Abstract
This review summarizes the progress over the last fifteen years in visible light reactive photocatalysts for environmental arsenic remediation. The design and performance of several materials including (1) doped and surface functionalized TiO2, (2) binary composites combining TiO2 with another semiconductor that absorbs visible light radiation or a metal (Pt), (3) ternary composites incorporating TiO2, a conductive polymer that can retard electron-hole recombination and an excellent adsorbent material for the removal of As(V), (4) tungsten, zinc, and bismuth oxides, (5) g-C3N4 based catalysts, and (6) M@AgCl core–shell structures. These results show that long reaction time remains a major challenge in achieving high As(III) oxidation.
1. Introduction
Arsenic is fairly abundant in Earth’s crust, with a natural occurrence of 2–3 ppm on average, which makes it the 20th most abundant element in the crust. It exists in four main oxidation states, namely +V (arsenate), +III (arsenite), 0 (arsenic), and −III (arsine), of which the first two are the most abundant in the environment. In oxygenated water (such as surface waters) arsenic usually occurs in the form of As(V), whereas under reducing conditions, such as those often found in deep lake sediments or groundwater, the predominant form is As(III) [1]. Organoarsenic compounds, such as dimethylarsinic acid or monomethylarsonic acid [1], are also found in water.
Arsenic can diffuse in the environment from both natural and anthropogenic sources; the latter are derived from activities such as coal mining, smelting, fertilizers, and pesticides, and from the hide tanning, semiconductor, and aluminum industries [2]. However, arsenic contamination of drinking water is generally derived from geological sources. Bangladesh and India (West Bengal) are among the countries with the highest levels of contamination. Levels of arsenic above safe limits were also found locally in several countries in Europe, Australia, New Zealand, Brazil, and Canada [3].
Through prolonged exposure to arsenic-contaminated drinking water, the element accumulates in tissues. Signs of chronic arsenicism include dermal lesions, peripheral neuropathy, bladder, lung, and kidney cancers, urinary tract infections, peripheral vascular disorders, and skin cancer [2]. For acute toxicity, arsine is considered to be the most toxic form, followed by arsenites (As(III)), arsenates (As(V)), and organic arsenic compounds. The World Health Organization (WHO) estimated that about 180 million people in 50 countries have been exposed to toxic arsenic levels (at least 10 μg/L in drinking water). Consequently, the guideline value for the permissible limit for arsenic in drinking water according to WHO is 10 μg/L; however, every effort should be made to maintain levels as low as possible [4].
For these reasons, abundant research has been devoted to the quest for remediation methods, especially for water treatment. It must be considered that none of the known technologies will work in every situation. The applicability of a given technology depends on the types and concentrations of the arsenic species, the pH of the medium, the presence of other dissolved species or organic contaminants, the volume of water to be treated, the cost, and the target value for concentration levels (namely, whether or not the obtained water must fit the requirements for drinking water).
Although the number of articles dealing with the application of photocatalysis to arsenic removal in water is relatively small, there are five recent reviews on the application of metal oxides for arsenic remediation in water under UV irradiation [1,5,6,7,8], none of which focused on visible light irradiation. One very recent review [9] deals with photocatalysis by both UV and visible light. Therefore, the present review emphasizes the visible-light-responsive performance of the semiconductor photocatalysts for arsenic remediation in water.
Methodologically, we decided to focus on photocatalytic methods of removal and to only take into consideration those papers meeting criteria that would ensure reproducibility of the results. We chose to take into consideration only those papers where all the following data are clearly stated: (i) As(III) or arsanilic acid concentrations; (ii) catalyst concentration (where applicable); (iii) required time; (iv) characteristics of the light source; and (v) percentage of removed pollutant.
2. Conventional Remediation Techniques
In many cases, arsenic levels in groundwater are much higher than the allowed limit of 10 μg/L; therefore, a variety of methods are used for decontamination. Mostly, they can be grouped into the categories of adsorption and ion exchange, coagulation–precipitation processes, membrane processes, and bioremediation.
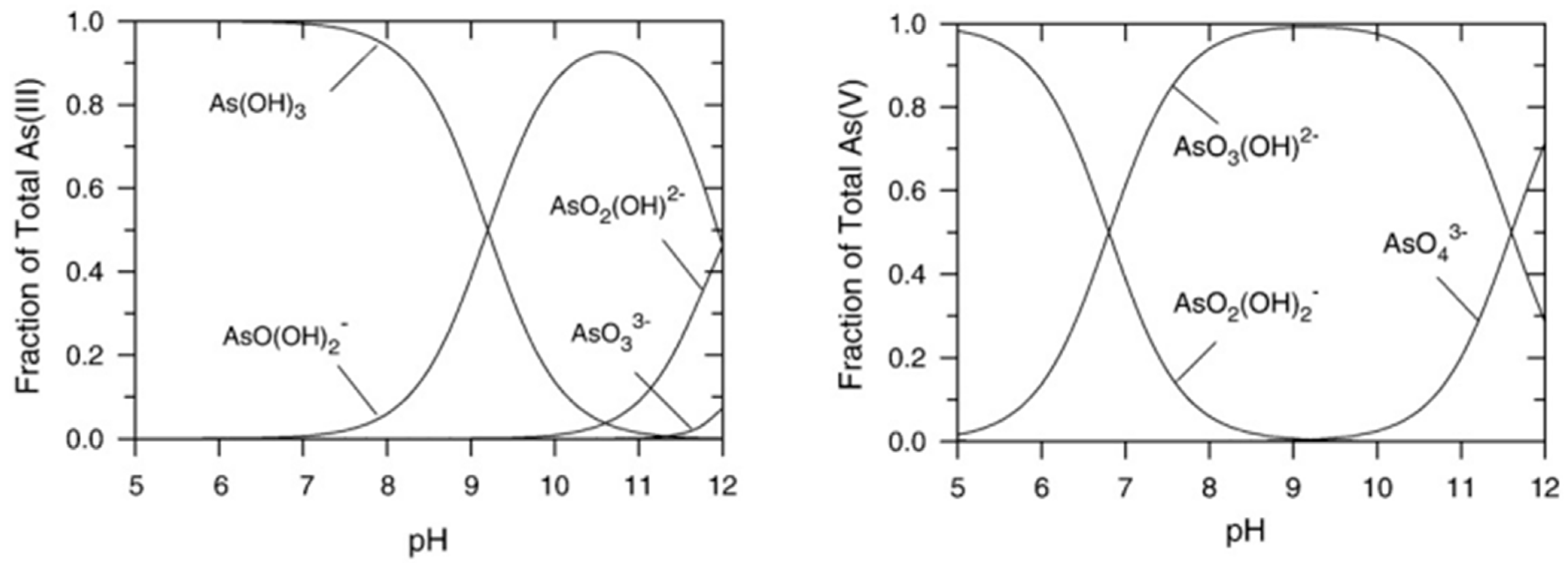
As the various forms of arsenic undergo acid–base equilibria, pH is of paramount importance in determining whether arsenic will be present in neutral or ionic form [1,10], as shown in Figure 1.
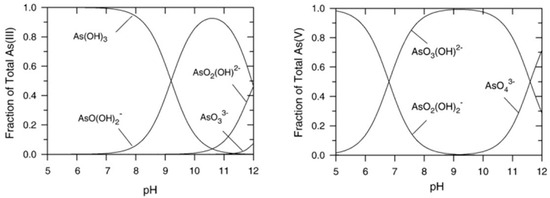
Figure 1.
Arsenic speciation in water as a function of pH: As(III) (left) and As(V) inorganic species (right). Reprinted with permission from Ref. [1]. Copyright 1999, Elsevier.
When the problem to be addressed is the treatment of drinking water, pH must be close to neutrality, at which value As(III) is mainly in a neutral form, while As(V) is in anionic form. In general, as arsenite is electrically neutral at a pH of 9.2, most current arsenic remediation methods for drinking water, which exploit the presence of surface charge, typically perform better in removing the less toxic As(V); therefore, peroxidation usually has to be applied for arsenite removal [11].
2.1. Oxidation
Oxidation is a preliminary technique used to convert As(III) to As(V); the latter must then be removed from water using a physical technique. The preferred oxidizing techniques include the use of chemicals, electrochemistry, and photochemistry.
Chemical oxidants include different species, such as ozone, chlorine, and KMnO4, which can rapidly and completely oxidize As(III) at concentrations of 50 μg/L or higher. However, in some cases, pH must be kept above 7.5, and other contaminants, such as sulfides, may interfere, slowing the process. Other oxidants, such as oxygen, air, chloramine (NH2Cl), chlorine dioxide, hydrogen peroxide, and ferrate, generally require longer contact times and fail to achieve complete oxidation of As(III) [12,13]. Hypochlorite may also be used, but complete oxidation requires a concentration of 500 μg/L [12,14]. If the oxidizing species is a solid, it also acts as an adsorbent for the resulting As(V) [13,15,16]. The oxidation of As(III) with H2O2 and Fe(II) is important in natural and technical systems at pH values up to 8, below which As(III) is not oxidized at appreciable rates by H2O2 alone [17].
One effective solution was to pump oxygenated water into the aquifer prior to arsenic removal to reduce As concentrations to <10 μg/L [18].
As(III) oxidation can also be achieved by biological methods by exploiting bacteria or algae [19].
Electrochemistry is a viable system for the anodic oxidation of arsenite without the use of noxious chemicals [20]. Oxidation may be direct, where the As(III) species are directly adsorbed on the electrode surface and oxidized, or indirect if the process involves the electrochemical generation of an oxidizing species, such as O2 [21]. A huge amount of research is presently devoted to the quest for an efficient electrode material [15,22]. Electrochemical methods seem promising owing to low energy consumption and good As removal, even down to <1 μg/L, although their efficacy in groundwater is lower than in synthetic water in several instances [20].
Photochemical oxidation of As(III) can be achieved when light-absorbing substances such as H2O2, Fe(III) complex, NO3−/NO2−, and I−, produce transient high-energy species under UV radiation [11]. Photocatalytic oxidation has often been used [23], in many cases by TiO2-based photocatalysts; however, owing to its wide bandgap, TiO2 is only activated by UV light, thus allowing the exploitation of only a small fraction of solar light or requiring the use of UV lamps. Photocatalysis by visible light is detailed below.
Table 1 sums up the main oxidation methods used for As(III), with their main advantages and drawbacks.

Table 1.
Main oxidation methods to convert As(III) into As(V).
In the following paragraphs, the main conventional techniques that can be applied after oxidation for the removal of arsenic from water are described.
2.2. Adsorption
The most widely used system for arsenic removal from groundwater is adsorption, due to its simple operation, potential for regeneration, and minimal toxic sludge generation. Processes based on the use of natural, locally available adsorbents are considered to be more accessible for low-income countries, as they have a smaller investment cost and a lower environmental impact (CO2 emission) [24]. Adsorption of As is highly dependent on several parameters, including pH, the presence of other cations, particle size, surface porosity, the oxidative state of As(III or V), contact time, and adsorbent dosage.
The optimal conditions are determined by the types of surface functional groups and surface charges present on the adsorbent being used. A wide variety of materials has been used for arsenic adsorption. Among them, rocks, minerals, and other materials containing iron oxides or hydroxides [25] and/or alumina, such as red mud from the treatment of bauxite, carbon-based materials such as activated carbon [26], which is frequently obtained during combustion of a wide variety of locally available agricultural wastes, biochar [27,28,29] graphene [30], and a huge variety of polymer-based materials and composites [31]. Frequently, these materials are manufactured in the form of nanoparticles, which have large surface areas, highly porous surfaces, and large numbers of active sites [8]. Other low-cost materials for arsenic adsorption are those classified as biosorbents, among which are biopolymers such as chitosane, alginate, or cellulose, raw waste material from agricultural activities such as rice husks, biomass from wood processing [32], and microalgae and fungal biomass [24]. All these materials have the advantage of being locally available and easily manageable, even in low-income countries.
An intrinsic problem of this remediation approach is the need for subsequent disposal of the contaminated sorbent. In principle, two approaches are possible: (a) desorption of arsenic from the adsorbent medium, to recover and reuse the latter and/or to obtain and utilize the arsenic itself; and (b) dumping of the contaminated adsorption material. As arsenic does not have many industrial applications, its recovery is usually not economical, so most of the time it is disposed of. However, it cannot be destroyed, and incineration of the sorbent is not viable due to the high volatility of arsenic compounds. Therefore, safe management of the spent sorbent medium requires prior application of stabilization/solidification techniques to avoid leaching of toxic compounds from landfills. For instance, it can be incorporated into building materials or in a cementitious binder [33]. Iron hydroxides can be thermally treated in order to promote arsenic fixation and, therefore, safer disposal of the solid residue [34]. Some instances instead use the recovery approach: about 85% of As adsorbed on δ-FeOOH nanoparticles was recovered by treatment with NaOH solution, to be used as a precursor for the synthesis of Ag3AsO4, which is a material with outstanding photocatalytic activity [35]. Desorption methods were also developed for the reuse of the sorbent material [36]. A recent review [2] dealt with the disposal of sorbents, whereas another [37] investigated plants performing arsenic adsorption from water in continuous mode and the latest progress in the regeneration and recovery of arsenic. The disposal of the exhausted bed was also discussed.
2.3. Precipitation
The precipitation methods are mainly used for the remediation of heavily arsenic-loaded wastewater, such as those derived from mining and related activities. In industrial wastewater, precipitation of As(III) in the form of sulfide As2S3 is almost complete at pH 2 [38]. Coagulation/flocculation is usually obtained by iron or aluminum salts used as flocculants. The technology of coagulation–flocculation is simple, only common chemicals are used, installation costs are low, and it can be easily applied to large volumes of water. During this process, As(V) is removed more effectively than As(III); however, As removal is hindered by the presence of sulfates, silicates, and phosphates, and simple setting of the solution is not sufficient for effective decontamination, and filtration is a necessary step [39]. During the precipitation processes, often owing to low selectivity, a large amount of sludge is produced [40], which is usually disposed of in landfills. Precipitation is sometimes proposed as a method for the treatment of wastewater produced by the management of arsenic-laden waste created during the regeneration of exhausted adsorbents [41].
As in the case of adsorption, the production of solid waste with high arsenic content during precipitation poses leaching problems [42]; however, as the amount of arsenic in this type of waste is important, remediation techniques are also frequently directed to the recovery of arsenic for reuse [43,44].
2.4. Membrane Technologies
Membrane technology is a promising area for arsenic removal from water. The most commonly developed membrane separation processes are reverse osmosis (RO), electrodialysis (ED), microfiltration (MF), ultrafiltration (UF), and nanofiltration (NF). Microfiltration, ultrafiltration, nanofiltration, and reverse osmosis are pressure-driven processes, whereas electrodialysis is an electro-driven process. For arsenic remediation, the most appropriate technique is nanofiltration due to its ability to eliminate multivalent ions in water [45]. A recent review [46] compared the various membrane techniques and discussed the research trends, indicating advantages and drawbacks, the latter of which were typically the issues of low durability [47] and fouling. Fouling is the deposition of solutes on the membrane surface and within pores, and it causes mass transfer resistance, low water flux, and low selectivity [45]. In another review [39], emergent membrane-based technologies such as forward osmosis, membrane distillation, and electrodialysis were discussed.
2.5. Other Techniques
Ion exchange was realized using a vast number of materials, such as metal–organic frameworks [48] or polymeric ion exchange resins containing dispersed metal oxide nanoparticles [49]. Resins require periodic regeneration via treatment with solutions of appropriate counterions and must eventually be disposed of in landfills, where they should not give rise to leachate.
Bioremediation using the biomass of bacteria, algae, fungi, and yeast has gained much attention from researchers in the last few decades as an environmentally friendly and cost-effective approach [50]. Arsenic-resistant bacterial strains can be genetically engineered for better performance. Microbial consortia, namely organized collectives of microorganisms, either natural or engineered, can work synergistically [51].
Although arsenic bioremediation using bacteria can be very effective within laboratory conditions, bacterial ecology in the environment may present important differences with respect to their behavior under controlled parameters. Hence, it is essential to develop bioremediation systems in field applications to enhance their commercial viability.
Electrochemical methods are interesting because of their flexibility, environmental compatibility, low cost, and high efficiency. They can be distinguished using separation and oxidation methods [21]. Separation methods include electrocoagulation, electrodialysis, and electrosorption.
Directional solvent extraction with decanoic acid was applied at low temperature to achieve purification from arsenic to meet drinking water standards [52].
Generally speaking, evaluation of the viability of a given decontamination technique depends on a vast number of factors, including economic issues, acceptability by the users, management of the resulting waste, ease of maintenance of the plant, and so on, and considerations may vary greatly based on the location of the plant and the characteristics of the contaminated water [53]. Particularly in low-income countries, even where decontamination plants have been built, their long-term efficiency is often not guaranteed [54], and residents often discontinue their use of water treatment plant for various reasons [55].
3. Heterogeneous Photocatalysis for the Removal of Arsenic in Water
Heterogeneous photocatalysis, which mainly refers to semiconductor photocatalysis or semiconductor-sensitized photoreactions, is considered a promising alternative to conventional processes used for the removal of toxic As(III) from water. The process mainly oxidizes As(III) into As(V), which may be then removed from the solution either by precipitation or by the addition of a suitable adsorbent, typically iron oxides such as Fe2O3 and Fe3O4.
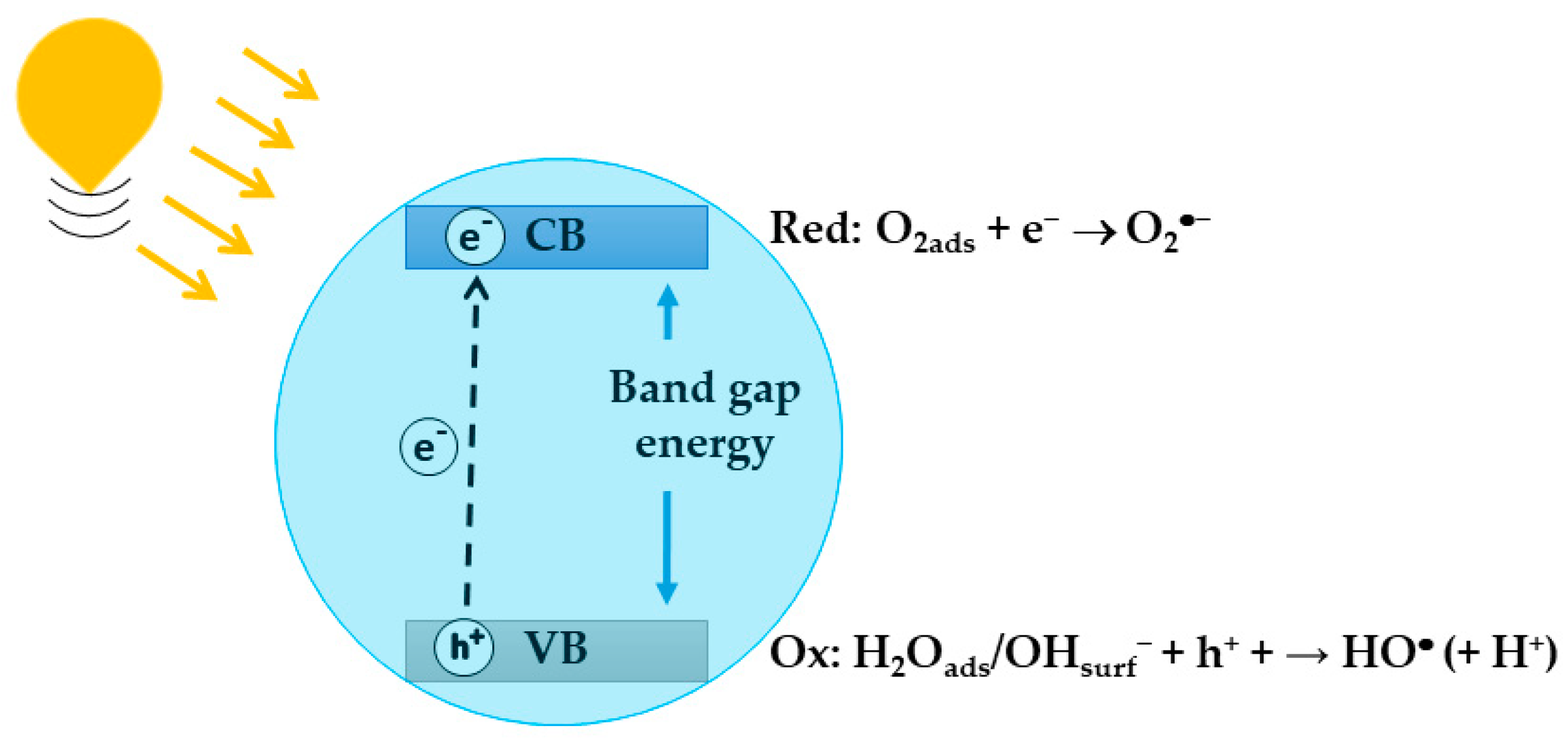
In the photocatalytic process, a chemical reaction is activated or its rate is increased when a semiconducting photocatalyst is irradiated using photons with energy that matches the band gap energy of the semiconductor, resulting in the formation of excited electron–hole (-) pairs. The excited electrons are promoted from the valence band (VB) to the conduction band (CB), thereby generating holes in the VB (Figure 2) [56]. Owing to the band bending, electrons and holes move to the surface of the semiconductor, where, in the absence of electron and hole scavengers, their recombination occurs in few nanoseconds. Recombination is avoided if a proper scavenger or surface defect state is available to trap the electron–hole pairs, and in this case, the electron and hole can be involved in thermodynamically facilitated electron transfer reactions with the solvent or other species. Adsorbed water or hydroxyl groups present on the semiconductor surface can be oxidized by , generating (Equation (1)), while adsorbed can be reduced by , generating superoxide radicals, (Equation (2)):
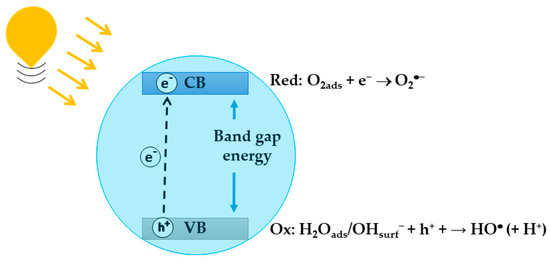
Figure 2.
Schematic representation of the formation of excited electron-hole pair.
The mechanism of photocatalytic oxidation of As(III) has been studied for many years, and research has focused on determining the major oxidant in the system, either , , or [57,58]. The oxidation of As(III) to As(V) proceeds through monoelectronic steps with the formation of As(IV), as described by the following equations:
Subsequently, As(IV) is readily oxidized to As(V) by , , or molecular [59]. With all these oxidants, the heterogeneous photocatalytic process is highly efficient at transforming As(III) into As(V) [60,61].
The most studied material for the photocatalytic oxidation of As(III) in water under UV light is TiO2 coupled with an iron oxide, such as α-Fe2O3, which acts as an adsorbent of As(V), improving the total arsenic removal. Although the band-gap energy of α-Fe2O3 is about 2.0 eV, TiO2/Fe2O3 is not efficient in the As(III) oxidation reaction under visible-light irradiation, because Fe2O3 is a rather poor photocatalyst due to rapid electron–hole recombination [62].
A good photocatalytic material must show chemical stability under a wide variety of environments. Metal oxide semiconductors with a band gap in the range of 2–4 eV generally possess this important property. In addition to being chemically robust, a good photocatalyst should exhibit other important properties such as (i) a high surface-to-mass ratio, (ii) efficient absorption of light in a useful range of frequencies, and (iii) efficient separation and transportation of photogenerated charge carriers. To achieve the first feature, the surface-area effects of metal oxides are tailored by preparing nanoparticles using suitable synthetic methods (such as hydrothermal/solvothermal and sol–gel syntheses) or, alternatively, as a result of deposition onto a nanostructured film. The importance of the second property is related to the fact that exploiting a light source in the visible region of the electromagnetic spectrum would make the management of wastewater treatment plants simpler and more economical. Several strategies are adopted to extend the optical response of photocatalytic materials to the visible region: among others, doping with metal and/or non-metal elements, surface functionalization, composites, and junction coupling [63,64]. In particular, nano-heterojunctions could enhance visible light absorption and induce band bending and the formation of an internal electric field [65]. Consequently, owing to specific band alignment and spatial charge separation, the photoactivity is improved.
3.1. Photocatalytic Oxidation of As(III) Using Modified TiO2
Titanium dioxide (TiO2) is an n-type semiconductor. It has been widely studied as a photocatalyst in the oxidative degradation of organic and inorganic water pollutants [66,67]. The interaction of TiO2 with UV radiation generates electron–hole pairs, which activate surface reactive processes [59,61]. Among the three common crystalline polymorphs of TiO2 (rutile, anatase, and brookite) the anatase phase exhibits higher photocatalytic behavior for oxidation processes. The relatively wide bandgap of TiO2 anatase, Eg = 3.2 eV corresponding to a threshold of 390 nm, hinders the exploitation of solar light in photocatalytic reactions. Moreover, the high recombination probability of the photogenerated electron–hole pairs results in low quantum efficiency of photocatalyzed reactions, which is a feature present in all semiconductor photocatalysts. Different approaches have been developed to modify the process involving light harvesting and excitation to increase visible light absorption of TiO2 and to maximize electron–hole separation. A widespread strategy consists of doping TiO2 with metal and/or non-metal elements; this is achieved through the incorporation of different elements into the TiO2 crystalline lattice, resulting in modified electronic properties. No previous literature discussing the photocatalytic oxidation of As(III) using TiO2 modified by a physical method (such as ion implantation) was found. Table 2 lists the examples of visible-light-activated chemically modified TiO2 covered in this review. A brief description of their performance for As(III) oxidation is also provided. Fe-doped TiO2 [68] outperformed other types of doped TiO2 such as N-doped TiO2 fiber [69], N–S co-doped TiO2 [70], and Er-doped TiO2 [71]. It is worth noting that in ref. [68], a very high (3 g/L) concentration of Fe-doped TiO2 was used to remove 100% of As(III) in 30 min. TiO2 doped with Er3+ 2.0 mol% and reduced graphene oxide (rGO) 10.0 mol% degrades 100% of arsanilic acid (p-ASA) in 50 min under visible light, and the resulting arsenic (mainly As(V)) is rapidly removed from the solution owing to the strong adsorption of rGO to inorganic arsenic [71].
Some studies that were carried out with the aim of investigating the mechanism of photocatalytic oxidation of As(III) in the presence of TiO2 were performed under visible-light irradiation and pH = 3 [72,73]. The visible-light-induced oxidation on dye-sensitized Pt-TiO2 (~80% in 60 min) [73] is as fast as the UV photooxidation on Pt-loaded TiO2. Nanostructured TiO2 nanotubes prepared by potentiostatic anodization of a titanium foil, combined with Pt-loading, are capable of harvesting visible light because of localized surface plasmon resonance (SPR) of Pt nanoparticles. Qin et al. reported that Pt nanoparticles on TiO2 nanotubes induce the oxidation of 83% of As(III) in 280 min [74].

Table 2.
As(III) oxidation to As(V) over modified TiO2 photocatalysts under visible light.
Table 2.
As(III) oxidation to As(V) over modified TiO2 photocatalysts under visible light.
| Photocatalyst | pH | Light Source | As(III) (μM) | Catalyst (g/L) | As(III) Oxidized (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|---|
| (Ru″L3) dye-sensitized Pt-loaded TiO2 | 3 | Xe 300 W λ > 420 nm | 500 | 0.5 | ~80 | 60 | [73] |
| 4-chlorophenol/TiO2 system | 3 | Xe 300 W λ > 420 nm | 200 | 0.5 | ~90 | 300 | [72] |
| Pt-loaded TiO2 nanotube | 7 | 300 W halogen λ > 420 nm | 45.4 | foil | 83 | 280 | [74] |
| N-doped TiO2 fiber | 7 | Xe 300 W λ > 420 nm | 133 | 0.5 | 100 | 90 | [69] |
| Fe-doped TiO2 | n.a. | LED 400–600 nm | 80 | 3 | 100 | 30 | [68] |
| N–S co-doped TiO2 | 9 | LED 650 W/m2 430–650 nm | 46 (p-ASA) | 1 | 98 | 300 | [70] |
| 2 mol% Er-doped TiO2 | n.a. | Xe 500 W λ > 400 nm | 23 (p-ASA) | 0.1 | ~70 | 90 | [71] |
| Er3+–rGO co-doped TiO2 | n.a. | Xe 500 W λ > 400 nm | 23 (p-ASA) | 0.1 | 100 | 50 | [71] |
As mentioned above, an adsorption step takes place before the reaction step. There is an attractive interaction between particles with opposite electric charges. The solution pH significantly affects the charge of the surface particles. The pH of zero-point charge (zpc) is the value at which the material surface is neutral (uncharged). Below this value, the photocatalyst is positively charged and attracts negative species. At pH values above the zpc, the photocatalyst surface is negatively charged and attracts positive species. Although the large band gap of TiO2 limits the utilization in visible light, many studies examined the arsenic adsorption performance of Degussa P25 TiO2. The results showed that at pH 4, adsorption for As(V) is much higher than for As(III) because of the negative and neutral speciation of As(V) and As(III) (see Figure 1), respectively. As(V) desorbs more effectively as pH increases, and at pH 9, the adsorption of As(III) is higher than As(V) [75]. The maximum adsorption capacity for As(III) (89 μM) is 2.4 mg/gTiO2, and that for As(V) is 9.7 mg/gTiO2 at pH 6.3 [76].
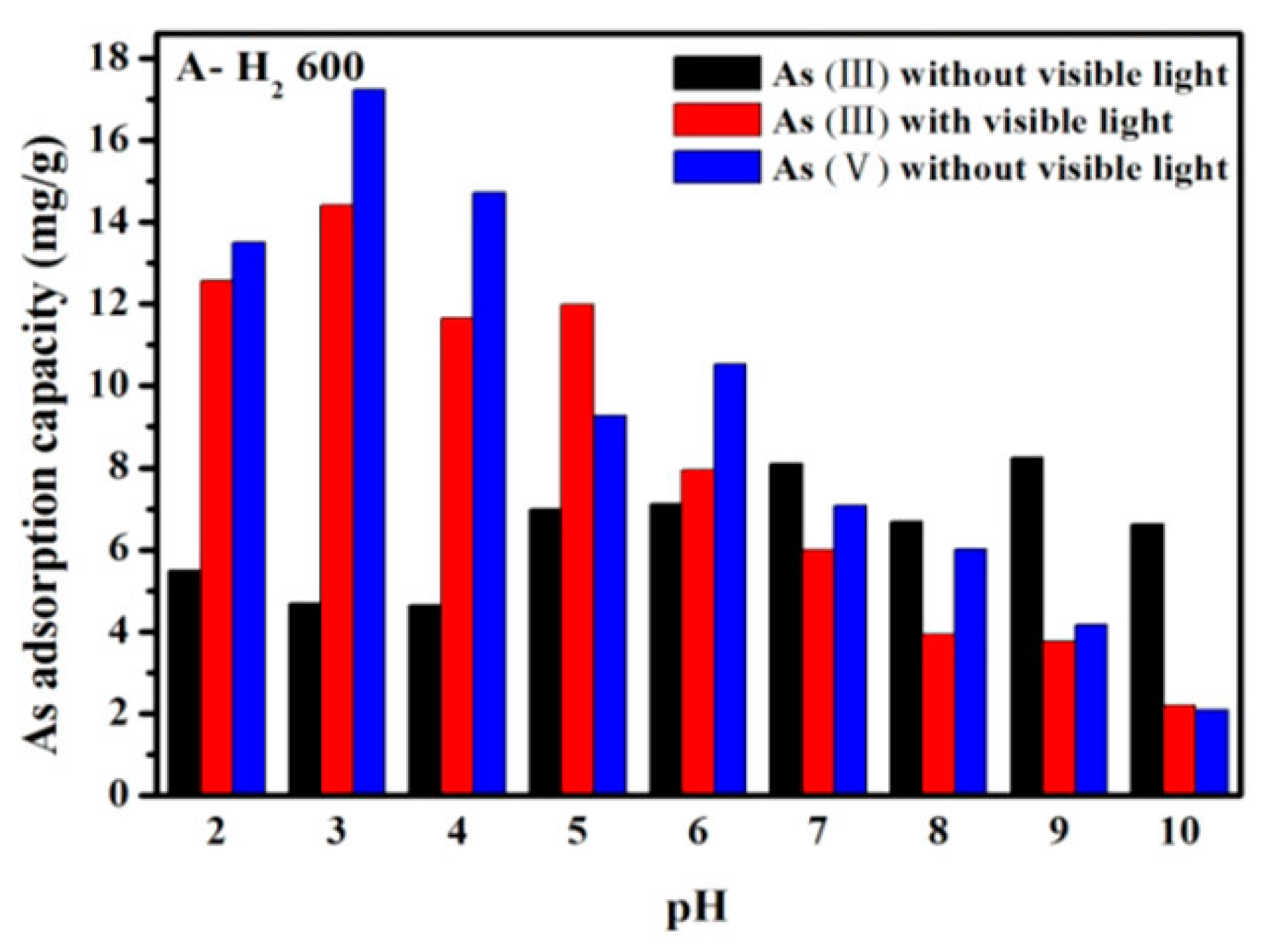
Colored TiO2−x shows photoactivity under visible light. As shown in Figure 3, As(III) adsorption diminished above zpc (at pH 6.3) under visible light owing to the conversion of As(III) to As(V) by photocatalytic oxidation (a conversion rate of about 2.85 mgAs/g h) [77].
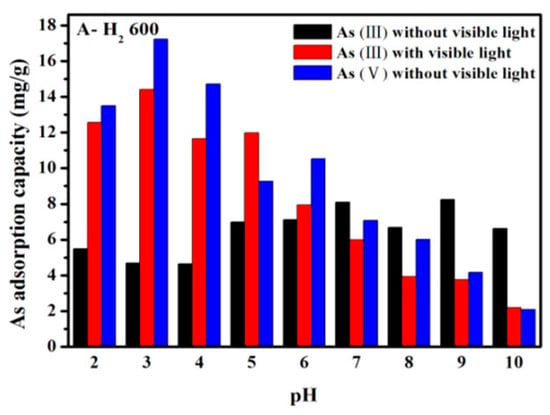
Figure 3.
Effect of pH on arsenic adsorption on TiO2−x with and without visible light. Reprinted with permission from ref. [77]). Copyright American Chemical Society 2020.
3.2. Photocatalytic Oxidation of As(III) Using TiO2-Based Nanocomposites
The design of TiO2-based nanocomposites is the most frequently used strategy to increase the efficiency of photocatalytic reaction under visible-light irradiation and, at the same time, to enhance the pollutant removal capacity. Interfacial interactions between the phases forming a nanocomposite make the physical properties different from those of its individual components. WO3 absorbs solar radiation better than TiO2 owing to its smaller band-gap energy of about 2.6 eV [78]. When TiO2 is coupled with WO3 (TiO2/WO3 nanocomposite), the photo-generated electrons of WO3 are transferred to the TiO2 conduction band, thus generating holes in the WO3 valence band [79]. TiO2/WO3 composite oxidizes about 75% of As(III) in 60 min, while V2O5@TiO2 composite with a core–shell sphere microstructure oxidizes 92% of As(III) in 80 min [80]. Hydrogenated black TiO2 (HBTiO2, anatase) and rutile-based inorganic hollow microspheres (RBIHM) oxidize 70.3% of arsenite in 30 min [81]. Coupling HBTiO2/RBIHM with MoS2 results in superior As(III) photooxidation of 96.6% in 30 min under visible light [81]. Repeated experiments of HBTiO2/RBIHM-MoS2 maintain 91.4% of As(III) photooxidation after 5 cycles.
Ternary composites incorporating (i) TiO2, (ii) a conductive polymer to retard electron–hole recombination, and (iii) an excellent adsorbent material for the removal of As(V) were investigated. Examples include γ-Fe2O3/polyaniline/TiO2 [82], MWCNT/polyaniline/TiO2 [83], Fe3O4/polyparaphenylene diamine/TiO2 [84], and γ-Fe2O3/polythiophene/TiO2 [85]. As shown in Table 3, the best results were obtained by γ-Fe2O3/polythiophene/TiO2, which oxidizes 99% of As(III) in 300 min and simultaneously adsorbs 90% of As(V) [85]. Recently, graphitic carbon nitride (g-C3N4,), which absorbs visible light (band-gap energy of 2.7 eV), but is a poor arsenic sorbent, was coupled with B-doped TiO2, showing a removal efficiency of 92% of As(III) in 300 min [86]. The literature reports the effect of oxyanions that are frequently present in water on photocatalysis reaction rates through competitive adsorption and surface-site blocking [87,88]. In particular, and have a weak inhibitory effect on the adsorption ability of γ-Fe2O3@polyaniline@TiO2, while the removal capacity of As decreased by 17% in the presence of and by 45% in the presence of [82].

Table 3.
As(III) oxidation to As(V) over TiO2-based nanocomposite photocatalysts under visible light. @ indicates core–shell structure.
3.3. Photocatalytic Oxidation of As(III) Using WO3, ZnO, and Bismuth Oxides
Although the semiconductor oxide most widely used as photocatalyst is TiO2 because of its low cost, stability, and reactivity, it is effective only under UV-light irradiation, which limits its utilization with solar light and increases costs and management problems. In addition to studying many types of modifications to widen the absorption range of TiO2, other semiconductor oxides that can absorb light in the visible range were studied.
Tungsten (VI) trioxide was studied as a photocatalyst because of its effective oxidation capacity of valence band (VB) holes, strong absorption of solar radiation, small band gap (2.4–2.8 eV), high oxidation power of valence band (VB) holes (+3.1−3.2 VNHE), and nontoxicity [89].
The photocatalytic oxidation of As(III) to As(V) on WO3 primarily proceeds through two-hole transfer, while hydroxyl radical (HO•) and hydrogen peroxide (H2O2) are only marginally involved in the oxidation of As(III) to As(V) [90]. However, the rapid recombination of photo-generated electron–hole pairs reduces the catalytic activity and calls for modifications aimed at increasing the efficiency of the electron–hole separation. As shown in Table 4, platinization is one such modification [89], the purpose of which is to enable O2 to serve as an electron acceptor despite the insufficient reduction potential of the conduction band electrons in WO3. The catalytic system was tested on several types of substrate; As(III) was in all cases successfully converted to As(V), but the oxidation is much faster with the platinized catalyst. Another way to increase the efficiency of WO3 is to add Cu2+ ions or CuO as a co-catalyst [91]. It was established that the presence of Cu2+ increases the arseniate oxidation rate by 3.6 times, while the rate for CuO increases by 2.3 times compared with pure WO3. In addition, pH plays an important role in increasing the oxidation rate of As(III): in the case of Cu2+–WO3, oxidation takes place at an acidic pH and is much faster than with CuO–WO3 at basic pH [91].

Table 4.
As(III) oxidation to As(V) over WO3-based photocatalysts under visible light.
Zinc (II) oxide is another oxide that has attracted the attention of researchers who are studying new, economically competitive methods for the oxidation of arsenic (III). This compound shows peculiar characteristics, such as a direct and wide band gap in the near-UV spectral region, strong oxidation ability, good photocatalytic properties, and a high free-exciton binding energy [92]. ZnO is also more affordable than TiO2. To make it active even under visible-light irradiation and to improve its photocatalytic performance, the literature suggests different solutions, including the introduction of different types of metal dopants into ZnO (Table 5). Cu is a cationic dopant that leads to a lower band gap energy value compared with undoped ZnO. The tests showed the complete conversion of As(III) to As(V) in the presence of Cu-doped ZnO under visible light [93], which did not happen in the presence of pure ZnO under the same conditions. The same result was also obtained using solar-simulated radiation. In 2021, to develop a catalyst that was simple to remove, Cu-doped ZnO was supported on polystyrene pellets [93]. The excellent performance of the photocatalyst, as proved in a previous paper [93], was also provided in this case. It was proved that the high photo-catalytic activity was preserved after several reuse cycles, and there was evidence that no significant release of photocatalyst particles from PS pellets occurred.

Table 5.
As(III) oxidation to As(V) over ZnO-based photocatalysts under visible light.
Bismuth oxyhalides BiOX (X = Cl, Br, I) are promising photocatalysts under UV or visible-light illumination due to their layered structure, high activity, and stability. In several papers, BiOI, bismuth oxyiodide, as such or modified, was used as a photocatalyst for As(III) oxidation. In [94], the efficiency of BiOI was related to its morphology, which was in turn dependent on the viscosity of the solvent used in its synthesis. With the highest viscosity, largest specific surface area, and thinnest nanosheets with exposed (0 0 1) surfaces, it could efficiently absorb visible light, subsequently generate a large number of carriers, and then rapidly move to the nanosheet surface in the role of a static electric field, thereby yielding the highest photocatalytic efficiency. The proposed mechanism proceeds through the excitation of the BiOI nanoparticles under >420 nm illumination to excite electrons to the CB; these could reduce to while the photogenerated holes cannot oxidize the hydroxyl groups due to unfavorable potential. Instead, they directly oxidize the As(III) adsorbed on the surface of BiOI.
Modifications included doping with La to obtain Bi0.9La0.1OI [95] to facilitate migration and hinder the recombination of photogenerated charge carriers. The contribution of La is due to free La3+ ions on the surfaces of BiOI nanosheets, which can form doping energy levels, trapping the photo-induced e− below the CB edge of Bi0.9La0.1OI and accelerating the formation of . The in situ introduction of La into the lattice structure of BiOI may provide more active sites for the formation of oxygen vacancies; these increase the amount of adsorbed oxygen and consequently induce the generation of superoxide radicals, suppressing the recombination of photogenerated electron–hole pairs, while the h+ may migrate to the surface of Bi0.9La0.1OI.
ZrO2-modified BiOCl0.5I0.5 composites (ZBCI) [96] can not only oxidize As(III) into As(V) with visible-light irradiation but can also effectively capture the generated As(V), leading to negligible residual As(III) or As(V) in aqueous solutions after a 90 min treatment. In the fabricated composites, ZrO2 acted as the main adsorption site, while BiOCl0.5I0.5 served as the primary photocatalysis center. Because of the hetero-structure of ZBCI, e- generated by BiOCl0.5I0.5 would be transferred to ZrO2 and an inhibited e−-h+ recombination rate, contributing to the improved photocatalytic efficiency. ZBCI can effectively remove As(III) over a broad pH range from 3 to 11. The high-resolution XPS spectra of ZBCI after the reaction showed that the As present on ZBCI surfaces was in the form of As(V), which further confirmed that As(III) was completely oxidized by photocatalysis.
BiOI@Fe2O3 core–shell nanoparticles [97] provided efficient removal of As(III) via a simultaneous photocatalytic oxidation–adsorption process with an As removal efficiency of 99.8% (3.99 mg/g) within 180 min. Such BiOI@Fe2O3 also demonstrated good As removal stability (3.8–4.0 mg/g) over a wide pH range of 2–8. Owing to the magnetic property, the BiOI@Fe2O3 nanocomposites can be rapidly recovered from the solution under an external magnetic field. Bi2.15WO6 (BWO) [98] can be used to treat wastewater containing both Mn(II) and As(III), as it can oxidize Mn(II) to amorphous manganese oxide (MnOx) under visible light. MnOx generated in this way can further transform As(III) to As(V). Conversion of As(III) to As(V) can be obtained either by the BWO-Mn(II) system under visible light or by the BWO-MnOx system in the dark, with the latter system providing slightly better results. BWO can remove Mn(II) and As(III) via adsorption and/or oxidation processes. As(III) was gradually converted into As(V) as the reaction progressed, and the oxidation was basically complete after 24 h. The remaining As(III) was only 0.021 mg L−1 after the reaction, and the removal percentage was as high as 97.9%. The adsorbed As in the BWO-MnOx system was mainly As(V). From the results reported in refs [97,98], although they are interesting, it is not possible to extrapolate the contribution of photooxidation versus absorption. To avoid inconsistencies with the other data in Table 6 (which refer to the oxidized amount), these results are omitted.

Table 6.
As(III) oxidation to As(V) over bismuth oxide photocatalysts under visible light.
Nanocrystalline Bi2Sn2O7, with a band gap of 2.88 eV, exhibits a high photocatalytic activity in the oxidation of As(III) (up to 96.8%) under visible-light irradiation [99]. As(III) species can adsorb readily on the Bi2Sn2O7 surface, which is beneficial for the subsequent oxidation of As(III) via photocatalyst. The total removal ratio of As(III) is up to 96.8% after 60 min of illumination.
3.4. Photocatalytic Oxidation of As(III) Using Graphitic Carbon Nitride Based Photocatalysts
Graphitic carbon nitride, often referred to as g-C3N4, recently gained significant attention owing to its distinct physicochemical characteristics. This layered structure, which is reminiscent of graphene, gained prominence for its stability, thermal resistance, and narrow band gap (around 2.7 eV). g-C3N4 comprises layers of bi-dimensional π-conjugated polymer structures, consisting of S-triazine or S-heptazine units and tertiary amine linkages. The appeal of g-C3N4 also stems from its environmental friendliness, straightforward preparation process, and cost-effectiveness. Nonetheless, pristine carbon nitride (pCN) exhibits limitations in terms of electronic conductivity, visible light absorption, and available surface active sites. These shortcomings were effectively addressed through diverse strategies, including the creation of heterojunctions, the introduction of heteroatoms, and nanostructural engineering. The material’s photocatalytic efficacy under visible light sparked interest in applications involving hydrogen generation through water splitting and degradation of pollutants at room temperature.
Table 6 reports the entries of the results obtained using g-C3N4 in various forms to photocatalytically oxidize As(III) species.
Although g-C3N4 by itself can act as a photocatalyst, its performance in the oxidation of As(III) to As(V) species is poor in many conditions due to the rapid recombination of the photogenerated electron–hole pairs. A conversion as low as 5% was reported by Kim et al. [100], while other authors obtained conversions up to a maximum of about 65% [101] depending on the experimental conditions. Variously modified types of g-C3N4 were usually able to improve the lifetime of the photogenerated species, reaching oxidation percentages of 41% [100], 67% [102], and 90% [101], and even complete oxidation in some cases [103,104].
For example, the oxidation ability was greatly enhanced in composite photocatalysts prepared by Wang et al. [103]: they produced a composite using bentonite clay by mixing and firing the two components at 450–550 °C, enhancing the oxidation capabilities of graphitic carbon nitride up to complete oxidation in 180 min of illumination with visible light, with only small changes in efficiencies by changing the solution pH from 3 to 8.5. The authors speculated that the improvement was caused by a change in the g-C3N4 structure in the composite, suggesting a negligible contribution of bentonite in the oxidation. Indeed, the oxidation ability was not linearly sensitive to the amount of bentonite (the 10% bentonite composite was the optimum composition, whereas the 5% and 20% composites had poorer performance). The structural change was also supported by the change in oxidation ability depending on the composite calcination temperature. Furthermore, a slight decrease in efficiency was observed when increasing the As(III) initial concentration.
The addition of amorphous iron to g-C3N4 was reported by Kim et al. [100] as a method of improving the removal ability of arsenic species in water. The photocatalysts were obtained in a single-step synthesis starting from melamine and iron and were compared with a mixture of g-C3N4 and crystalline hematite. The authors demonstrated a synergy between the oxidation of As(III) by the graphitic carbon nitride under illumination (both in UV and visible light) and the absorption of both As(III) and As(V) by the amorphous iron oxide species in the structure of the composite. Visible light seems to have a role in the process; nevertheless, the oxidation efficiency seems quite small (only about 40% of the absorbed As is in the form of As(V)), and the reported results seems mainly due to the absorption process; since the overall As removal is 41%, the oxidation efficiency can be calculated to be about 16%.
In 2017, Sun et al. [104] developed a α-Fe2O3/g-C3N4 heterojunction, exploiting the relative positions of the conduction and valence bands of α-Fe2O3 and g-C3N4 to allow the electrons in the CB of g-C3N4 to transfer to the CB of α-Fe2O3, thus enhancing the lifetime of the photogenerated electron–hole pairs and inhibiting their rapid recombination. Indeed, the oxidation ability of the composite largely exceeded the ability of g-C3N4 alone (38%) and that of α-Fe2O3 (21%). They found the optimum amount of α-Fe2O3 in the composite (8%) that allowed the oxidization of about 94% of the starting As(III) at neutral pH in 500 min of irradiation.
Wang et al. [101] used a different approach, modifying the g-C3N4 structure with pyromellitic diimide (PDI) doping. The modification gave rise to a high oxidation ability, so to produce high amounts of H2O2 in water. The photocatalyst was then applied to the simultaneous photooxidation of As(III) and photoreduction of Cr(VI) in high concentrations for the first time, so as to simulate Acid Mine Drainage composition. Nonetheless, we only analyze the results of As oxidation. At pH = 4 and after 120 min of visible light irradiation, the oxidation efficiency of As(III) reached 65% for the pure g-C3N4, which increased to a value of about 90% under the same conditions for the g-C3N4/PDI composite.
Later, Lei et al. [102] applied a different approach for a similar purpose: this time a direct Z-scheme photocatalyst was prepared by combining a magnetic semiconductor oxide ZnFe2O4 with g-C3N4 and, it was tested using simultaneous As(III) oxidation and Cr(VI) reduction. The as-prepared catalyst yielded a 67% oxidation of As(III) in two hours of reaction, starting from a mixture of both As(III) and Cr(VI). The result was enhanced by the addition of oxalate ions in the reaction mixture, obtaining virtually complete oxidation of As(III) in the same amount of time at pH = 5.
The same authors also employed a different approach using similar materials (core–shell structures of ZnFe2O4 supported on polyaniline (PANI) [105] that, for the sake of clarity, are analyzed in the following paragraph and reported in Table 7, together with other core–shell photocatalysts.

Table 7.
As(III) oxidation to As(V) over g-C3N4-based photocatalysts.
3.5. Photocatalytic Oxidation of As(III) Using Core–Shell Structure Photocatalysts
The approach of Lei et al. [105] involved the use of sulfate and hydroxide () radicals, obtained by activation of sulfite radicals ( via ZnFe2O4@PANI photocatalyst as the active species for As(III) oxidation. The efficacy of the photocatalyst was attributed by the authors to the core–shell structure of the catalyst. Indeed, the effect of PANI covering the ZnFe2O4 surface was emphasized by the increase in oxidation efficiency that spans from 60% with bare ZnFe2O4 to complete oxidation using ZnFe2O4@PANI in just 60 min. Moreover, the method is fairly independent of the solution pH in the range of 3–10, and the magnetic properties of the semiconductor oxide allow for easy recovery. A minor leaching of iron ions into the solution was reported, but less than observed for the bare ZnFe2O4.
Other core–shell structure catalysts that gained attention are silver-based compounds, especially silver halides, owing to their ability to absorb visible light and efficiently attain charge separation and charge transfer. In turn, core–shell structures allow for easy tunability of the catalyst properties.
Quin et al. [106] reported on the preparation and testing of Ag@AgCl core–shell nanowires. With an optimized composition, they obtained an oxidation efficiency of 40% in acidic conditions (pH = 3), 76% at neutral and 83% in basic conditions (pH = 10), after two hours of illumination (Table 8). The authors also tested the photocatalysts for several cycles, unfortunately showing a washout of the catalyst that decreased the efficiency to about 22% after 20 cycles.

Table 8.
As(III) oxidation to As(V) over core–shell structured photocatalysts.
The same authors also modified the pristine Ag@AgCl by incorporating iron [107] in a ratio of Ag:Fe = 1:1, which allowed for the reduction of the band gap of the photocatalyst from 3.25 eV to 3.04. They used the nanowire photocatalysts that were originally developed for the inactivation of Escherichia coli (E. coli) bacteria, in the concurrent oxidation of As(III) and inactivation of E.coli. The authors reported that the presence of the bacteria did not affect the efficiency of the catalyst on the photooxidation of As(III) until a very high amount (108 cfu/mL). The iron-modified photocatalyst resulted in an As(III) oxidation percentage of about 92% after 180 min of reaction under visible-light irradiation at neutral pH, increasing the performance of the iron-free photocatalyst, which, under the same conditions, only attained 39% oxidation.
4. Conclusions and Perspectives
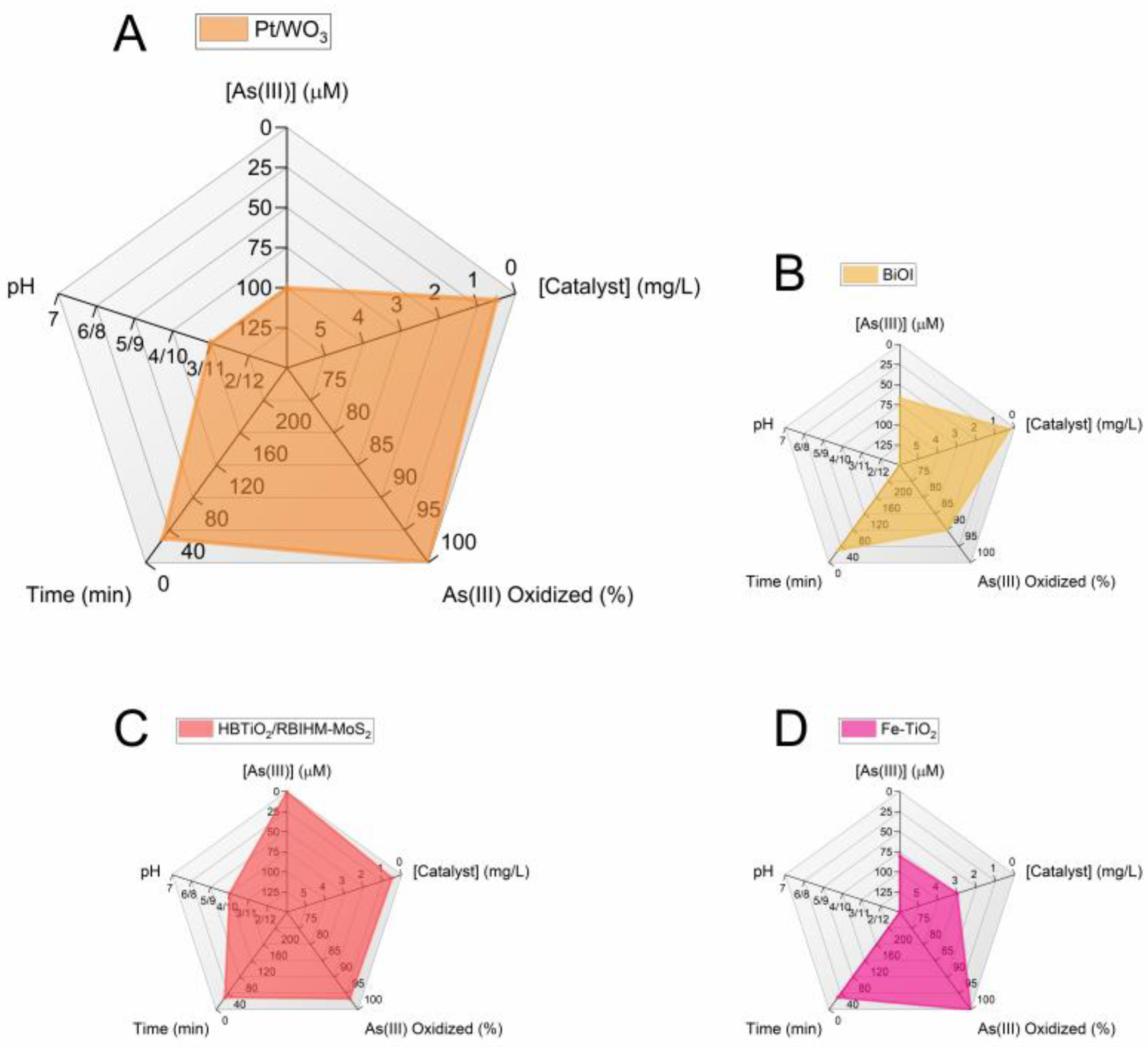
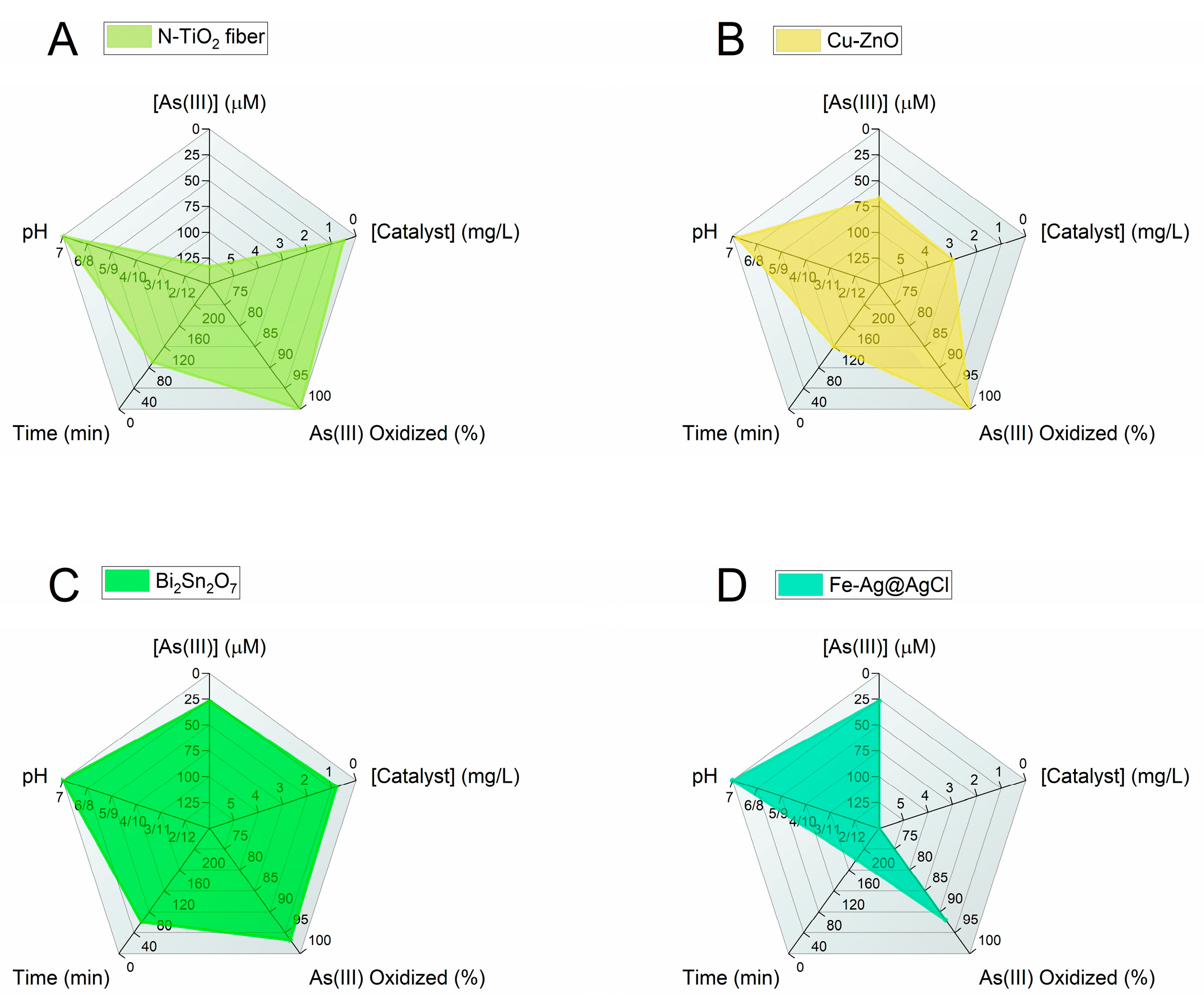
In this review, the most advanced photocatalytic materials for arsenic removal are described with a focus on semiconductor catalysts activated by visible light, including the fundamental aspects of their design and applications. In addition, the importance of coupling materials for excellent oxidation of As(III) to As(V) by photocatalysis with highly adsorbent materials is highlighted. Figure 4 and Figure 5 summarize the conclusions of the review in two radar charts. Figure 4 groups the best photocatalysts regardless of the working pH, while Figure 5 reports only the catalysts for which results were obtained at pH ≈ 7. The radar charts allow the comparison of an arbitrary number of variables. The variables compared in these graphs are (clockwise, starting from the top): (i) [As(III)]: the molar concentration of As(III) used in the experiments in μmol/L(μM). The lower the better, as the concentrations usually found in groundwater are very low and the limit for arsenic in drinking water is around 10 μg/L (0.1 μM) [4]. (ii) [Catalyst]: the concentration of catalyst used (in mg/L). The lower the better (scale from 0 to 6 mg/L). (iii) Amount of As(III) oxidized to As(V) (in %): this is the efficiency of the photocatalyst in oxidizing As(III). The higher the better. (iv) Time (in minutes) required to attain the reported % of As(III) oxidation. The lower the better. (v) pH: this is the specific pH condition at which the experiments were performed. The closer the pH is to 7 the better, the farther the pH deviates from 7 (either towards acidic or basic conditions), the worse.
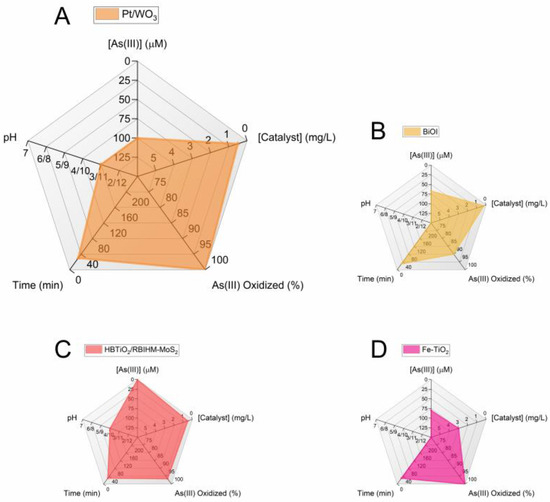
Figure 4.
Radar graphs comparing the performance of the best visible-light photocatalytic systems. The first graph is enlarged to enhance the readability of the axis scales. The data used to prepare the figures refer to the following References: [89] (A); [94] (B); [81] (C); [68] (D).
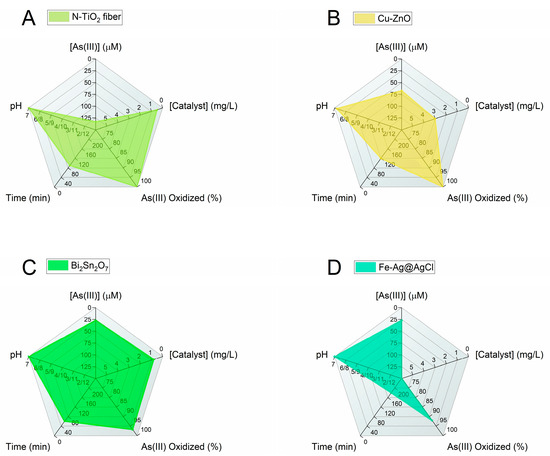
Figure 5.
Radar charts comparing the performance of the best visible-light photocatalytic systems operating at pH ≈ 7. References: [69] (A); [92] (B); [99] (C); [107] (D).
The scales of each variable are designed so that the better the catalyst, the larger the filled area in the graph. When data are unavailable, the parameter is assigned the worst possible value.
Currently, the most efficient performance under 30 min of visible-light irradiation is offered by the following photocatalysts (Figure 4): (1) Fe-doped TiO2 with 100% As(III) oxidation [68], HBTiO2/RBIHM-MoS2 with 96.6% As(III) oxidation [81], Pt/WO3 with 100% As(III) oxidation [89], and BiOI with 90% As(III) oxidation [94]. It is worth noting that in these studies, the pH of the starting As(III) solution is 3 (Pt/WO3) or 10 (HBTiO2/RBIHM-MoS2) or is not available (Fe-doped TiO2, BiOI). For a practical application of the oxidation of As(III), it is necessary to work with an arsenic solution at a pH of approximately 7. Among the studies carried out at pH ≈ 7, the most interesting are the following (Figure 5): N-doped TiO2 fiber with 100% As(III) oxidation under 90 min visible-light irradiation [69]; 1.08 mol% Cu-doped ZnO with 100% As(III) oxidation (120 min) [92]; Bi2Sn2O7 with 83% As(III) oxidation (60 min) [99]; and Fe-Ag@AgCl with ~92% As(III) oxidation (180 min) [107]. These results show that long reaction time remains a major challenge in achieving high As oxidation.
For photocatalysis technology to find use in small low-resource communities, the photocatalyst must be robust and inexpensive. However, this cannot be the only criterion in the selection of a photocatalyst, as the effect of oxyanions, which are frequently present in water, on photocatalysis reaction rate through competitive adsorption and surface-site blocking can hinder As(III) oxidation.
Author Contributions
Conceptualization, I.N.S.; formal analysis, I.N.S., F.F., R.P., and B.B.; writing—original draft preparation, I.N.S., F.F., R.P., and B.B.; writing—review and editing, I.N.S., F.F., and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, V.K.; Sohn, M. Aquatic Arsenic: Toxicity, Speciation, Transformations, and Remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.D.; Rene, E.R.; Giri, B.S.; Pandey, A.; Singh, H. Adsorptive and Photocatalytic Properties of Metal Oxides towards Arsenic Remediation from Water: A Review. J. Environ. Chem. Eng. 2021, 9, 106376. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The Global Menace of Arsenic and Its Conventional Remediation—A Critical Review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Exposure to Arsenic: A Major Public Health Concern; Preventing Disease through Healthy Environments; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic Contamination, Consequences and Remediation Techniques: A Review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Marinho, B.A.; Cristóvão, R.O.; Boaventura, R.A.R.; Vilar, V.J.P. As(III) and Cr(VI) Oxyanion Removal from Water by Advanced Oxidation/Reduction Processes—A Review. Environ. Sci. Pollut. Res. 2019, 26, 2203–2227. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising Prospects of Nanomaterials for Arsenic Water Remediation: A Comprehensive Review. Process Saf. Environ. Prot. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Kuroda, K.; Lu, B.; Hama, Y.; Yang, Y. Recent Progress in Photocatalysts for Oxidation of As(III) and Photocatalyst-Impregnated Adsorbents for Removing Aqueous Arsenic. Curr. Opin. Environ. Sci. Health 2023, 35, 100498. [Google Scholar] [CrossRef]
- Issa, N.B.; Rajaković-Ognjanović, V.N.; Marinković, A.D.; Rajaković, L.V. Separation and Determination of Arsenic Species in Water by Selective Exchange and Hybrid Resins. Anal. Chim. Acta 2011, 706, 191–198. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, Y.; Wang, L. Abiotic Oxidation of Arsenite in Natural and Engineered Systems: Mechanisms and Related Controversies over the Last Two Decades (1999–2020). J. Hazard. Mater. 2021, 414, 125488. [Google Scholar] [CrossRef]
- Nicomel, N.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Qu, J. Review on Heterogeneous Oxidation and Adsorption for Arsenic Removal from Drinking Water. J. Environ. Sci. 2021, 110, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Mahamallik, P.; Swain, R. A Mini-Review on Arsenic Remediation Techniques from Water and Future Trends. Water Sci. Technol. 2023, 87, 3108–3123. [Google Scholar] [CrossRef] [PubMed]
- ALSamman, M.T.; Sotelo, S.; Sánchez, J.; Rivas, B.L. Arsenic Oxidation and Its Subsequent Removal from Water: An Overview. Sep. Purif. Technol. 2023, 309, 123055. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.; Zhang, L.; Lu, X.; Liu, Y.; Li, X.; Wang, Y.; Wang, S. Arsenic Oxidation and Removal from Water via Core–Shell MnO2@La(OH)3 Nanocomposite Adsorption. Int. J. Environ. Res. Public. Health 2022, 19, 10649. [Google Scholar] [CrossRef] [PubMed]
- Hug, S.J.; Leupin, O. Iron-Catalyzed Oxidation of Arsenic(III) by Oxygen and by Hydrogen Peroxide: pH-Dependent Formation of Oxidants in the Fenton Reaction. Environ. Sci. Technol. 2003, 37, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, B.; Chatterjee, S.; Rott, U.; Kauffman, H.; Bandopadhyay, A.; DeGroot, W.; Nag, N.K.; Carbonell-Barrachina, A.A.; Mukherjee, S. A Simple Chemical Free Arsenic Removal Method for Community Water Supply—A Case Study from West Bengal, India. Environ. Pollut. 2009, 157, 3351–3353. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lin, G.; Zeng, J.; Yang, Z.; Wang, L. Construction of Algal-Bacterial Consortia Using Green Microalgae Chlorella vulgaris and As(III)-Oxidizing Bacteria: As Tolerance and Metabolomic Profiling. J. Environ. Sci. 2024, 139, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kraaijeveld, E.; Rijsdijk, S.; Van Der Poel, S.; Van Der Hoek, J.P.; Rabaey, K.; Van Halem, D. Electrochemical Arsenite Oxidation for Drinking Water Treatment: Mechanisms, by-Product Formation and Energy Consumption. Water Res. 2024, 253, 121227. [Google Scholar] [CrossRef]
- Syam Babu, D.; Nidheesh, P.V. A Review on Electrochemical Treatment of Arsenic from Aqueous Medium. Chem. Eng. Commun. 2021, 208, 389–410. [Google Scholar] [CrossRef]
- Miao, X.; Shen, J.; Ji, W.; Zhang, T.C.; Liang, Y.; Yuan, S. Boosting Electrochemical Oxidation of As(III) on Fe-Doped RuO2/PEDOT/SnO2 Nanocomposite Anode: Fabrication, Performance and Mechanism. J. Mater. Sci. Technol. 2024, 180, 243–258. [Google Scholar] [CrossRef]
- Litter, M.I.; Candal, R.J.; Meichtry, J.M. (Eds.) Reduction of Pentavalent and Trivalent Arsenic by TiO2-Photocatalysis: An Innovative Way of Arsenic Removal. In Advanced Oxidation Technologies; CRC Press: Boca Raton, FL, USA, 2014; pp. 61–80. ISBN 978-0-429-22744-8. [Google Scholar]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (Modified) Natural Adsorbents for Arsenic Remediation: A Review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A Critical Review on Arsenic Removal from Water Using Iron-Based Adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lin, J.; Luo, J.; Sun, S.; Zhang, X.; Ma, R.; Peng, J.; Ji, F.; Zheng, S.; Tian, Z.; et al. Rapid Immobilization of Arsenic in Contaminated Soils by Microwave Irradiation Combined with Magnetic Biochar: Microwave-Induced Electron Transfer for Oxidation and Immobilization of Arsenic (III). Sci. Total Environ. 2024, 919, 170916. [Google Scholar] [CrossRef]
- Siddiq, O.M.; Tawabini, B.S.; Soupios, P.; Ntarlagiannis, D. Removal of Arsenic from Contaminated Groundwater Using Biochar: A Technical Review. Int. J. Environ. Sci. Technol. 2022, 19, 651–664. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, R.; Singh, R.K.; Sharma, P.; Ghosh, A. Review on Arsenic Removal Using Biochar-Based Materials. Groundw. Sustain. Dev. 2022, 17, 100740. [Google Scholar] [CrossRef]
- Foti, C.; Mineo, P.G.; Nicosia, A.; Scala, A.; Neri, G.; Piperno, A. Recent Advances of Graphene-Based Strategies for Arsenic Remediation. Front. Chem. 2020, 8, 608236. [Google Scholar] [CrossRef]
- Shan, H.; Liu, Y.; Zeng, C.; Peng, S.; Zhan, H. On As(III) Adsorption Characteristics of Innovative Magnetite Graphene Oxide Chitosan Microsphere. Materials 2022, 15, 7156. [Google Scholar] [CrossRef]
- Saqib, A.N.S.; Waseem, A.; Khan, A.F.; Mahmood, Q.; Khan, A.; Habib, A.; Khan, A.R. Arsenic Bioremediation by Low Cost Materials Derived from Blue Pine (Pinus wallichiana) and Walnut (Juglans regia). Ecol. Eng. 2013, 51, 88–94. [Google Scholar] [CrossRef]
- Mondal, M.K.; Garg, R. A Comprehensive Review on Removal of Arsenic Using Activated Carbon Prepared from Easily Available Waste Materials. Environ. Sci. Pollut. Res. 2017, 24, 13295–13306. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Souza, T.G.F.; Mohallem, N.D.S.; Ciminelli, V.S.T. Removal and Environmentally Safe Disposal of As(III) and As(V)-Loaded Ferrihydrite/Biosilica Composites. J. Environ. Manag. 2023, 335, 117489. [Google Scholar] [CrossRef]
- Hott, R.C.; Andrade, T.G.; Santos, M.S.; Lima, A.C.F.; Faria, M.C.S.; Bomfeti, C.A.; Barbosa, F.; Maia, L.F.O.; Oliveira, L.C.A.; Pereira, M.C.; et al. Adsorption of Arsenic from Water and Its Recovery as a Highly Active Photocatalyst. Environ. Sci. Pollut. Res. 2016, 23, 21969–21979. [Google Scholar] [CrossRef] [PubMed]
- Taleb, K.; Markovski, J.; Milosavljević, M.; Marinović-Cincović, M.; Rusmirović, J.; Ristić, M.; Marinković, A. Efficient Arsenic Removal by Cross-Linked Macroporous Polymer Impregnated with Hydrous Iron Oxide: Material Performance. Chem. Eng. J. 2015, 279, 66–78. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Current Trends of Arsenic Adsorption in Continuous Mode: Literature Review and Future Perspectives. Sustainability 2021, 13, 1186. [Google Scholar] [CrossRef]
- Ostermeyer, P.; Bonin, L.; Folens, K.; Verbruggen, F.; García-Timermans, C.; Verbeken, K.; Rabaey, K.; Hennebel, T. Effect of Speciation and Composition on the Kinetics and Precipitation of Arsenic Sulfide from Industrial Metallurgical Wastewater. J. Hazard. Mater. 2021, 409, 124418. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.S.; Coutinho de Paula, E.; Amaral, M.C.S. Arsenic Contamination, Effects and Remediation Techniques: A Special Look onto Membrane Separation Processes. Process Saf. Environ. Prot. 2021, 148, 604–623. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Kumar, M.; Agrawal, P.K.; Singh, D.K. Perspectives on Arsenic Toxicity, Carcinogenicity and Its Systemic Remediation Strategies. Environ. Technol. Innov. 2019, 16, 100462. [Google Scholar] [CrossRef]
- Pranudta, A.; Patra, S.; Amonpattaratkit, P.; Klysubun, W.; Saiyasombat, C.; El-Moselhy, M.M.; Nguyen, T.T.; Padungthon, S. Immobilization of Arsenic in Wastewater from Regeneration of Fixed-Bed Adsorbent by Co-Precipitation with Zirconium Nano-Sludge for Disposal in Landfills. J. Environ. Chem. Eng. 2022, 10, 107756. [Google Scholar] [CrossRef]
- Camacho, J.; Wee, H.-Y.; Kramer, T.A.; Autenrieth, R. Arsenic Stabilization on Water Treatment Residuals by Calcium Addition. J. Hazard. Mater. 2009, 165, 599–603. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, Q.; Shao, L.-M.; He, P.-J. Recovery of Arsenic Trioxide from a Sludge-Like Waste by Alkaline Leaching and Acid Precipitation. Waste Biomass Valoriz. 2014, 5, 255–263. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Zhang, X.; Sun, W.; Han, H.; Yu, Z.; Yue, T. A Novel Scheme for Safe Disposal and Resource Utilization of Arsenic-Alkali Slag. Process Saf. Environ. Prot. 2021, 156, 429–437. [Google Scholar] [CrossRef]
- Siddique, T.; Gangadoo, S.; Quang Pham, D.; Dutta, N.K.; Choudhury, N.R. Antifouling and Antimicrobial Study of Nanostructured Mixed-Matrix Membranes for Arsenic Filtration. Nanomaterials 2023, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Worou, C.N.; Chen, Z.-L.; Bacharou, T. Arsenic Removal from Water by Nanofiltration Membrane: Potentials and Limitations. Water Pract. Technol. 2021, 16, 291–319. [Google Scholar] [CrossRef]
- Algieri, C.; Pugliese, V.; Coppola, G.; Curcio, S.; Calabro, V.; Chakraborty, S. Arsenic Removal from Groundwater by Membrane Technology: Advantages, Disadvantages, and Effect on Human Health. Groundw. Sustain. Dev. 2022, 19, 100815. [Google Scholar] [CrossRef]
- Sharma, S.; Desai, A.V.; Joarder, B.; Ghosh, S.K. A Water-Stable Ionic MOF for the Selective Capture of Toxic Oxoanions of SeVI and AsV and Crystallographic Insight into the Ion-Exchange Mechanism. Angew. Chem. Int. Ed. 2020, 59, 7788–7792. [Google Scholar] [CrossRef] [PubMed]
- Padungthon, S.; German, M.; Wiriyathamcharoen, S.; SenGupta, A.K. Polymeric Anion Exchanger Supported Hydrated Zr(IV) Oxide Nanoparticles: A Reusable Hybrid Sorbent for Selective Trace Arsenic Removal. React. Funct. Polym. 2015, 93, 84–94. [Google Scholar] [CrossRef]
- Bukhari, D.A.; Rehman, A. Metal-Resistant Bacteria as a Green Bioresource for Arsenic Remediation in Wastewaters. Curr. Opin. Green Sustain. Chem. 2023, 40, 100785. [Google Scholar] [CrossRef]
- Kaya, C.; Uğurlar, F.; Ashraf, M.; Hou, D.; Kirkham, M.B.; Bolan, N. Microbial Consortia-Mediated Arsenic Bioremediation in Agricultural Soils: Current Status, Challenges, and Solutions. Sci. Total Environ. 2024, 917, 170297. [Google Scholar] [CrossRef]
- Guo, J.; Luo, S.; Liu, Z.; Luo, T. Direct Arsenic Removal from Water Using Non-Membrane, Low-Temperature Directional Solvent Extraction. J. Chem. Eng. Data 2020, 65, 2938–2946. [Google Scholar] [CrossRef]
- German, M.S.; Watkins, T.A.; Chowdhury, M.; Chatterjee, P.; Rahman, M.; Seingheng, H.; SenGupta, A.K. Evidence of Economically Sustainable Village-Scale Microenterprises for Arsenic Remediation in Developing Countries. Environ. Sci. Technol. 2019, 53, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Sengupta, M.K.; Ahamed, S.; Rahman, M.M.; Mondal, D.; Lodh, D.; Das, B.; Nayak, B.; Roy, B.K.; Mukherjee, A.; et al. Ineffectiveness and Poor Reliability of Arsenic Removal Plants in West Bengal, India. Environ. Sci. Technol. 2005, 39, 4300–4306. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, I.M.; McBean, E.A. Beyond Appropriate Technology: Social Considerations for the Sustainable Use of Arsenic–Iron Removal Plants in Rural Bangladesh. Technol. Soc. 2015, 41, 1–9. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Photocatalytic Oxidation of Arsenite on TiO2: Understanding the Controversial Oxidation Mechanism Involving Superoxides and the Effect of Alternative Electron Acceptors. Environ. Sci. Technol. 2006, 40, 7034–7039. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Leng, W.; Li, X.; Cheng, X.; Xu, Y.; Zhang, J.; Cao, C. Photocatalytic Oxidation of Arsenite over TiO2: Is Superoxide the Main Oxidant in Normal Air-Saturated Aqueous Solutions? Environ. Sci. Technol. 2011, 45, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Litter, M.I. Last Advances on TiO2-Photocatalytic Removal of Chromium, Uranium and Arsenic. Curr. Opin. Green Sustain. Chem. 2017, 6, 150–158. [Google Scholar] [CrossRef]
- García, F.E.; Litter, M.I.; Sora, I.N. Assessment of the Arsenic Removal From Water Using Lanthanum Ferrite. ChemistryOpen 2021, 10, 790–797. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P. Arsenic Removal from Water by Coupling Photocatalysis and Complexation-Ultrafiltration Processes: A Preliminary Study. Water Res. 2017, 109, 327–336. [Google Scholar] [CrossRef]
- Pendlebury, S.R.; Wang, X.; Le Formal, F.; Cornuz, M.; Kafizas, A.; Tilley, S.D.; Grätzel, M.; Durrant, J.R. Ultrafast Charge Carrier Recombination and Trapping in Hematite Photoanodes under Applied Bias. J. Am. Chem. Soc. 2014, 136, 9854–9857. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent Development in Band Engineering of Binary Semiconductor Materials for Solar Driven Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, H.G.; Lee, J.S. Heterojunction Semiconductors: A Strategy to Develop Efficient Photocatalytic Materials for Visible Light Water Splitting. Catal. Today 2012, 185, 270–277. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical Properties of TiO2 Photocatalyst and Its Applications for Environmental Purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 Photocatalyst for Water Treatment Applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Rizzo, L. Visible Light Active Fe-Doped TiO2 for the Oxidation of Arsenite to Arsenate in Drinking Water. Chem. Eng. Trans. 2018, 70, 1573–1578. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, M.; Liu, Y.; Lang, X.; Liu, L.; Liu, H.; Qu, J.; Li, J. Visible-Light Induced Photocatalytic Activity of Electrospun-TiO2 in Arsenic(III) Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Diaz, D.; Hernandez-Ramirez, A.; Guzman-Mar, J.; Villanueva-Rodriguez, M.; Maya-Trevino, L.; Hinojosa-Reyes, L. N-S Co-Doped TiO2 Synthesized by Microwave Precipitation Method: Effective Photocatalytic Performance for the Removal of Organoarsenic Compounds. J. Environ. Chem. Eng. 2021, 9, 106683. [Google Scholar] [CrossRef]
- Ren, X.; Yao, H.; Tang, R.; Rong, A.; Yuan, S.; Wang, W.; Ali, I.; Hu, Z. Modification of TiO2 by Er3+ and rGO Enhancing Visible Photocatalytic Degradation of Arsanilic Acid. Environ. Sci. Pollut. Res. 2022, 30, 35023–35033. [Google Scholar] [CrossRef]
- Choi, W.; Yeo, J.; Ryu, J.; Tachikawa, T.; Majima, T. Photocatalytic Oxidation Mechanism of As(III) on TiO2: Unique Role of As(III) as a Charge Recombinant Species. Environ. Sci. Technol. 2010, 44, 9099–9104. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Effects of TiO2 Surface Modifications on Photocatalytic Oxidation of Arsenite: The Role of Superoxides. Environ. Sci. Technol. 2004, 38, 2928–2933. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, Y.; Tian, Z.; Wu, Y.; Cui, Y. Efficiently Visible-Light Driven Photoelectrocatalytic Oxidation of As(III) at Low Positive Biasing Using Pt/TiO2 Nanotube Electrode. Nanoscale Res. Lett. 2016, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Ray, A.K.; Sharma, V.K.; Millero, F.J. Adsorption of Arsenate and Arsenite on Titanium Dioxide Suspensions. J. Colloid Interface Sci. 2004, 278, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.; Hoffmann, M.R.; Hering, J.G. TiO2-Photocatalyzed As(III) Oxidation in Aqueous Suspensions: Reaction Kinetics and Effects of Adsorption. Environ. Sci. Technol. 2005, 39, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, B.; Yang, J.; Wu, J.; Zhai, W.; Yang, B.; Liu, M. Rapid Preparation of TiO2 and Its Photocatalytic Oxidation for Arsenic Adsorption under Visible Light. Langmuir 2020, 36, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- González-Borrero, P.P.; Sato, F.; Medina, A.N.; Baesso, M.L.; Bento, A.C.; Baldissera, G.; Persson, C.; Niklasson, G.A.; Granqvist, C.G.; Ferreira Da Silva, A. Optical Band-Gap Determination of Nanostructured WO3 Film. Appl. Phys. Lett. 2010, 96, 061909. [Google Scholar] [CrossRef]
- Ezaki, M.; Kusakabe, K. Highly Crystallized Tungsten Trioxidc Loaded Titania Composites Prepared by Using Ionic Liquids and Their Photocatalytic Behaviors. Evergreen 2014, 1, 18–24. [Google Scholar] [CrossRef]
- Xie, L.; Liu, P.; Zheng, Z.; Weng, S.; Huang, J. Morphology Engineering of V2O5/TiO2 Nanocomposites with Enhanced Visible Light-Driven Photofunctions for Arsenic Removal. Appl. Catal. B Environ. 2016, 184, 347–354. [Google Scholar] [CrossRef]
- Balati, A.; Matta, A.; Nash, K.; Shipley, H.J. Heterojunction of Vertically Aligned MoS2 Layers to Hydrogenated Black TiO2 and Rutile Based Inorganic Hollow Microspheres for the Highly Enhanced Visible Light Arsenic Photooxidation. Compos. Part B—Eng. 2020, 185, 107785. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Zhang, T.C.; Xiang, G.; Wang, X.; Pehkonen, S.; Yuan, S. A Magnetic γ-Fe2O3@PANI@TiO2core-Shell Nanocomposite for Arsenic Removalviaa Coupled Visible-Light-Induced Photocatalytic Oxidation-Adsorption Process. Nanoscale Adv. 2020, 2, 2018–2024. [Google Scholar] [CrossRef]
- Ouyang, L.; Wang, Y.; Zhang, P.; Wang, X.; Yuan, S. Heterostructured MWCNTs@PANI@TiO2 Nanocomposites for Enhanced Adsorption of As(III) from Aqueous Solution: Adsorption and Photocatalytic Oxidation Behaviors. Ind. Eng. Chem. Res. 2020, 59, 11743–11756. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, T.C.; Xiang, G.; Wang, X.; Yuan, S. Removal of Trace Arsenite through Simultaneous Photocatalytic Oxidation and Adsorption by Magnetic Fe3O4@PpPDA@TiO2 Core-Shell Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 8495–8504. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhang, T.; Xiang, G.; Wang, X.; Yuan, S. One-Pot Synthesis of a Magnetic TiO2/PTh/Gamma-Fe2O3 Heterojunction Nanocomposite for Removing Trace Arsenite via Simultaneous Photocatalytic Oxidation and Adsorption. Ind. Eng. Chem. Res. 2021, 60, 528–540. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhang, Y.; Wang, Y.; Wang, X.; Yuan, S. Insights into the Adsorption and Photocatalytic Oxidation Behaviors of Boron-Doped TiO2/g-C3N4 Nanocomposites toward As(III) in Aqueous Solution. Ind. Eng. Chem. Res. 2021, 60, 7003–7013. [Google Scholar] [CrossRef]
- Bolognino, I.; Pelosato, R.; Marcì, G.; Natali Sora, I. Comparison of Ten Metal-Doped LaFeO3 Samples on Photocatalytic Degradation of Antibiotics in Water under Visible Light: Role of Surface Area and Aqueous Phosphate Ions. Molecules 2023, 28, 3807. [Google Scholar] [CrossRef]
- Katz, A.; McDonagh, A.; Tijing, L.; Shon, H.K. Fouling and Inactivation of Titanium Dioxide-Based Photocatalytic Systems. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1880–1915. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Choi, W. Platinized WO3 as an Environmental Photocatalyst That Generates OH Radicals under Visible Light. Environ. Sci. Technol. 2010, 44, 6849–6854. [Google Scholar] [CrossRef]
- Kim, J.; Moon, G.; Kim, S.; Kim, J. Photocatalytic Oxidation Mechanism of Arsenite on Tungsten Trioxide under Visible Light. J. Photochem. Photobiol. Chem. 2015, 311, 35–40. [Google Scholar] [CrossRef]
- Samad, A.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Highly Efficient Visible Light-Induced Photocatalytic Oxidation of Arsenite with Nanosized WO3 Particles in the Presence of Cu2+ and CuO. Environ. Technol. 2022, 44, 3096–3107. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-Doped ZnO as Efficient Photocatalyst for the Oxidation of Arsenite to Arsenate under Visible Light. Appl. Catal. B Environ. 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Vaiano, V.; Chianese, L.; Rizzo, L.; Iervolino, G. Visible Light Driven Oxidation of Arsenite to Arsenate in Aqueous Solution Using Cu-Doped ZnO Supported on Polystyrene Pellets. Catal. Today 2021, 361, 69–76. [Google Scholar] [CrossRef]
- Hu, J.; Weng, S.; Zheng, Z.; Pei, Z.; Huang, M.; Liu, P. Solvents Mediated-Synthesis of BiOI Photocatalysts with Tunable Morphologies and Their Visible-Light Driven Photocatalytic Performances in Removing of Arsenic from Water. J. Hazard. Mater. 2014, 264, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, L.; Chen, M.; Zhang, Q.; Dai, S.; Zhao, T. Mechanochemical Construction of Bi1-xLaxOI Solid Solution with Abundant Oxygen Vacancies for Enhanced Photocatalytic Oxidation of Inorganic/Organic Arsenic. Appl. Surf. Sci. 2022, 602, 154250. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, M.; Guo, J.; Liu, W.; Tong, M. Facile Synthesis of ZrO2 Coated BiOCl0.5I0.5 for Photocatalytic Oxidation-Adsorption of As(III) under Visible Light Irradiation. Chemosphere 2018, 211, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Chen, Q.; Zhang, T.C.; Ouyang, L.; Yuan, S. Simultaneous Photocatalytic Oxidation and Adsorption for Efficient As(III) Removal by Magnetic BiOI/γ-Fe2O3 Core–Shell Nanoparticles. Mater. Today Chem. 2022, 24, 100823. [Google Scholar] [CrossRef]
- Ren, H.-T.; Jing, M.-Z.; Liang, Y.; Li, T.-T.; Jiang, S.-M.; Lou, C.-W.; Lin, J.-H.; Han, X. Performance and Mechanism Involved in the Cascade Oxidation of Mn(II) and As(III) by Bi2.15WO6 under Alkaline Conditions. J. Environ. Chem. Eng. 2021, 9, 106196. [Google Scholar] [CrossRef]
- Tian, Q.; Zhuang, J.; Wang, J.; Xie, L.; Liu, P. Novel Photocatalyst, Bi2Sn2O7, for Photooxidation of As(III) under Visible-Light Irradiation. Appl. Catal. Gen. 2012, 425–426, 74–78. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, H.-B.; Choi, J.-H.; Baek, K. Bifunctional Iron-Modified Graphitic Carbon Nitride (g-C3N4) for Simultaneous Oxidation and Adsorption of Arsenic. Environ. Res. 2020, 188, 109832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Murugananthan, M.; Zhang, Y. Graphitic Carbon Nitride Based Photocatalysis for Redox Conversion of Arsenic(III) and Chromium(VI) in Acid Aqueous Solution. Appl. Catal. B Environ. 2019, 248, 349–356. [Google Scholar] [CrossRef]
- Lei, D.; Xue, J.; Peng, X.; Li, S.; Bi, Q.; Tang, C.; Zhang, L. Oxalate Enhanced Synergistic Removal of Chromium(VI) and Arsenic(III) over ZnFe2O4/g-C3N4: Z-Scheme Charge Transfer Pathway and Photo-Fenton like Reaction. Appl. Catal. B Environ. 2021, 282, 119578. [Google Scholar] [CrossRef]
- Wang, C.; Dai, Y.; Fu, X.; Lu, H.; Zhang, J. A Novel Layer-Layer Crossed Structure of Bentonite/g-C3N4 for Enhanced Photocatalytic Oxidation of Arsenic(III) in a Wide pH Range. Surf. Interfaces 2021, 26, 101365. [Google Scholar] [CrossRef]
- Sun, S.; Ji, C.; Wu, L.; Chi, S.; Qu, R.; Li, Y.; Lu, Y.; Sun, C.; Xue, Z. Facile One-Pot Construction of α-Fe2O3/g-C3N4 Heterojunction for Arsenic Removal by Synchronous Visible Light Catalysis Oxidation and Adsorption. Mater. Chem. Phys. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Lei, D.; Xue, J.; Bi, Q.; Tang, C.; Zhang, L.; Zhang, J. Visible-Light Activation of Sulfite by ZnFe2O4@PANI Photocatalyst for As(III) Removal: The Role of Radicals and Fe(IV). Appl. Surf. Sci. 2022, 578, 151940. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, Y.; Tian, Z.; Wu, Y.; Li, Y. Synthesis of AG@AgCl Core–Shell Structure Nanowires and Its Photocatalytic Oxidation of Arsenic (III) Under Visible Light. Nanoscale Res. Lett. 2017, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ye, Q.; Wang, H.; Duo, X.; Peng, L.; Dong, W.; Cui, X.; Lu, Y.; Li, Y. Photocatalytic and Oxidation Mechanisms of Fe–Ag@AgCl: Effect on Co-Existing Arsenic (III) and Escherichia coli. Environ. Res. 2023, 217, 114913. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).