A Systematic Review of Heterogeneous Catalysis Applied to the Treatment of Pharmaceutical Wastewater: Operational Conditions and Statistical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review
2.2. Systematic Analysis of the FBP
2.3. Meta-Analysis Study Design
3. Results and Discussion
3.1. Systematic Review
3.2. FBP Analysis
3.2.1. Operating Parameters
3.2.2. Radiation Types
3.2.3. Concentrations of the Catalyst and Drug Pollutant
3.2.4. Type of Water Matrix
3.2.5. pH and Catalyst Concentration
3.3. Meta-Analysis Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging pollutants in the urban water cycle in Latin America: A review of the current literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.F.; Jardim, W.F. A fotocatálise Heterogênea E sua aplicação ambiental. Química Nova 1998, 21, 69–72. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Starling, M.C.V.; Amorim, C.C.; Leão, M.M.D. Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2013, 12, 27–47. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.; Silva, A.M. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Janani, F.Z.; Elhalil, A.; Mahjoubi, F.Z. Removal of emerging pharmaceutical pollutants: A systematic mapping study review. J. Environ. Chem. Eng. 2020, 8, 104251. [Google Scholar] [CrossRef]
- Sánchez-Barboza, L.; Villagran-Sánchez, P.S.; Armenise-Gil, S.A. Removal of estrone in water and wastewater by photocatalysis: A systematic review. Producción+ Limpia 2019, 14, 18–32. [Google Scholar] [CrossRef]
- Noguera-Oviedo, K.; Aga, D.S. Lessons learned from more than two decades of research on emerging contaminants in the environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M. Homogeneous and Heterogeneous Photocatalysis for the Treatment of Pharmaceutical Industry Wastewaters: A Review. Toxics 2022, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Kosma, C.; Albanis, T.; Konstantinou, I. An overview of homogeneous and heterogeneous photocatalysis applications for the removal of pharmaceutical compounds from real or synthetic hospital wastewaters under lab or pilot scale. Sci. Total Environ. 2021, 765, 144163. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, R.C.; Rocha EM, R.; Lucena, L.G.; Cahino, A.M. Tratamento fotocatalítico de fármacos utilizando TiO2: Uma análise sistêmica dos mecanismos de degradação, reusabilidade e viabilidade do processo em escala real. Rev. AIDIS Ing. Cienc. Ambient. Investig. Desarro. Práctica 2023, 834–857. [Google Scholar] [CrossRef]
- Lucena, L.G.; de Carvalho, N.A.; Segundo, I.D.B.; Melo, M.M.M.; Cahino, A.M.; Rocha, E.M.R. A Systematic Review on Fenton Optimization for Leachate Treatment Via Response Surface Methodology from 2005 to 2015. J. Solid Waste Technol. Manag. 2021, 47, 409–416. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, L.; Ferronato, C.; Yang, X.; Salvador, A.; Zeng, C.; Chovelon, J.-M. Photocatalytic degradation of atenolol in aqueous titanium dioxide suspensions: Kinetics, intermediates and degradation pathways. J. Photochem. Photobiol. A Chem. 2013, 254, 35–44. [Google Scholar] [CrossRef]
- Hu, J.; Jing, X.; Zhai, L.; Guo, J.; Lu, K.; Mao, L. BiOCl facilitated photocatalytic degradation of atenolol from water: Reaction kinetics, pathways and products. Chemosphere 2019, 220, 77–85. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Dantas, R.F.; Bayarri, B.; González, O.; Giménez, J.; Esplugas, S.; Machulek, A. Synthesis and characterization of B-doped TiO2 and their performance for the degradation of metoprolol. Catal. Today 2015, 252, 27–34. [Google Scholar] [CrossRef]
- Romero, V.; De la Cruz, N.; Dantas, R.F.; Marco, P.; Giménez, J.; Esplugas, S. Photocatalytic treatment of metoprolol and propranolol. Catal. Today 2011, 161, 115–120. [Google Scholar] [CrossRef]
- Santiago-Morales, J.; Agüera, A.; Gómez, M.d.M.; Fernández-Alba, A.R.; Giménez, J.; Esplugas, S.; Rosal, R. Transformation products and reaction kinetics in simulated solar light photocatalytic degradation of propranolol using Ce-doped TiO2. Appl. Catal. B Environ. 2013, 129, 13–29. [Google Scholar] [CrossRef]
- De la Cruz, N.; Dantas, R.F.; Giménez, J.; Esplugas, S. Photolysis and TiO2 photocatalysis of the pharmaceutical propranolol: Solar and artificial light. Appl. Catal. B Environ. 2013, 130–131, 249–256. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.J. Zr-doped TiO2 supported on delaminated clay materials for solar photocatalytic treatment of emerging pollutants. J. Hazard. Mater. 2017, 322, 233–242. [Google Scholar] [CrossRef]
- Chang, C.T.; Wang, J.J.; Ouyang, T.; Zhang, Q.; Jing, Y.H. Photocatalytic degradation of acetaminophen in aqueous solutions by TiO2/ZSM-5 zeolite with low energy irradiation. Mater. Sci. Eng. B 2015, 196, 53–60. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Yanyan, L.; Ouyang, T.; Albadarin, A.B.; Walker, G. BaTiO3/TiO2 composite-assisted photocatalytic degradation for removal of acetaminophen from synthetic wastewater under UV–vis irradiation. Mater. Sci. Semicond. Process. 2018, 73, 42–50. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodriguez, J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Martínez, C.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl. Catal. B Environ. 2011, 107, 110–118. [Google Scholar] [CrossRef]
- Brazón, E.M.; Piccirillo, C.; Moreira, I.S.; Castro, P.M.L. Photodegradation of pharmaceutical persistent pollutants using hydroxyapatite-based materials. J. Environ. Manag. 2016, 182, 486–495. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Belver, C.; Bedia, J.; Amara, A.B.H.; Ruiz-Hitzky, E. ZnO/sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl. Clay Sci. 2018, 156, 104–109. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Eslami, A.; Amini, M.M.; Yazdanbakhsh, A.R.; Mohseni-Bandpei, A.; A Safari, A.; Asadi, A. N,S co-doped TiO2 nanoparticles and nanosheets in simulated solar light for photocatalytic degradation of non-steroidal anti-inflammatory drugs in water: A comparative study. J. Chem. Technol. Biotechnol. 2016, 91, 2693–2704. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Kansal, S.K. Sunlight-driven photocatalytic degradation of non-steroidal anti-inflammatory drug based on TiO2 quantum dots. J. Colloid Interface Sci. 2015, 459, 257–263. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. TiO2 photocatalysis of naproxen: Effect of the water matrix, anions and diclofenac on degradation rates. Chemosphere 2015, 139, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Koltsakidou, A.; Katsiloulis, C.; Εvgenidou, Ε.; Lambropoulou, D.A. Photolysis and photocatalysis of the non-steroidal anti-inflammatory drug Nimesulide under simulated solar irradiation: Kinetic studies, transformation products and toxicity assessment. Sci. Total Environ. 2019, 689, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Yanyan, L.; Kurniawan, T.A.; Ying, Z.; Albadarin, A.B.; Walker, G. Enhanced photocatalytic degradation of acetaminophen from wastewater using WO3/TiO2/SiO2 composite under UV–VIS irradiation. J. Mol. Liq. 2017, 243, 761–770. [Google Scholar] [CrossRef]
- Lin, C.-J.; Yang, W.-T. Ordered mesostructured Cu-doped TiO2 spheres as active visible-light-driven photocatalysts for degradation of paracetamol. Chem. Eng. J. 2013, 237, 131–137. [Google Scholar] [CrossRef]
- Borges, M.E.; García, D.M.; Hernández, T.; Ruiz-Morales, J.C.; Esparza, P. Supported photocatalyst for removal of emerging contaminants from wastewater in a continuous packed-bed photoreactor configuration. Catalysts 2015, 5, 77–87. [Google Scholar] [CrossRef]
- Motlagh, P.Y.; Khataee, A.; Hassani, A.; Rad, T.S. ZnFe-LDH/GO nanocomposite coated on the glass support as a highly efficient catalyst for visible light photodegradation of an emerging pollutant. J. Mol. Liq. 2020, 302, 112532. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Ji, Y.; Tian, J.; Du, Z. Photocatalytic degradation of four non-steroidal anti-inflammatory drugs in water under visible light by P25-TiO2/tetraethyl orthosilicate film and determination via ultra performance liquid chromatography electrospray tandem mass spectrometry. Chem. Eng. J. 2015, 262, 1108–1115. [Google Scholar] [CrossRef]
- He, L.; Dong, Y.; Zheng, Y.; Jia, Q.; Shan, S.; Zhang, Y. A novel magnetic MIL-101(Fe)/TiO2 composite for photo degradation of tetracycline under solar light. J. Hazard. Mater. 2019, 361, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kansal, S.K. Bi2WO6 nanocuboids: An efficient visible light active photocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 2016, 302, 194–203. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Kockler, J.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin. Appl. Catal. B Environ. 2015, 166–167, 45–55. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO 2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Rožman, M.; Ašperger, D.; Ćurković, L.; Babić, S. Photocatalytic degradation of azithromycin by nanostructured TiO2 film: Kinetics, degradation products, and toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Esrafili, A.; Jafari, A.J.; Gholami, M.; Sobhi, H.R.; Nourbakhsh, M.; Akbari-Adergani, B. Photocatalytic degradation of cefixime with MIL-125(Ti)-mixed linker decorated by g-C3N4 under solar driven light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123874. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Evgenidou E, Ν.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef]
- Han, C.; Likodimos, V.; Khan, J.A.; Nadagouda, M.N.; Andersen, J.; Falaras, P.; Rosales-Lombardi, P.; Dionysiou, D.D. UV–visible light-activated Ag-decorated, monodisperse TiO2 aggregates for treatment of the pharmaceutical oxytetracycline. Environ. Sci. Pollut. Res. 2014, 21, 11781–11793. [Google Scholar] [CrossRef]
- Pereira, J.H.; Vilar, V.J.; Borges, M.T.; González, O.; Esplugas, S.; Boaventura, R.A. Photocatalytic degradation of oxytetracycline using TiO2 under natural and simulated solar radiation. Sol. Energy 2011, 85, 2732–2740. [Google Scholar] [CrossRef]
- Ahmadi, M.; Motlagh, H.R.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorfi, S. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Kan, E. Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. J. Environ. Manag. 2016, 180, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhao, L.; Luo, X.; Luo, S.; Dionysiou, D.D. Highly efficient visible-light photocatalytic performance of Ag/AgIn5S8 for degradation of tetracycline hydrochloride and treatment of real pharmaceutical industry wastewater. Chem. Eng. J. 2018, 333, 423–433. [Google Scholar] [CrossRef]
- Khodadadi, M.; Ehrampoush, M.H.; Ghaneian, M.T.; Allahresani, A.; Mahvi, A.H. Synthesis and characterizations of FeNi3@SiO2@TiO2 nanocomposite and its application in photo- catalytic degradation of tetracycline in simulated wastewater. J. Mol. Liq. 2018, 255, 224–232. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Song, J.; Zhang, G.; Li, C.; Zheng, S. Synthesis of novel ternary heterogeneous BiOCl/TiO2/sepiolite composite with enhanced visible-light-induced photocatalytic activity towards tetracycline. J. Colloid Interface Sci. 2019, 533, 238–250. [Google Scholar] [CrossRef]
- Oros-Ruiz, S.; Zanella, R.; Prado, B. Photocatalytic degradation of trimethoprim by metallic nanoparticles supported on TiO2-P25. J. Hazard. Mater. 2013, 263, 28–35. [Google Scholar] [CrossRef]
- Mehrotra, K.; Yablonsky, G.S.; Ray, A.K. Kinetic studies of photocatalytic degradation in a TiO2 slurry system: Distinguishing working regimes and determining rate dependences. Ind. Eng. Chem. Res. 2003, 42, 2273–2281. [Google Scholar] [CrossRef]
- Ghaly, M.Y.; Jamil, T.S.; El-Seesy, I.E.; Souaya, E.R.; Nasr, R.A. Treatment of highly polluted paper mill wastewater by solar photocatalytic oxidation with synthesized nano TiO2. Chem. Eng. J. 2011, 168, 446–454. [Google Scholar] [CrossRef]
- Carabin, A.; Drogui, P.; Robert, D. Photocatalytic Oxidation of Carbamazepine: Application of an Experimental Design Methodology. Water Air Soil Pollut. 2016, 227, 122. [Google Scholar] [CrossRef]
- dos Santos, M.C.; de Mello Oehninger, I.; Willig, J.C.M.; da Rosa, M.F. Utilização de Fotocatálise Heterogênea para a Degradação de Contaminantes Emergentes: Cloridrato de Norfloxacino. Revista de Química Industrial. 2017. Available online: https://www.abq.org.br/rqi/2014/758/RQI-758-pagina25-Artigo-Tecnico.pdf (accessed on 24 April 2024).
- Montagner, C.C.; Vidal, C.; Acayaba, R.D. Contaminantes emergentes em matrizes aquáticas do Brasil: Cenário atual e aspectos analíticos, ecotoxicológicos e regulatórios. Química Nova 2017, 40, 1094–1110. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Beatriz, B.; Tonin, F.; Zamora, P.; Wagner, R.; Gomes, E. Micropoluentes emergentes de origem farmacêutica em matrizes aquosas do Brasil: Uma revisão sistemática. Ciência Nat. 2015, 37, 725–739. [Google Scholar]


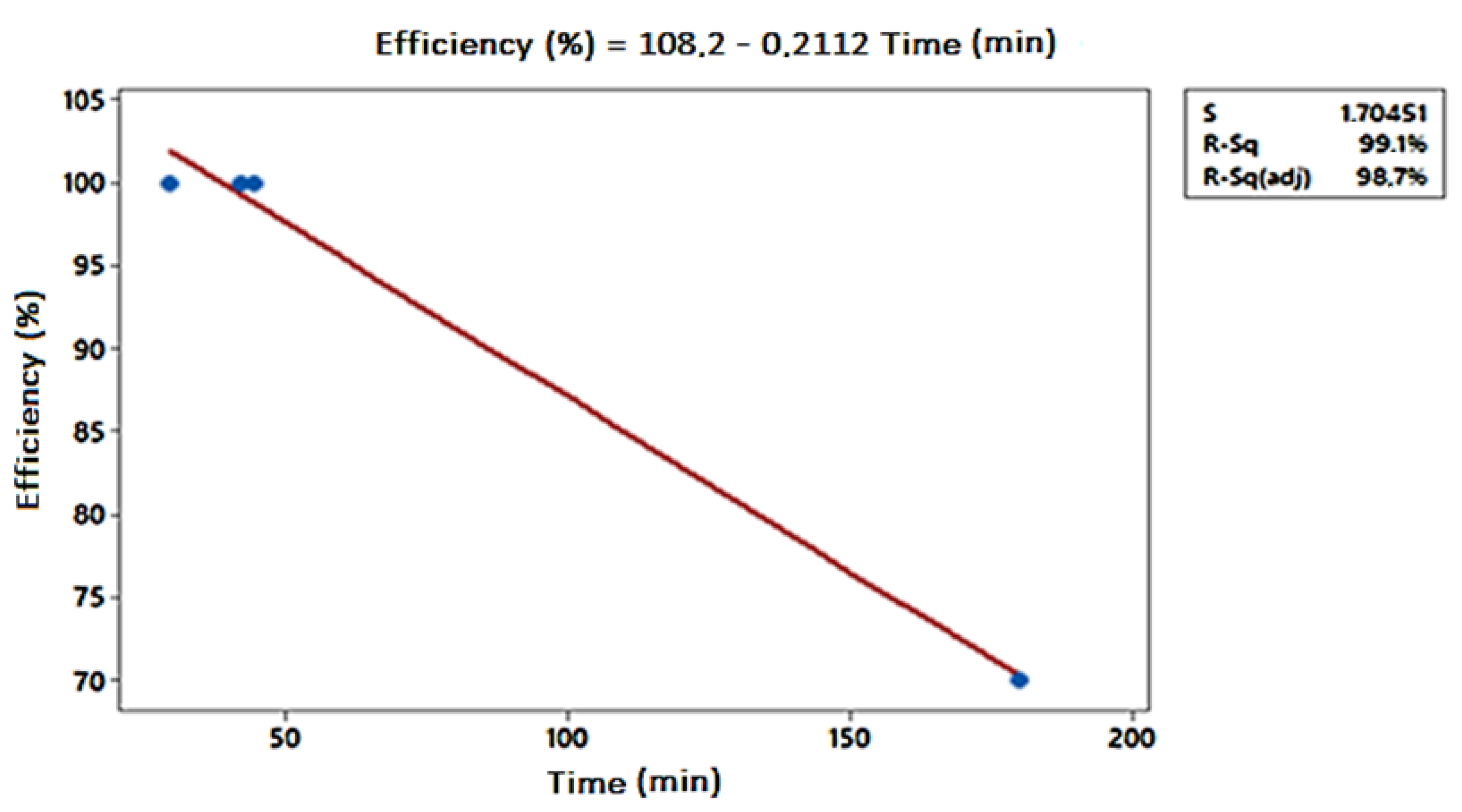
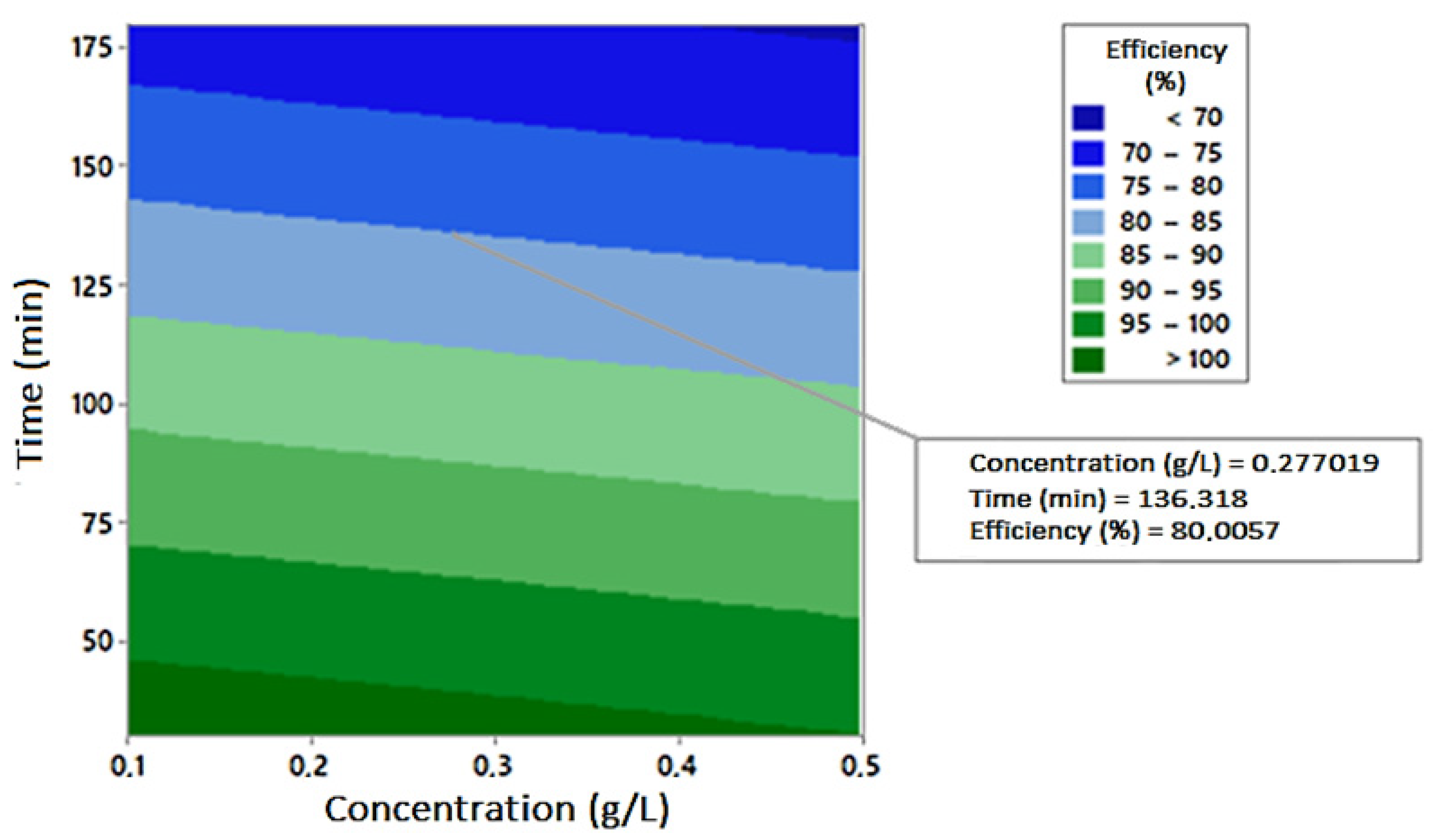
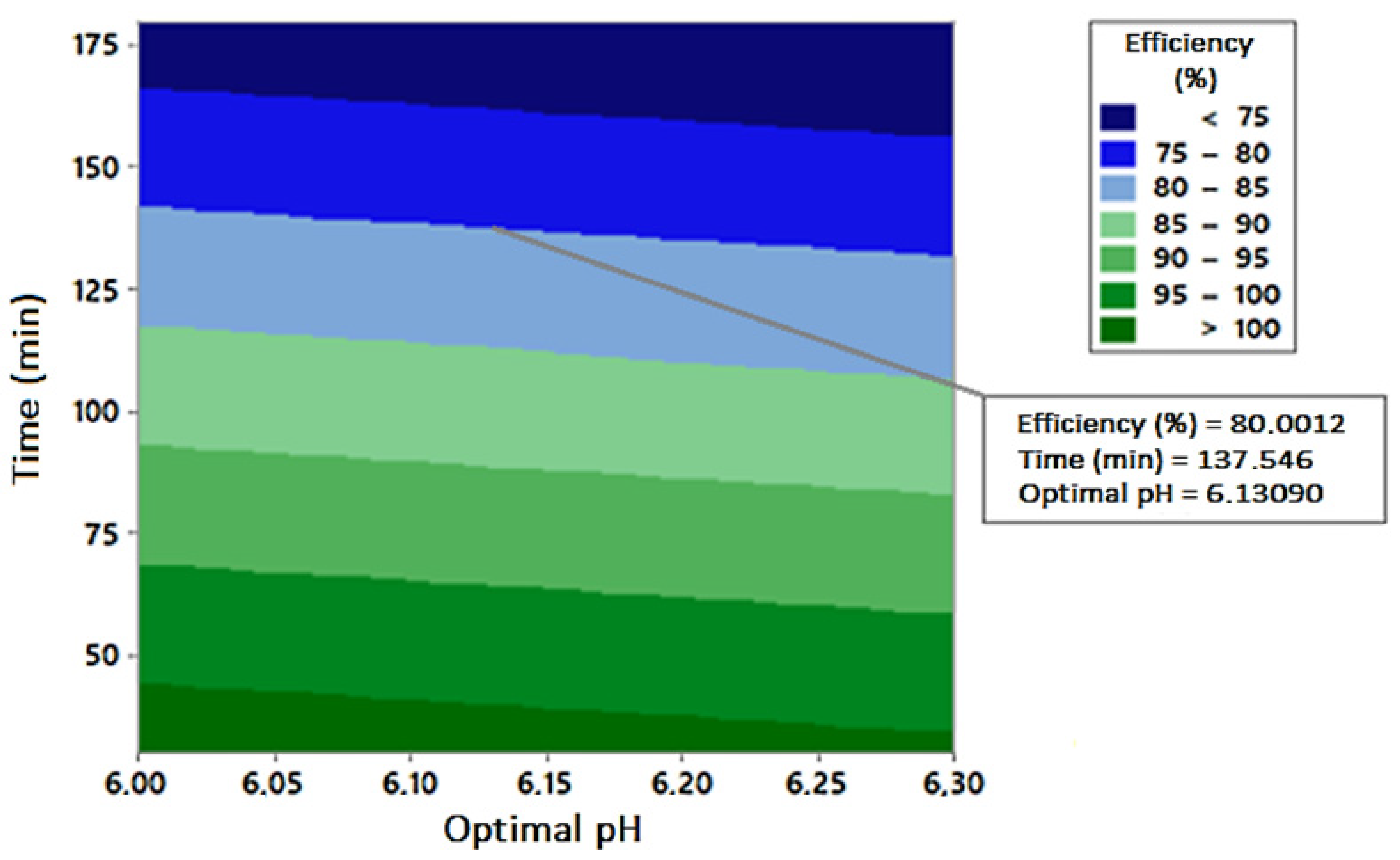
| Compounds | Operational Conditions | Removal Rate (%)—Time (min) | References | |
|---|---|---|---|---|
| ANTIHYPERTENSIVES | Atenolol | λ = 365 nm; LA (1); SINT (2) and AR (3); TiO2 (2.0 g/L); pH 6.8; [C] (4) 37.6 μM | SINT 100%—60 min AR 100%—180 min | [19] |
| Atenolol | λ = 290–400 nm; LA; SINT; bismuth oxychloride (0.4 g/L); pH 5; [C] 10 mM | 100% < 50 min | [20] | |
| Metoprolol | λ = 280 nm; LA; SINT and ES; 5%B-TiO2 (0.4 g/L AU; 2.0 g/L ES); pH 6.3; [C] 50 mg/L | SINT 70%—180 min ES 44%—180 min | [21] | |
| Metoprolol and propranolol | λ = 290–400 nm; LA; SINT; TiO2 (0.4 g/L); pH 4.2 and 5.7; [C] 50 mg/L | 100%—300 min 100%—360 min | [22] | |
| Propranolol | λ = 290–400 nm; LA; SINT and ES (5); 5%Ce-TiO2 (0.14 g/L); pH 7.5; [C] 25 mg/L | SINT 100%—90 min ES 100%—360 min | [23] | |
| Propranolol | λ = 280 nm; LA and natural light; SINT; TiO2 (0.4 g/L); [C] 50 mg/L | Artificial 94%—240 min Solar 81%—240 min | [24] | |
| Antipyrine | λ = 300–400 nm; LA; SINT; Zr-TiO2 (0.25 g/L); [C] 10 mg/L | 90%—360 min | [25] | |
| Acetaminophen | λ = 254 nm; LA; SINT; 40% TiO2/zeolite (1.0 g/L); pH 6.4; [C] 15 mg/L | 96%—180 min | [26] | |
| Acetaminophen | λ = 800–200 nm; LA; SINT; BaTiO3/TiO2 (1 g/L); pH 7; [C] 5 mg/L | 95%—240 min | [27] | |
| Acetaminophen and antipyrine | λ = 320 nm; LA; SINT; TiO2-0.5%ZnO/clay (0.25 g/L); [C] 10 mg/L | 95%—360 min | [28] | |
| ANTI-INFLAMMATORY ANALGESICS | Diclofenac | λ = 366 nm; LA; SINT; TiO2 anatase (0.5 g/L); pH 6.22; [C] 8 mg/L | 100%—30 min | [29] |
| Diclofenac and fluoxetine | λ = 365 nm; LA; ES; hydroxyapatite (4 g/L); pH 6.1; [C] 5 mg/L | 92%—24 h 100%—24 h | [30] | |
| Ibuprofen; acetaminophen; antipyrine | λ = 320 nm; LA; SINT; ZnO/sepiolite (0.25 g/L); [C] 10 mg/L | 100%—600 min 85%—600 min 70%—600 min | [31] | |
| Ibuprofen | λ = 382 nm; LA; SINT and FARM (6); TiO2 (1.0 g/L); pH 5.3; [C] 213 mg/L | SINT—100%—30 min FARM—18%—30 min | [32] | |
| Ibuprofen Naproxen | λ = 380–900 nm; LA; SINT; nanoparticles and nanosheets; N, S-TiO2; (2 g/L); pH 6.0; [C] 2.5–10 mg/L | NanoP 85%, NanoS 71%—90 min; NanoP 99%, NanoS 99%—90 min | [33] | |
| Ketorolac tromethamine | Natural light; SINT; TiO2/quantum dots (0.5 g/L); pH 4.4; [C] 750 mg/L | 99%—120 min | [34] | |
| Naproxen | λ = 200–600 nm; LA; SINT; TiO2 (0.1 g/L); [C] 30 mg/L | 100%—25 min | [35] | |
| Nimesulide | λ = 290 nm; LA; SINT; TiO2 (0.1 g/L); pH 6.0; [C] 5 mg/L | 100%—45 min | [36] | |
| Paracetamol | λ = 200–800 nm; LA; SINT; 3%WO3/TiO2/SiO2 (1.5 g/L); pH 9; [C] 5 mg/L | 95%—240 min | [37] | |
| Paracetamol | λ = 420 nm; LA; SINT; 0.1%Cu-TiO2 (4 g/L); pH 6.0; [C] 50 mg/L | 100%—180 min | [38] | |
| Paracetamol | LA, ES; TiO2/glass (0.50 g/L); [C] 51 mg/L | 100%—240 min | [39] | |
| Phenazopyridine | λ = 400 nm; LA; SINT; graphene oxide hydroxide/sulfate (0.015 mg/L); pH 8.0; [C] 15 mg/L | 60%—150 min | [40] | |
| Salicylic acid; ibuprofen; naproxen; diclofenac | SINT; natural light, P25-TiO2 tetraethyl orthosilicate; (3 mg); pH 6.0; [C] 5 mg/L | 76%—600 min 85%—600 min 94%—600 min 65%—600 min | [41] | |
| ANTIBIOTICS | Tetracycline | λ = 253–2500 nm; LA and natural; SINT; metal–organic structure Fe/TiO2 (1 g/L); pH 7; [C] 20 mg/L | Natural (solar) 92.76%—10 min Visible 91.24%—360 min | [42] |
| Levofloxacin | λ = 400–520 nm; LA; SINT; bismuth tungstate (0.1 g/L); pH 7; [C] 750 mg/L | 80%—180 min | [43] | |
| Amoxicillin | λ = 200 and 600 nm; LA; SINT; integrated photocatalytic adsorbents/TiO2/zeolite (2 g/L); [C] 30 mg/L | 88%—240 min | [44] | |
| Amoxicillin | λ = 350–400 nm; LA; SINT e ES; TiO2 Degussa P25 (0.25 g/L); pH 5 (AUP); 7.5 (ES); [C] 10 mg/L (0.2 g/L); pH 5; [C] 10 mg/L | SINT 100%—25 min ES 100%—60 min | [45] | |
| Amoxicillin Cloxacillin Ampicillin | λ = 365 nm; LA; SINT; TiO2 (1 g/L); pH 5.0; [C] 104 AMOX, 105 CLOX, 103 AMP mg/L | 100%—30 min | [46] | |
| Azithromycin | λ = 254–185 nm; LA UV-C; SINT and ES; TiO2/glass; pH 7; [C] 10 mg/L | SINT 100%—30 min ES 95%—120 min | [47] | |
| Cefixime | λ = 320–780 nm; LA; SINT; mixed-binder MIL-125 (Ti)/nanocomposite g-C3N4 (0.3 g/L); pH 4.0; [C] 20 mg/L | 985—120 min | [48] | |
| Isoniazid; metronidazole; sulfadiazine; sulfamethoxazole; trimethoprim; norfloxacin; moxifloxacin; lincomycin | Natural light, LA; SINT and ES; ethylene terephthalate–TiO2 (0.5 g/L); [C] 1 mg/L | SINT 100%; ES 90%—10 h SINT 100%; ES 90%—10 h SINT 100%; ES 80%—14 h SINT 100%; ES 80%—14 h SINT 100%; ES 70%—14 h SINT 100%; ES 90%—10 h SINT 100%; ES 90%—10 h SINT 100%; ES 90%—10 h | [49] | |
| Oxytetracycline | λ = 420 nm; LA; SINT; TiO2-Ag (0.5 g/L); pH 5.8; [C] 0.5 mg/L | 100% UV-Vis—60 min 100% Visible—360 min | [50] | |
| Oxytetracycline | λ = 290–400 nm; LA and natural light; SINT; TiO2 (0.5 g/L); [C] 20 mg/L | Artificial 95%—35 min Solar 95%—35 min | [51] | |
| Tetracycline | λ = 240 nm; LA; FARM; multi-walled carbon nanotubes/TiO2 | 100%—100 min | [52] | |
| Tetracycline hydrochloride | λ = 254 nm; LA; SINT; TiO2/biochar (0.5 g); pH 4; [C] 5 mg/L | 91%—360 min | [53] | |
| Tetracycline hydrochloride | λ = 400 nm; LA; FARM; Ag/AgIn5S8 (0.3 g/L); [C] 10 mg/L | 95%—120 min | [54] | |
| Tetracycline | λ = 254 nm; LA; SINT; FeNi3/SiO2/TiO2 (0.005 g/L); pH 9.0; [C] 10 mg/L | 100%—200 min | [55] | |
| Tetracycline | λ = 400 nm; LA; ES; BiOCl/TiO2/sepiolite (60 mg/100 mL); [C] 10μM | 92%—180 min | [56] | |
| Trimethoprim | λ = 254 nm; LA; SINT; TiO2-P25-doped Au/Ag/Cu/Ni (0.25 g/250 mL); [C] 40 mg/L | 100%—40 min | [57] |
| Pollutants | Radiation Source | Photocatalyst | Catalyst Dose Range (g/L) | Optimal Dosage (g/L) | Efficiency (%) | References |
|---|---|---|---|---|---|---|
| Amoxicillin and ampicillin | UV | TiO2 | 0.5–2 | 1.0 | 100 | [46] |
| Atenolol | UV | BiOCl | 0.05–0.4 | 0.3 | 100 | [56] |
| Cefixime | UV | MIL-125 (Ti)/ g-C3N4 | 0.01–0.04 | 0.03 | 98 | [48] |
| Diclofenac | UV-Vis | TiO2 anatase P25 | 0.1–2 | 0.5 | 100 | [29] |
| Diclofenac Fluoxetine | UV | Hydroxyapatite | 1–4 | 4.0 | 92 100 | [30] |
| Ibuprofen | LED | TiO2 | 0.5–1.5 | 1.5 | SYNT 1—100 SE 2—18 | [32] |
| Ibuprofen Naproxen | UV | N, S-TiO2 | 0.5–5 | 2.0 | 78 99 | [33] |
| Naproxen | UV | TiO2 | 0.01–2 | 0.1 | 100 | [35] |
| Nimesulide | UV | TiO2 | 0.05–0.4 | 0.1 | 100 | [36] |
| Propranolol | Solar UV | TiO2 Degussa P25 | 0.1–0.4 | 0.4 | 81 94 | [24] |
| Tetracycline | UV | MWCNTs/TiO2 | 0.1–0.4 | 0.2 | 100 | [52] |
| Pollutant | Radiation Source | Photocatalyst | Catalyst Dose Range (g/L) | Optimal Dosage (g/L) | Efficiency (%) | References |
|---|---|---|---|---|---|---|
| Acetaminophen | UV | TiO2/zeolite | 0.0075–0.06 | 0.015 | 96 | [26] |
| Amoxicillin | UV | TiO2 Degussa P25 | 0.0025–0.03 | 0.01 | SYNT 1 100 SE 2 100 | [45] |
| Cefixime | UV | MIL-125 (Ti)/g-C3N4 | 0.015–0.025 | 0.02 | 98 | [48] |
| Diclofenac and fluoxetine | UV | Hydroxyapatite | 0.002–0.008 | 0.005 | 92 100 | [30] |
| Ketorolac tromethamine | Solar | TiO2/quantum dots | 0.01–0.04 | 0.01 | 80 | [34] |
| Phenazopyridine | LED | Graphene oxide/sulfate/hydroxide | 0.005–0.025 | 0.015 | 60 | [40] |
| Tetracycline | UV | MWCNTs/TiO2 | 0.0005–0.03 | 0.01 | 100 | [52] |
| Class/Pollutant | Raw Effluent | Treated Effluent | Surface Water | Tap Water | Groundwater |
|---|---|---|---|---|---|
| Pharmaceuticals | 13.9–3800 ng/L | 680–3800 ng/L | 0.50–30.421 ng/L | 18.5 ng/L | - |
| Analgesics and anti-inflammatories | - | 60.00 µg/L | 5.00 µg/L | 0.12 µg/L | - |
| β blockers | - | 9.00 µg/L | 2.00 µg/L | 0.27 µg/L | - |
| Antibiotics | - | 6.00 µg/L | 1.90 µg/L | - | 0.20 µg/L |
| Paracetamol | - | 0.13 µg/L | 0.84 µg/L | - | - |
| Acetaminophen | - | - | 0.01 µg/L | - | - |
| Diclofenac | - | 0.27 µg/L | 0.10 µg/L | - | - |
| Atenolol | - | - | 0.98 µg/L | - | - |
| Ibuprofen | - | 0.23 µg/L | 0.50 µg/L | - | - |
| Naproxen | - | 0.07 µg/L | 0.39 µg/L | - | - |
| Propranolol | - | - | 0.04 µg/L | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, R.C.d.; Cahino, A.; Lucena, L.G.; Barbosa Segundo, I.D.; Espíndola, J.C.; Rocha, E.M.R. A Systematic Review of Heterogeneous Catalysis Applied to the Treatment of Pharmaceutical Wastewater: Operational Conditions and Statistical Analysis. Photochem 2024, 4, 285-301. https://doi.org/10.3390/photochem4030017
Nascimento RCd, Cahino A, Lucena LG, Barbosa Segundo ID, Espíndola JC, Rocha EMR. A Systematic Review of Heterogeneous Catalysis Applied to the Treatment of Pharmaceutical Wastewater: Operational Conditions and Statistical Analysis. Photochem. 2024; 4(3):285-301. https://doi.org/10.3390/photochem4030017
Chicago/Turabian StyleNascimento, Raqueline Caldas do, Arthur Cahino, Larissa Granjeiro Lucena, Inalmar D. Barbosa Segundo, Jonathan Cawettiere Espíndola, and Elisângela M. R. Rocha. 2024. "A Systematic Review of Heterogeneous Catalysis Applied to the Treatment of Pharmaceutical Wastewater: Operational Conditions and Statistical Analysis" Photochem 4, no. 3: 285-301. https://doi.org/10.3390/photochem4030017
APA StyleNascimento, R. C. d., Cahino, A., Lucena, L. G., Barbosa Segundo, I. D., Espíndola, J. C., & Rocha, E. M. R. (2024). A Systematic Review of Heterogeneous Catalysis Applied to the Treatment of Pharmaceutical Wastewater: Operational Conditions and Statistical Analysis. Photochem, 4(3), 285-301. https://doi.org/10.3390/photochem4030017







