Fluorescence Quantum Yields and Lifetimes of Aqueous Natural Dye Extracted from Tradescantia pallida purpurea at Different Hydrogen Potentials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Spectroscopic Techniques
2.3. Thermo-optical Techniques
2.3.1. Conical Diffraction (CD)
2.3.2. Single Arm Double Interferometer
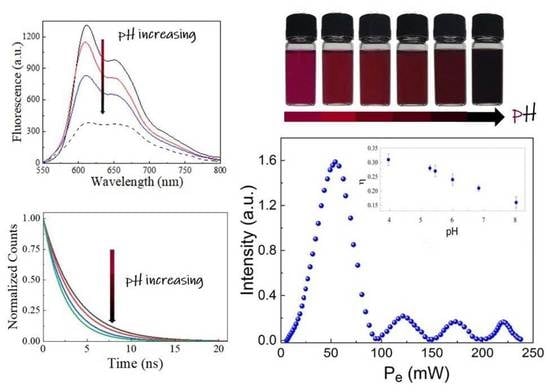
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural Products from Endophytic Microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-inspired Sustainable and Durable Superhydrophobic Materials: From Nature to Market. J. Mater. Chem. A 2019, 7, 16643. [Google Scholar] [CrossRef]
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural Plant Chemicals: Sources of Industrial and Medicinal Materials. Science 1985, 228, 1154–1160. [Google Scholar] [CrossRef]
- Blaszczyk, A.; Joachimiak-Lechman, K.; Sady, S.; Tański, T.; Szindler, M.; Drygata, A. Environmental Performance of Dye-Sensitized Solar Cells Based on Natural Dyes. Sol. Energy 2021, 215, 346–355. [Google Scholar] [CrossRef]
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Stanys, V.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From Plant Pigments to Health Benefits at Mitochondrial Level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- De Lima, S.R.; Felisbino, D.G.; Lima, M.R.S.; Chang, R.; Martins, M.M.; Goulart, L.R.; Andrade, A.A.; Messias, D.N.; Dos Santos, R.R.; Juliatti, F.C.; et al. Fluorescence quantum yield of natural dye extracted from Tradescantia Pallida Purpurea as a function of the seasons: Preliminary bioapplication as a fungicide probe for necrotrophic fungi. J. Photochem. Photobiol. B 2019, 200, 111631. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Hallagan, J.B.; Allen, D.C.; Borzelleca, J.F. The safety and regulatory status of food, drug and cosmetics colour additives exempt from certification. Food Chem. Toxicol. 1995, 33, 515–528. [Google Scholar] [CrossRef]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo Dyes: Past, Present and the Future. Environ. Rev. 2011, 19, 350–370. [Google Scholar] [CrossRef]
- Ardila-Leal, L.D.; Poutou-Pinales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.J.; Markakis, P.C. Food colorans: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Rhim, J.-W. Anthocyanin Food Colorant and Its Application in pH-Reponsive Color Change Indicator Films. Crit. Rev. Food Sci. Nutr. 2020, 61, 2297–2325. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, R. Chemical Structure of Anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 1–40. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary Anthocyanin-Rich Plants: Biochemical Basis and Recent Progress in Health Benefits Studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; de Freitas, V.; Mateus, N.; Calhau, C. Blueberry Anthocyanins and Pyruvic Acid Adducts: Anticancer Properties in Breast Cancer Cell Lines. Phytother. Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef] [Green Version]
- Rechner, A.R.; Kroner, C. Anthocyanins and Colonic Metabolites of Dietary Polyphenols Inhibit Platelet Function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary Cyanidin 3-O-β-D-Glucoside-Rich Purple Corn Color Prevents Obesity and Ameliorates Hyperglycemia in Mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba Extract and Bilberry Anthocyanins Improve Visual Function in Patients with Normal Tension Glaucoma. J. Med. Food 2012, 15, 818–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genskowsky, E.; Puente, L.A.; Pérez-Alvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of Polyphenolic Profile, Antioxidante Activity and Antibacterial Properties of Maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean Blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Roopchand, D.E.; Graf, B.L.; Cheng, D.M.; Ribnicky, D.; Fridlender, B.; Raskin, I. Role of Anthocyanins in Skin Aging and UV-Induced Skin Damage. In Anthocyanins in Health and Disease; Wallace, T.C., Giusti, M.M., Eds.; CRC Press: Boca Raton, MA, USA, 2013; pp. 309–322. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Heng, L.Y.; Salam, F.; Zaid, M.H.M.; Hanifah, S.A. A Colorimetric pH Sensor Based on Clitoria sp and Brassica sp for Monitoring of Food Spoilage Using Chromametry. Sensors 2019, 19, 4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, R.S.; Elsherif, M.; Moreddu, R.; Rashid, I.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Anthocyanin-Functionalized Contact Lens Sensors for Ocular pH Monitoring. ACS Omega 2019, 4, 21792–21798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, T.-V.; Dang, T.-H.; Chen, B.-H. Synthesis of Intelligent pH Indicative Films from Chitosan/Poly(vinyl alcohol)/Anthocyanin Extracted from Red Cabbage. Polymers 2019, 11, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH Indicator Film Composed of Agar/Potato Starch and Anthocyanin Extracts from Purple Sweet Potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef]
- Madushan, R.; Vidanarachchi, J.K.; Prasanna, P.H.P.; Werellagama, S.; Priyashantha, H. Use of Natural Plant Extracts as a Novel Microbiological Quality Indicator in Raw Milk: An Alternative for Resazurin Dye Reduction Method. LWT- Food Sci. Technol. 2021, 144, 111221. [Google Scholar] [CrossRef]
- Mendes, V.; De Lima, S.R.; Torres, J.O.B.; Antunes, A.; Messias, D.N.; Andrade, A.A.; Dantas, N.O.; Zilio, S.C.; Pilla, V. Preliminary spectroscopic and thermo-optical characterization of anthocyanin unpurified crude extracted from Tradescantia Pallida Purpurea. Dyes Pigm. 2016, 135, 57–63. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-Garcìa, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [Green Version]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Spectral and Colorimetric Characteristics of Metal Chelates of Acylated Cyanidin Derivatives. Food Chem. 2017, 221, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Timrov, I.; Binnie, S.; Biancardi, A.; Calzolari, A.; Baroni, S. Accurate and Inexpensive Prediction of the Color Optical Properties of Anthocyanins in Solution. J. Phys. Chem. A 2015, 119, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Timrov, I.; Micciarelli, M.; Rosa, M.; Calzolari, A.; Baroni, S. Multimodel Approach to the Optical Properties of Molecular Dyes in Solution. J. Chem. Theory Comput. 2016, 12, 4423–4429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacelli, I.; Ferretti, A.; Prampolini, G. Predicting Light Absorption Properties of Anthocyanidins in Solution: A Multi-Level Computational Approach. Theor. Chem. Acc. 2016, 135, 156. [Google Scholar] [CrossRef]
- De Lima, S.R.; Pereira, G.J.; Messias, D.N.; Andrade, A.A.; Oliveira, E.; Lodeiro, C.; Zilio, S.C.; Pilla, V. Fluorescence Quantum Yield Determination of Molecules in Liquids by Thermally Driven Conical Diffraction. J. Lumin. 2018, 197, 175–179. [Google Scholar] [CrossRef]
- Domenegueti, J.F.M.; Andrade, A.A.; Pilla, V.; Zilio, S.C. Simultaneous Measurement of Thermo-Optic and Thermal Expansion Coefficients with a Single Arm Double Interferometer. Opt. Express 2017, 25, 313–319. [Google Scholar] [CrossRef]
- Yang, D.; Wang, H.; Sun, C.; Zhao, H.; Hu, K.; Qin, W.; Ma, R.; Yin, F.; Qin, X.; Zhang, Q.; et al. Development of a High Quantum Yield Dye for Tumour Imaging. Chem. Sci. 2017, 8, 6322–6326. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.-Y.; Li, C.-Y.; Li, Y.-F.; Fei, J.; Xu, F.; Ou-Yang, J.; Liu, J. Near-Infrared Fluorescent Probe with High Quantum Yield and Its Application in the Selective Detection of Glutathione in Living Cells and Tissues. Anal. Chem. 2016, 88, 9746–9752. [Google Scholar] [CrossRef]
- Santos, T.T.S.; Lourenço, L.R.; de Lima, S.R.; Goulart, L.R.; Messias, D.N.; Andrade, A.A.; Pilla, V. Fluorescence Quantum Yields and Lifetimes of Annatto Aqueous Solutions Dependent on Hydrogen Potenatial: Applications in Adulterated Milk. J. Photochem. Photobiol. 2021, 8, 100080. [Google Scholar] [CrossRef]
- Pilla, V.; Gonçalves, A.C.; dos Santos, A.A.; Lodeiro, C. Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions. Chemosensors 2018, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Pilla, V.; Munin, E.; Gesualdi, M.R.R. Measurement of the Thermo-Optic Coefficient in Liquids by Laser-Induced Conical Diffraction and Thermal Lens Techniques. J. Opt. A: Pure Appl. Opt. 2009, 11, 105201. [Google Scholar] [CrossRef]
- Iwazaki, A.N.; Pilla, V.; Dias, V.M.; Munin, E.; Andrade, A.A. Self-Induced Phase Modulation for Thermo-Optical Characterization of Annatto Extracted Using Different Solvents. Appl. Spectrosc. 2011, 65, 1393–1397. [Google Scholar] [CrossRef]
- Durbin, S.D.; Arakelian, S.M.; Shen, Y.R. Laser-Induced Diffraction Rings from a Nematic-Liquid-Crystal Film. Opt. Lett. 1981, 6, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Song, C.; Xiang, Y.; Dai, X. Recent Advances in Spatial Self-Phase Modulation with 2D Materials and its Applications. Ann. Phys. 2020, 532, 200322. [Google Scholar] [CrossRef]
- Pilla, V.; Menezes, L.D.S.; Alencar, M.A.R.; de Araújo, C.B. Laser-Induced Conical Diffraction due to Cross-Phase Modulation in a Transparente Medium. J. Opt. Soc. Am. B 2003, 20, 1269–1272. [Google Scholar] [CrossRef]
- Catunda, T.; Baesso, M.L.; Messaddeq, Y.; Aegerter, M.A. Time-Resolved Z-scan and Thermal Lens Measurements in Er+3 and Nd+3 Doped Fluoroindate Glasses. J. Non-Cryst Solids 1997, 213–214, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Brouillard, R. Origin of the Exceptional Colour Stability of the Zebrina Anthocyanin. Phytochemistry 1981, 20, 143–145. [Google Scholar] [CrossRef]
- Baublis, A.; Spomer, A.; Berber-Jiménez, M.D. Anthocyanin Pigments: Comparison of Extract Stability. J. Food Sci. 1994, 59, 1219–1221. [Google Scholar] [CrossRef]
- Shi, Z.; Lin, M.; Francis, F.J. Anthocyanins of Tradescantia pallida. Potential Food Colorants. J. Food Sci. 1992, 57, 761–765. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated Anthocyanins from Edible Sources and their Applications in Food Systems. Bioch. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Radha, N.; Maheswari, G.; Anandan, S.; Manoharan, S.; Williams, R.V. Betalain and Anthocyanin Dye-Sensitized Solar Cells. J. Appl. Electrochem. 2016, 46, 929–941. [Google Scholar] [CrossRef]
- Munawaroh, H.; Fadillah, G.; Saputri, L.N.M.Z.; Hanif, Q.A.; Hidayat, R.; Wahyuningsih, S. The Co-Pigmentation of Anthocyanin Isolated from Mangosteen Pericarp (Garcinia mangostana L.) as Natural Dye for Dye-Sensitized Solar Cells (DSSC). IOP Conf. Ser.: Mater. Sci. Eng. 2016, 107, 012061. [Google Scholar] [CrossRef]
- Favaro, L.I.L.; Balcão, V.M.; Rocha, L.K.H.; Silva, E.C.; Oliveira, J.M., Jr.; Vila, M.M.D.C.; Tubino, M. Physicochemical Characterization of a Crude Anthocyanin Extract from the Fruits of Jussara (Euterpe edulis Martius): Potential for Food and Pharmaceutical Applications. J. Braz. Chem. Soc. 2018, 29, 2072–2088. [Google Scholar] [CrossRef]
- Jiang, X.; Guan, Q.; Feng, M.; Wang, M.; Yang, N.; Wang, M.; Xu, L.; Gui, Z. Preparation and pH Controlled Release of Fe3O4/Anthocyanin Magnetic Biocomposites. Polymers 2019, 11, 2077. [Google Scholar] [CrossRef] [Green Version]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The Effect of pH and Color Stability of Anthocyanin on Food Colorant. IOP Conf. Ser. Mat. Sci. Eng. 2017, 193, 012047. [Google Scholar] [CrossRef] [Green Version]
- Hurst, W.J. Methods of Analysis for Functional Foods and Nutraceuticals. In Functional Foods & Nutraceuticals Series; CRC Press: New York, NY, USA, 2002; Book 4. [Google Scholar]
- Baublis, A.J.; Berber-Jiménez, M.D. Structural and Conformational Characterization of a Stable Anthocyanin from Tradescantia pallida. J. Agric. Food Chem. 1995, 43, 640–646. [Google Scholar] [CrossRef]
- Shi, Z.; Daun, H.; Francis, F.J. Major Anthocyanin from Tradescantia pallida: Identification by LSI-MS and Chemical Analyses. J. Food Sci. 1993, 58, 1068–1069. [Google Scholar] [CrossRef]
- Brochard, P.; Grolier-Mazza, V.; Cabanel, R. Thermal Nonlinear Refraction in Dye Solutions: A Study of the Transient Regime. J. Opt. Soc. Am. B 1997, 14, 405–414. [Google Scholar] [CrossRef]
- Pina, F.; Oliveira, J.; de Freitas, V. Anthocyanins and Derivatives are more than Flavylium Cations. Tetrahedron 2015, 71, 3107–3114. [Google Scholar] [CrossRef]
- Houbiers, C.; Lima, J.C.; Maçanita, A.L.; Santos, H. Color Stabilization of Malvidin 3-Glucoside: Self-Aggregation of the Flavylium Cation and Copigmentation with the Z-Chalcone Form. J. Phys. Chem. B 1998, 102, 3578–3585. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Tuzi, A.; Piotto, S.; Concilio, S.; Caruso, U. An Amphiphilic Pyridinoyl-hydrazone Probe for Colorimetric and Fluorescence pH Sensing. Molecules 2019, 24, 3833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementi, C.; Basconi, G.; Pellegrino, R.; Romani, A. Carthamus tinctorius L.: A photophysical Study of the Main Coloured Species for Artwork Diagnostic Purposes. Dyes Pigm. 2014, 103, 127–137. [Google Scholar] [CrossRef]
- Zhegalova, N.G.; He, S.; Zhou, H.; Kim, D.M.; Berezin, M.Y. Minimization of Self-Quenching Fluorescence on Dyes Conjugated to Biomolecules with Multiple Labeling Sites via Asymmetrically Charged NIR Fluorophores. Contrast Media Mol. Imaging 2014, 9, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.M.; House, J.K.; Hazelton, M.S.; Bosward, K.L.; Mohler, V.L.; Maunsell, F.P.; Sheehy, P.A. Milk Acidification to Control the Growth of Mycoplasma Bovis and Salmonella Dublin in Contaminated Milk. J. Dairy Sci. 2016, 99, 9875–9884. [Google Scholar] [CrossRef] [Green Version]
- Karshima, N.S.; Pam, V.A.; Bata, S.I.; Dung, P.A.; Paman, N.D. Isolation of Salmonella Species from Milk and Locally Processed Milk Products Traded for Human Consumption and Associated Risk Factors in Kanam, Plateau State, Nigeria. J. Anim. Prod. Adv. 2013, 3, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.; Cerqueira, L.; Azevedo, N.F.; Vieira, M.J. Detection of Salmonella Enterica Serovar Enteritidis Using Real Time PCR, Immunocapture Assay, PNA FISH and Standard Culture Methods in Different Types of Food Samples. In. J. Food Microbiol. 2013, 161, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Azad, T.; Ahmed, S. Common Milk Adulteration and Their Detection Techniques. Int. J. Food Contam. 2016, 3, 22. [Google Scholar] [CrossRef]
- Goodarzi, M.M.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Development of an easy-to-use colorimetric pH label with starch and carrot anthocyanins for milk shelf life assessment. Int. J. Biol Macromol. 2020, 153, 240–247. [Google Scholar] [CrossRef]
- Pereira Jr, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Terra, A.L.M.; Moreira, J.B.; Costa, J.A.V.; de Morais, M.G. Development of time-pH indicator nanofibers from natural pigments: An emerging processing technology to monitor the quality of foods. LWT Food Sci. Technol. 2021, 142, 111020. [Google Scholar] [CrossRef]
- Liang, T.; Wang, L. A pH-Sensing Film from Tamarind Seed Polysaccharide with Litmus Lichen Extract as an Indicator. Polymers 2018, 10, 13. [Google Scholar] [CrossRef] [PubMed]









| Sample | pH (± 0.005) | Peak3/Peak2 | <λem> (nm) | Stoke′s Shift (nm) |
|---|---|---|---|---|
| a | 3.960 | 0.394 | 656 | 25 |
| b | 5.280 | 0.567 | 648 | 24 |
| c | 5.450 | 0.394 | 652 | 26 |
| d | 6.000 | 0.514 | 650 | 25 |
| e | 6.830 | 0.593 | 651 | 23 |
| f | 8.020 | 1.215 | 655 | 27 |
| Sample | pH | η | τ (ns) | χ2 |
|---|---|---|---|---|
| a | 3.960 | (0.31 ± 0.02) | (3.68 ± 0.06) | (0.99 ± 0.04) |
| b | 5.250 | (0.28 ± 0.01) | (3.24 ± 0.05) | (1.11 ± 0.06) |
| c | 5.500 | (0.27 ± 0.02) | (3.30 ± 0.06) | (1.15 ± 0.05) |
| d | 6.000 | (0.24 ± 0.02) | (3.17 ± 0.06) | (1.11 ± 0.05) |
| e | 6.890 | (0.21 ± 0.01) | (2.74 ± 0.04) | (1.10 ± 0.04) |
| f | 8.020 | (0.16 ± 0.02) | (2.39 ± 0.03) | (1.07 ± 0.07) |
| Natural Dye | Extraction | Applications |
|---|---|---|
| Anthocyanin | Brassica oleraceae var. capitata (Red Cabbage) [73] | Development of chitosan/PVA films doped with anthocyanins to indicate food quality due to pH change. Potential application in the evaluation of milk quality [73]. |
| Hibiscus rosa-sinensis L. flowers, Clitoria ternatea flowers, Beta vulgaris roots, Opuntia dillenii pricklypears [30] | Application of liquid coloring in raw milk to measure microbiological quality control [30]. | |
| Black carrot [72] | Starch film creation with anthocyanin to evaluate milk shelf-life assessment [72]. | |
| Curcumin, Quercetin, and Phycocyanin | Vegetable plants, and microalga Spirulina [74] | Introduction of natural dyes in nanofibers for use as pH colorimetric indicators as a function of time to monitor food quality [74]. |
| Annatto | Seeds of the shrub Bixa Orellana L. [41] | The natural dyes were tested in adulterated milk as colorimetric and/or fluorometric pH-biosensor probes [41]. |
| Litmus | Lichens [75] | Development of a pH-sensing film from polysaccharide extracted from tamarind and litmus lichen, as an indicator of deterioration of cream milk [75]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lima, S.R.; Lourenço, L.R.; Thomaz, M.; Messias, D.N.; Andrade, A.A.; Pilla, V. Fluorescence Quantum Yields and Lifetimes of Aqueous Natural Dye Extracted from Tradescantia pallida purpurea at Different Hydrogen Potentials. Photochem 2023, 3, 1-14. https://doi.org/10.3390/photochem3010001
De Lima SR, Lourenço LR, Thomaz M, Messias DN, Andrade AA, Pilla V. Fluorescence Quantum Yields and Lifetimes of Aqueous Natural Dye Extracted from Tradescantia pallida purpurea at Different Hydrogen Potentials. Photochem. 2023; 3(1):1-14. https://doi.org/10.3390/photochem3010001
Chicago/Turabian StyleDe Lima, Sthanley R., Larissa R. Lourenço, Marina Thomaz, Djalmir N. Messias, Acácio A. Andrade, and Viviane Pilla. 2023. "Fluorescence Quantum Yields and Lifetimes of Aqueous Natural Dye Extracted from Tradescantia pallida purpurea at Different Hydrogen Potentials" Photochem 3, no. 1: 1-14. https://doi.org/10.3390/photochem3010001
APA StyleDe Lima, S. R., Lourenço, L. R., Thomaz, M., Messias, D. N., Andrade, A. A., & Pilla, V. (2023). Fluorescence Quantum Yields and Lifetimes of Aqueous Natural Dye Extracted from Tradescantia pallida purpurea at Different Hydrogen Potentials. Photochem, 3(1), 1-14. https://doi.org/10.3390/photochem3010001