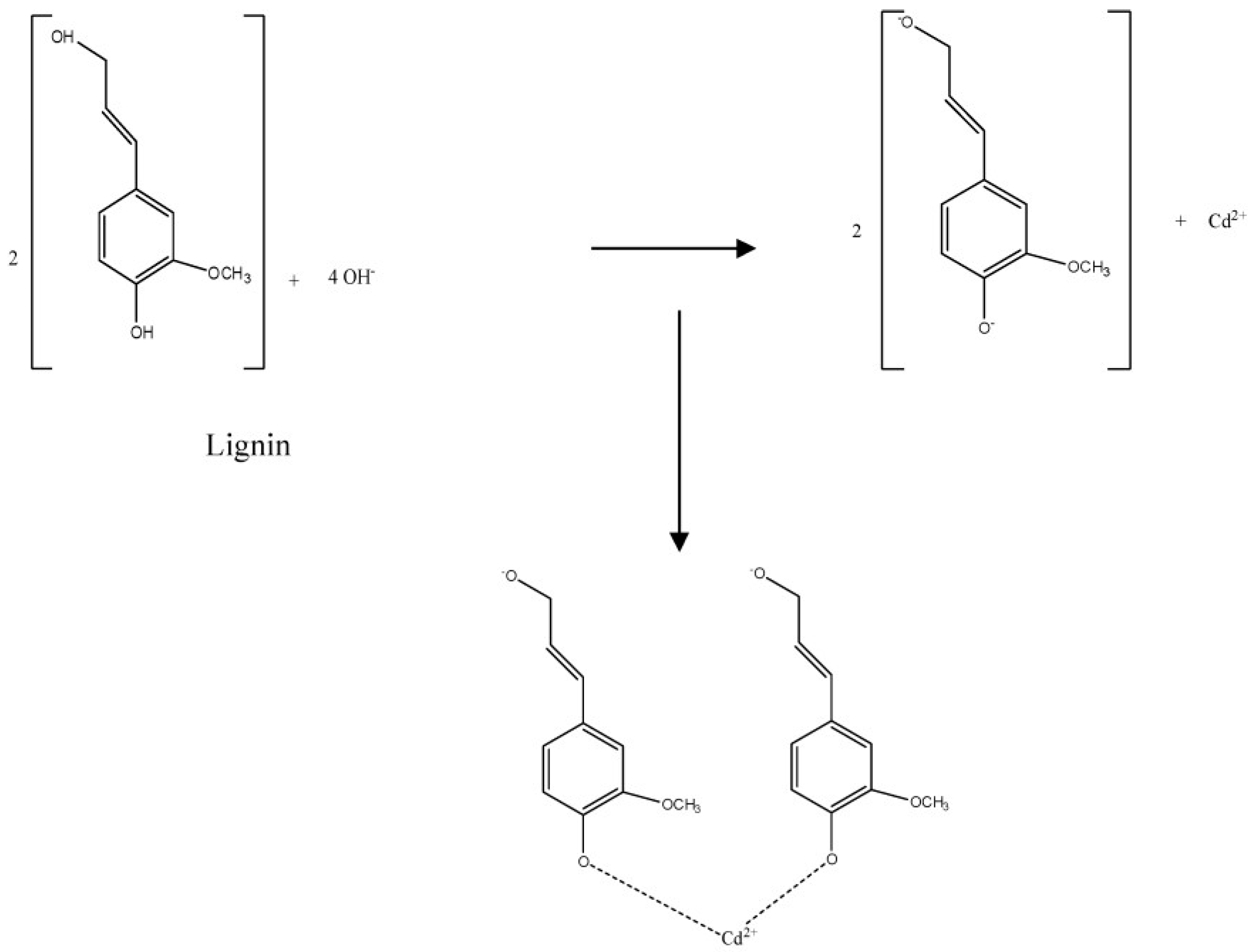
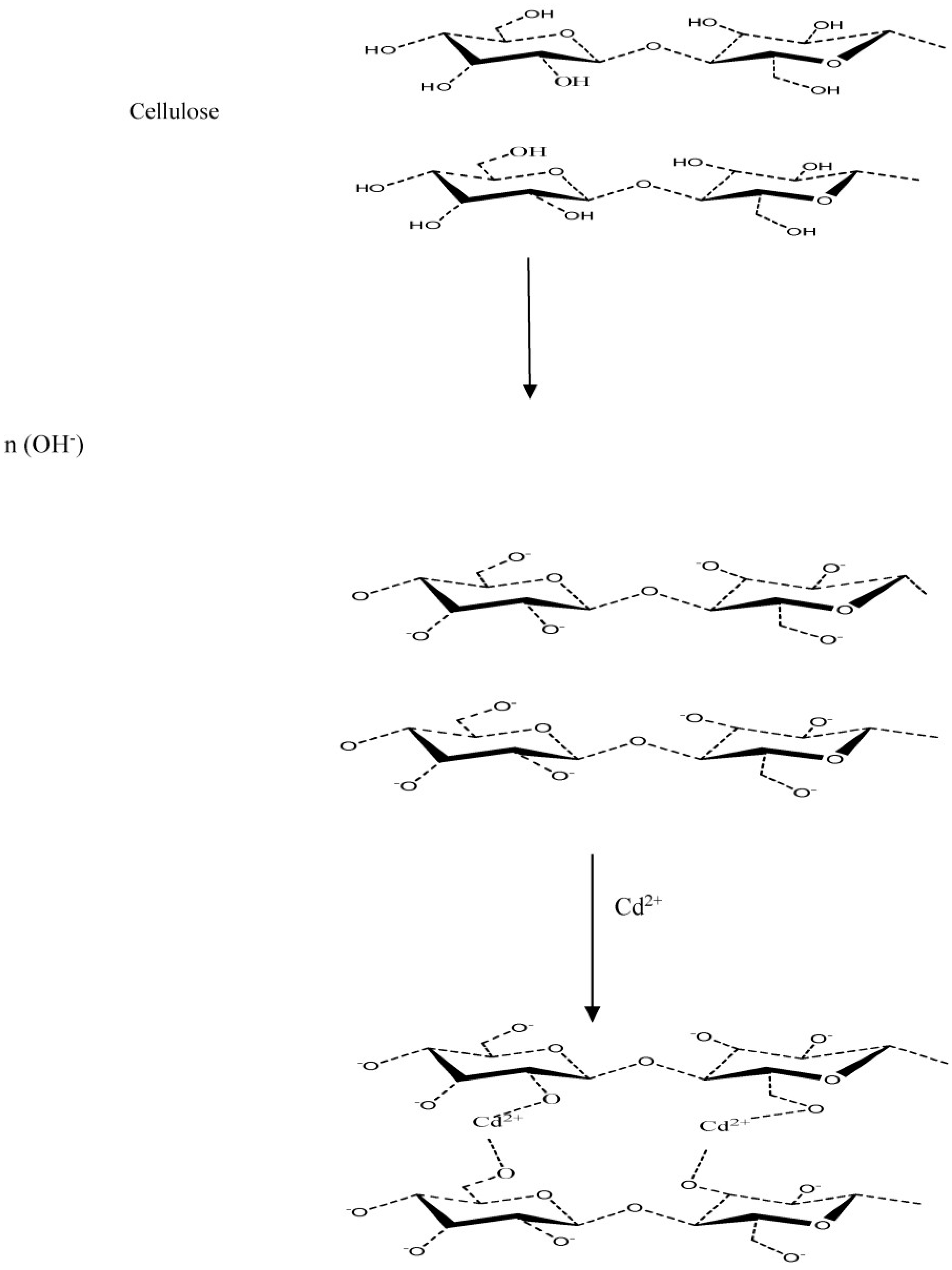
3.7. Proposed Adsorption Mechanisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamid, N.H.A.; Rushdan, A.I.; Nordin, A.H.; Husna, S.M.N.; Faiz Norrrahim, M.N.; Knight, V.F.; Tahir, M.I.H.M.; Li, G.X.; Quan, T.L.; Abdullah, A.M.; et al. A state-of-art review on the sustainable technologies for cadmium removal from wastewater. Water Reuse 2024, 14, 312–341. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, H.; Geng, J.; Chen, J. Facile one-pot preparation of nitrogen-doped ultra-light graphene oxide aerogel and its prominent adsorption performance of Cr(VI). Chem. Eng. J. 2018, 338, 62–71. [Google Scholar] [CrossRef]
- Fulke, A.B. Understanding heavy metal toxicity: Implications on human health, marine ecosystems and bioremediation strategies. Mar. Pollut. Bull. 2024, 206, 116707. [Google Scholar] [CrossRef]
- Guarín-Romero, J.R.; Rodríguez-Estupiñán, P.; Giraldo, L.; Moreno-Piraján, J.C. Simple and Competitive Adsorption Study of Nickel(II) and Chromium(III) on the Surface of the Brown Algae Durvillaea antarctica Biomass. ACS Omega 2019, 4, 18147–18158. [Google Scholar] [CrossRef]
- Dhanda, A.; Lamba, R.; Deepika, S.; Prakash, R. Cadmium in Environment—An Overview; Springer: Berlin/Heidelberg, Germany, 2024; pp. 3–20. [Google Scholar] [CrossRef]
- Kouzbour, S.; Gourich, B.; Gros, F.; Vial, C.; Allam, F.; Stiriba, Y. Comparative analysis of industrial processes for cadmium removal from phosphoric acid: A review. Hydrometallurgy 2019, 188, 222–247. [Google Scholar] [CrossRef]
- Suciu, N.A.; De Vivo, R.; Rizzati, N.; Capri, E. Cd content in phosphate fertilizer: Which potential risk for the environment and human health? Curr. Opin. Environ. Sci. Health 2022, 30, 100392. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. J. Int. Assoc. Geochem. Cosmochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.H.; Wahab, M.M.A. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef]
- Rashid, A.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Fungal biomass composite with bentonite efficiency for nickel and zinc adsorption: A mechanistic study. Ecol. Eng. 2016, 91, 459–471. [Google Scholar] [CrossRef]
- Qasem, N.; Mohammed, R.; Lawal, D. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Nascimento, T.L.; Oliveira, K.F.; Junior, J.O.; Pimenta, A.S.; Melo, D.M.; Melo, M.A.; Braga, R.M. Biosorption of nickel and cadmium using Pachira aquatica Aubl. peel biochar. Sci. Rep. 2024, 14, 5086. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Ansias-Manso, L.; Fernández-Calviño, D.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Pine bark as bio-adsorbent for Cd, Cu, Ni, Pb and Zn: Batch-type and stirred flow chamber experiments. J. Environ. Manag. 2014, 144, 258–264. [Google Scholar] [CrossRef]
- Gondhalekar, S.C.; Shukla, S.R. Biosorption of cadmium metal ions on raw and chemically modified walnut shells. Environ. Prog. Sustain. Energy 2015, 34, 1613–1619. [Google Scholar] [CrossRef]
- Kaikake, K.; Hoaki, K.; Sunada, H.; Dhakal, R.; Baba, Y. Removal Characteristics of Metal Ions Using Degreased Coffee Beans: Adsorption Equilibrium of Cadmium(II). Bioresour. Technol. 2007, 98, 2787–2791. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lalhmunsiama, C.S.-I.; Tiwari, D. Manganese and iron oxide immobilized activated carbons precursor to dead biomasses in the remediation of cadmium-contaminated waters. Environ. Sci. Pollut. Res. Int. 2013, 20, 7464–7477. [Google Scholar] [CrossRef]
- N’goran, P.D.A.; Diabaté, D.; Yao, K.M.; Berenger, K.; Gnonsoro, U.; Kinimo, K.; Trokourey, A. Lead and cadmium removal from natural freshwater using mixed activated carbons from cashew and shea nut shells. Arab. J. Geosci. 2018, 11, 498. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef]
- Kamsonlian, S.; Suresh, S.; Majumder, C.B.; Chand, S. Biosorption of As(III) from contaminated water onto low cost palm bark biomass. Int. J. Curr. Eng. Technol. 2012, 2, 153–158. [Google Scholar]
- Weshahy, A.R.; Sakr, A.K.; Gouda, A.A.; Atia, B.M.; Somaily, H.H.; Hanfi, M.Y.; Gado, M.A. Selective re-covery of cadmium, cobalt, and nickel from spent Ni–Cd batteries using Adogen® 464 and mesoporous silica deriva-tives. Int. J. Mol. Sci. 2022, 23, 8677. [Google Scholar] [CrossRef]
- Najah El Idrissi, A.; Benbrahim, M.; Rassai, N. Effect of the particle size argan nut shell (ANS) biomass on combustion parameters in stirling engine in Morocco. Results Eng. 2023, 18, 101202. [Google Scholar] [CrossRef]
- Boundati, Y.; Ziat, K.; Naji, A.; Saidi, M.; Rghioui, L. Copper biosorption on argan nut shell: Kinetic and thermodynamic studies. Int. J. Adv. Res. 2018, 6, 300–309. [Google Scholar] [CrossRef]
- Millett, M.A.; Baker, A.J.; Satter, L.D. Physical and chemical pretreatments for enhancing cellulose saccharification. Biotechnol. Bioeng. Symp. 1976, 6, 125–153. [Google Scholar]
- Benadjemia, M.; Milliere, L.; Reinert, L.; Benderdouche, N.; Duclaux, L. Preparation, characterization and Meth-ylene Blue adsorption of phosphoric acid activated carbons from globe artichoke leaves. Fuel Energy Abstr. 2011, 92, 1203–1212. [Google Scholar] [CrossRef]
- Bencheikh, I.; Azoulay, K.; Mabrouki, J.; El Hajjaji, S.; Moufti, A.; Labjar, N. The use and the performance of chemically treated artichoke leaves for textile industrial effluents treatment. Chem. Data Collect. 2021, 31, 100597. [Google Scholar] [CrossRef]
- Es-said, A.; Hamdaoui, L.; El Moussaouiti, M.; Bchitou, R. Esterification optimization of cellulose with p-Iodobenzoyl chloride using experimental design method. J. Polym. Res. 2019, 26, 237. [Google Scholar] [CrossRef]
- Benallou Benzekri, M.; Benderdouche, N.; Bestani, B.; Douara, N.; Duclaux, L. Valorization of olive stones into a granular activated carbon for the removal of Methylene blue in batch and fixed bed modes. J. Mater. Environ. Sci. 2018, 9, 272–284. [Google Scholar] [CrossRef]
- Olabinjo, O.O. Response Surface Techniques as an Inevitable Tool in Optimization Process. In Response Surface Methods—Theory, Applications and Optimization Techniques; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Duan, R.; Fedler, C.; Jiao, X. Adsorption of pyridine from aqueous solutions onto polyaluminium chloride and anionic polyacrylamide water treatment residuals. Water Sci. Technol. 2021, 83, 1753–1763. [Google Scholar] [CrossRef]
- Güray, T.; Menevşe, B.; Yavuz, A.A. Determination of optimization parameters based on the Box-Behnken design for cloud point extraction of quinoline yellow using Brij 58 and application of this method to real samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 243, 118800. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Olsson, A.-M.; Salmén, L. The association of water to cellulose and hemicellulose in paper examined by FTIR spectroscopy. Carbohydr. Res. 2024, 339, 813–818. [Google Scholar] [CrossRef]
- Paiva, M.; Ammar, I.; Campos, A.R.; Cheikh, R.; Cunha, A. Alfa fibres: Mechanical, morphological and interfacial characterization. Compos. Sci. Technol. 2007, 67, 1132–1138. [Google Scholar] [CrossRef]
- Arrakhiz, F.Z.; El Achaby, M.; Malha, M.; Bensalah, M.O.; Fassi-Fehri, O.; Bouhfid, R.; Benmoussa, K.; Qaiss, A. Mechanical and thermal properties of natural fibers reinforced polymer composites: Doum/low density polyethylene. Mater. Des. 2013, 43, 200–205. [Google Scholar] [CrossRef]
- Liu, D.; Han, G.; Huang, J.; Zhang, Y. Composition and structure study of natural Nelumbo nucifera fiber. Carbohydr. Polym. 2009, 75, 39–43. [Google Scholar] [CrossRef]
- Sen, T.K. Agricultural Solid Wastes Based Adsorbent Materials in the Remediation of Heavy Metal Ions from Water and Wastewater by Adsorption: A Review. Molecules 2023, 28, 5575. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Benbouzid, M.; Azoulay, K.; Bencheikh, I.; Al-Jadab, N.; Bensemlali, M.; Aarfane, A.; Nasrellah, H.; El Hajjaji, S.; Labjar, N. Evaluation of natural porous material as media filters for domestic wastewater treatment using infiltration percolation process. Euro-Mediterr. J. Environ. Integr. 2024, 1–16. [Google Scholar] [CrossRef]
- Tong, K.S.; Kassim, M.J.; Azraa, A. Adsorption of copper ion from its aqueous solution by a novel biosorbent Uncaria gambir: Equilibrium, kinetics, and thermodynamic studies. Chem. Eng. J. 2011, 170, 145–153. [Google Scholar] [CrossRef]
- Fadil, M.; Farah, A.; Ihssane, B.; Haloui, T.; Rachiq, S. Optimisation des paramètres influençant l’hydrodistillation de Rosmarinus officinalis L. par la méthodologie de surface de réponse Optimization of parameters influencing the hydrodistillation of Rosmarinus officinalis L. by response surface methodology. J. Mater. Environ. Sci. 2015, 6, 2346–2357. [Google Scholar]
- Azoulay, K.; Bencheikh, I.; Mabrouki, J.; Samghouli, N.; Moufti, A.; Dahchour, A.; El Hajjaji, S. Adsorption mecha-nisms of azo dyes binary mixture onto different raw palm wastes. Int. J. Environ. Anal. Chem. 2023, 103, 1633–1652. [Google Scholar] [CrossRef]
- Vishan, I.; Saha, B.; Sivaprakasam, S.; Kalamdhad, A. Evaluation of Cd(II) biosorption in aqueous solution by using lyophilized biomass of novel bacterial strain Bacillus badius AK: Biosorption kinetics, thermodynamics and mechanism. Environ. Technol. Innov. 2019, 14, 100323. [Google Scholar] [CrossRef]
- Ma, P.; Ma, M.; Wu, J.; Qian, Y.; Wu, D.; Zhang, X. The effect of plastic on performance of activated carbon and study on adsorption of methylene blue. J. Mater. Res. 2019, 34, 3040–3049. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Lu, S.; Chen, S.-S.; Nguyen, N.C.; Zhang, X.; Wang, J.; Wu, Y. A breakthrough biosorbent in removing heavy metals: Equilibrium, kinetic, thermodynamic and mechanism analyses in a lab-scale study. Sci. Total Environ. 2016, 542, 603–611. [Google Scholar] [CrossRef]
- Putro, J.N.; Kurniawan, A.; Ismadji, S.; Ju, Y.-H. Nanocellulose based biosorbents for wastewater treatment: Study of isotherm, kinetic, thermodynamic and reusability. Environ. Nanotechnol. Monit. Manag. 2017, 8, 134–149. [Google Scholar] [CrossRef]
- Kluczka, J.; Pudło, W.; Krukiewicz, K. Boron adsorption removal by commercial and modified activated carbons. Chem. Eng. Res. Des. 2019, 147, 30–42. [Google Scholar] [CrossRef]
- Kavakli, C.; Akkaş Kavaklı, P.; Turan, B.; Hamurcu, A.; Güven, O. Quaternized dimethylaminoethyl methacrylate strong base anion exchange fibers for As(V) adsorption. Radiat. Phys. Chem. 2014, 102, 84–95. [Google Scholar] [CrossRef]
- Abid, M.; Niazi, N.K.; Bibi, I.; Farooqi, A.; Ok, Y.S.; Kunhikrishnan, A.; Ali, F.; Ali, S.; Igalavithana, A.D.; Arshad, M. Arsenic(V) biosorption by charred orange peel in aqueous environments. Int. J. Phytoremediat. 2016, 18, 442–449. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Yang, Z.; Gluesenkamp, K.R.; Frazzica, A. Equilibrium vapor pressure properties for absorbent and adsorbent materials. Int. J. Refrig. 2021, 124, 134–166. [Google Scholar] [CrossRef]
- Shehu, A.; Ibrahim, M.B. Equilibrium Adsorption Isotherm of Methylene Blue and Rhodamine B Using Shea Butter Leaves as a Low-Cost Adsorbent. Appl. J. Environ. Eng. Sci. 2023, 9. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H. Raw and calcination-modified coal waste as adsorbents to remove cadmium from simulated mining wastewater. Int. J. Environ. Sci. Technol. 2014, 11, 987–996. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Mishra, P.K.; Ekielski, A. A Review on Polyacrylonitrile as an Effective and Economic Constituent of Adsorbents for Wastewater Treatment. Molecules 2022, 27, 8689. [Google Scholar] [CrossRef] [PubMed]
- Isaac, R.; Siddiqui, S.; Aldosari, O.F.; Uddin, M.K. Magnetic biochar derived from Juglans regia for the adsorption of Cu2+ and Ni2+: Characterization, modelling, optimization, and cost analysis. J. Saudi Chem. Soc. 2023, 27, 101749. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N.M. Adsorption mechanisms of re-moving heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520. [Google Scholar] [CrossRef] [PubMed]



| Variables | Code | −1 | 0 | +1 |
|---|---|---|---|---|
| [Cd2+]i (mg·L−1) | X1 | 5 | 12.5 | 20 |
| pH | X2 | 4 | 6 | 8 |
| ArS dose (g·L−1) | X3 | 1 | 2.5 | 4 |
| Carboxylic Groups (mmol·g−1) | Phenolic Groups (mmol·g−1) | Lactonic Groups (mmol·g−1) | Total Acid (mmol·g−1) | Total Basic (mmol·g−1) | pHzpc | Surface Area (m2·g−1) | |
|---|---|---|---|---|---|---|---|
| ArS | 0.025 | 0.015 | 0.093 | 0.133 | 0.085 | 3.6 | 31.99 |
| Experiment | pH | ArS Dose (g·L−1) | [Cd2+]i (mg·L−1) | q(Cd2+) (mg·g−1) |
|---|---|---|---|---|
| 1 | 6 | 2.5 | 12.5 | 2.121 |
| 2 | 4 | 1 | 12.5 | 0.951 |
| 3 | 8 | 4 | 12.5 | 2.748 |
| 4 | 6 | 1 | 20 | 1.756 |
| 5 | 8 | 2.5 | 20 | 3.824 |
| 6 | 4 | 2.5 | 20 | 1.678 |
| 7 | 6 | 4 | 20 | 2.898 |
| 8 | 4 | 2.5 | 5 | 0.095 |
| 9 | 6 | 1 | 5 | 0.449 |
| 10 | 8 | 1 | 12.5 | 2.361 |
| 11 | 8 | 2.5 | 5 | 0.863 |
| 12 | 6 | 2.5 | 12.5 | 1.973 |
| 13 | 6 | 4 | 5 | 0.464 |
| 14 | 6 | 2.5 | 12.5 | 2.367 |
| 15 | 4 | 4 | 12.5 | 1.486 |
| Degrees of Freedom | Sum of Squares | Mean Square | F-Ratio | |
|---|---|---|---|---|
| Model | 9 | 14.967247 | 1.66303 | 47.8409 |
| Residual | 5 | 0.173808 | 0.03476 | Prob. > F |
| Total | 14 | 15.141055 | 0.0003 * |
| Term | Estimation | Standard Error | t-Ratio | Prob. > |t| |
|---|---|---|---|---|
| Constant | 2.1538235 | 0.107644 | 20.01 | <0.0001 * |
| pH (4.8) | 0.6982206 | 0.065918 | 10.59 | 0.0001 * |
| m (1.4) | 0.2600368 | 0.065918 | 3.94 | 0.0109 * |
| cc (5.20) | 1.0357132 | 0.065918 | 15.71 | <0.0001 * |
| pH × m | −0.036985 | 0.093222 | −0.40 | 0.7079 |
| pH × cc | 0.3443676 | 0.093222 | 3.69 | 0.0141 * |
| m × cc | 0.2820588 | 0.093222 | 3.03 | 0.0292 * |
| pH × pH | −0.021926 | 0.097029 | −0.23 | 0.8302 |
| m × m | −0.245206 | 0.097029 | −2.53 | 0.0527 |
| cc × cc | −0.516706 | 0.097029 | −5.33 | 0.0031 * |
| Kinetic Model | Parameters | ArS/Cd2+ | |
|---|---|---|---|
| Pseudo-first-order [45] (5) | R2 | 0.171 | |
| k1 (min−1) | 0.373 | ||
| qetheo (mg·g−1) | 2.30 | ||
| qeexp (mg·g−1) | 4.73 | ||
| Pseudo-second-order [42] | R2 | 0.987 | |
| k2 (g/mg·min) | −0.065 | ||
| qetheo (mg·g−1) | 4.166 | ||
| qeexp (mg·g−1) | 4.73 | ||
| Intra-particle diffusion [44] | Type I | R2 | 1 |
| kd (mg·g1 min0.5) | 1.14 | ||
| I | −0.335 | ||
| Type II | R2 | 0.942 | |
| kd (mg·g1 min0.5) | 0.003 | ||
| I | 4.698 | ||
| Type III | R2 | 1 | |
| kd (mg/g1 min0.5) | 0.025 | ||
| I | 3.413 | ||
| Type IV | R2 | 0.566 | |
| kd (mg/g1 min0.5) | −0.014 | ||
| I | 5.49 | ||
| T | ΔG° (KJ·mol−1) | ΔH° (KJ·mol−1) | ΔS° (J/mol·K) | R2 |
|---|---|---|---|---|
| 298.15 | 2.26 | 20.26 | 60.36 | 0.913 |
| 308.15 | 1.43 | |||
| 323.15 | 1.24 | |||
| 338.15 | −0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abahdou, F.-Z.; Benbouzid, M.; Bouiti, K.; Nasrellah, H.; Bensemlali, M.; Labjar, N.; El Hajjaji, S. Enhanced Cadmium Removal by Raw Argan Shell Adsorbent: Experimental and Theoretical Investigations for Ecological Applications. Physchem 2025, 5, 13. https://doi.org/10.3390/physchem5010013
Abahdou F-Z, Benbouzid M, Bouiti K, Nasrellah H, Bensemlali M, Labjar N, El Hajjaji S. Enhanced Cadmium Removal by Raw Argan Shell Adsorbent: Experimental and Theoretical Investigations for Ecological Applications. Physchem. 2025; 5(1):13. https://doi.org/10.3390/physchem5010013
Chicago/Turabian StyleAbahdou, Fatima-Zahra, Maria Benbouzid, Khalid Bouiti, Hamid Nasrellah, Meryem Bensemlali, Najoua Labjar, and Souad El Hajjaji. 2025. "Enhanced Cadmium Removal by Raw Argan Shell Adsorbent: Experimental and Theoretical Investigations for Ecological Applications" Physchem 5, no. 1: 13. https://doi.org/10.3390/physchem5010013
APA StyleAbahdou, F.-Z., Benbouzid, M., Bouiti, K., Nasrellah, H., Bensemlali, M., Labjar, N., & El Hajjaji, S. (2025). Enhanced Cadmium Removal by Raw Argan Shell Adsorbent: Experimental and Theoretical Investigations for Ecological Applications. Physchem, 5(1), 13. https://doi.org/10.3390/physchem5010013






