Abstract
The global demand for sustainable energy sources has led to extensive research regarding (green) hydrogen production technologies, with water splitting emerging as a promising avenue. In the near future the calculated hydrogen demand is expected to be 2.3 Gt per year. For green hydrogen production, 1.5 ppm of Earth’s freshwater, or 30 ppb of saltwater, is required each year, which is less than that currently consumed by fossil fuel-based energy. Functional ceramics, known for their stability and tunable properties, have garnered attention in the field of water splitting. This review provides an in-depth analysis of recent advancements in functional ceramics for water splitting, addressing key mechanisms, challenges, and prospects. Theoretical aspects, including electronic structure and crystallography, are explored to understand the catalytic behavior of these materials. Hematite photoanodes, vital for solar-driven water splitting, are discussed alongside strategies to enhance their performance, such as heterojunction structures and cocatalyst integration. Compositionally complex perovskite oxides and high-entropy alloys/ceramics are investigated for their potential for use in solar thermochemical water splitting, highlighting innovative approaches and challenges. Further exploration encompasses inorganic materials like metal oxides, molybdates, and rare earth compounds, revealing their catalytic activity and potential for water-splitting applications. Despite progress, challenges persist, indicating the need for continued research in the fields of material design and synthesis to advance sustainable hydrogen production.
1. Introduction
The continuously growing global demand for sustainable and renewable energy sources has spurred many research and development (R&D) activities in academia and industry with respect to efficient technologies for hydrogen production. In the near future the calculated hydrogen demand is expected to be 2.3 Gt per year [1,2]. For green hydrogen production, 1.5 ppm of Earth’s freshwater, or 30 ppb of saltwater, is required each year, which is less than that currently consumed by fossil fuel-based energy. Among the many methods for producing hydrogen, water splitting has emerged as the green avenue in this quest [3,4]. Among the various materials explored for water splitting, functional ceramics have gained significant attention due to their unique combination of properties, including high chemical stability, robust mechanical strength, and tunable electronic and optical characteristics [5,6,7,8,9,10]. This review aims to provide a comprehensive overview of the recent advancements in the field of functional ceramics for water splitting applications, shedding light on the key mechanisms, challenges, and prospects associated with this burgeoning research area. Hydrogen, as a clean and versatile energy carrier, holds immense potential for addressing the escalating energy and environmental challenges facing the biosphere of our planet. Water splitting, a process wherein water molecules are dissociated into hydrogen and oxygen gases, is a particularly attractive method for hydrogen production [11]. While various approaches, such as photoelectrochemical cells and electrolysis, have been explored for water splitting, the choice of materials plays a pivotal role in determining the overall efficiency and viability of these technologies [12,13,14]. Functional ceramics, with their diverse range of compositions and inherent catalytic properties, have emerged as frontrunners in the race to develop cost-effective, sustainable, and thus, long-term running water splitting devices [15,16,17]. The unique electronic structure and catalytic activity of functional ceramics, such as perovskites, oxides, and nitrides, enable them to effectively participate in the intricate electrochemical reactions involved in water splitting [15]. Figure 1 depicts an exemplary functional ceramic. In this context, this review will explore recent breakthroughs in the design and synthesis of functional ceramics, highlighting innovative approaches to enhance their stability, conductivity, and overall efficiency in water-splitting applications. Despite the rapid progress in this field, several challenges persist, ranging from limited long-term stability to the scarcity of certain raw materials. The process is hindered by unfavorable thermodynamics; slow reaction kinetics, particularly for the oxygen evolution reaction (OER); the presence of dissolved oxygen; and significant contributions of backward reactions. Consequently, producing hydrogen through photocatalytic water splitting remains difficult, both now and in the foreseeable future [18]. This review will critically examine these challenges, offering insights into ongoing research efforts aimed at overcoming these hurdles.
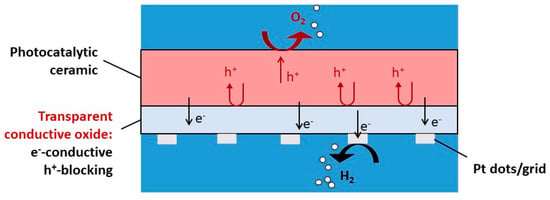
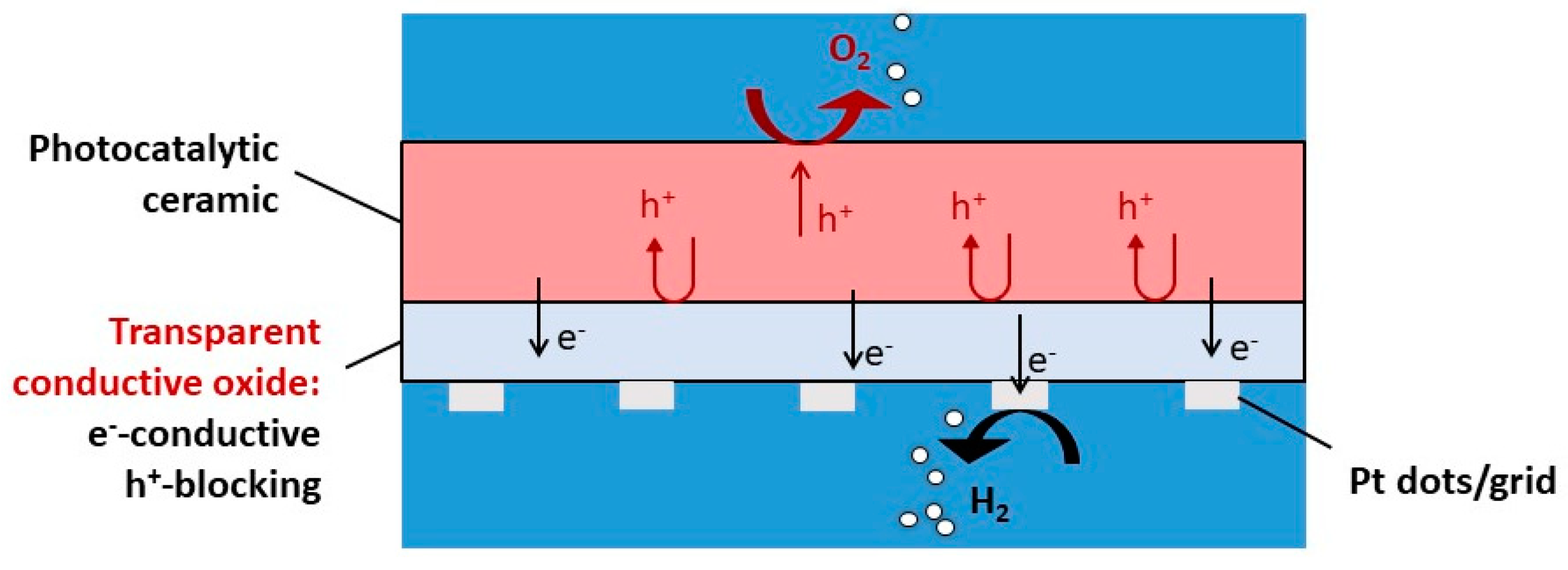
Figure 1.
Sketch of a functional ceramic for water splitting.
2. Theoretical Aspects
The theoretical underpinnings of functional ceramics for water splitting involve a nuanced exploration of various facets, each contributing to the overall efficacy of these materials in catalyzing the essential electrochemical reactions. At the heart of water splitting lies the OER and the hydrogen evolution reaction (HER), both orchestrated at the surface of catalyst materials [19].
HER: 2 H+ + 2 e− → H2 [20]
OER: 2 H2O + 4 H+ → O2 + 4 e− [21]
A fundamental grasp of these mechanisms is imperative for optimizing the catalytic prowess of functional ceramics in the realm of water-splitting applications. Electronic structure is at the forefront of theoretical considerations. Band engineering, a technique that tailors the electronic band structure through controlled doping or alloying, emerges as a key player in augmenting water-splitting efficiency [22,23,24,25,26,27,28,29,30,31,32,33].
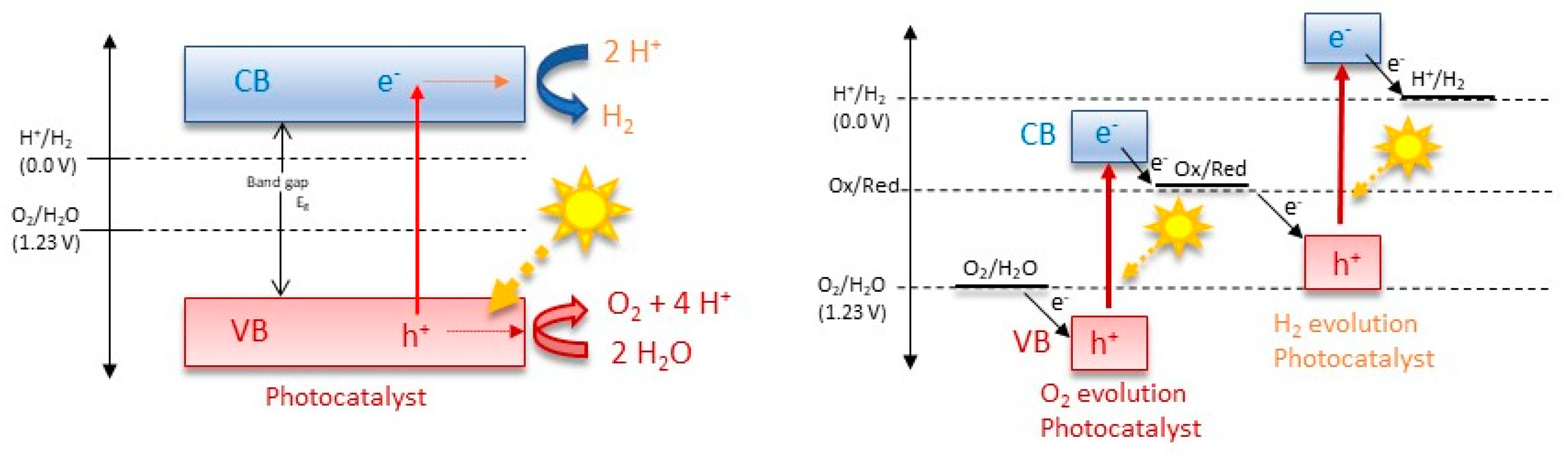
Overall, there are two main mechanisms of photocatalysis towards water splitting: the one-step and the two-step photoexcitation system (Figure 2). Semiconductor photocatalysts designed for water splitting via single-step photoexcitation must possess conduction band minimum (CBM) and valence band maximum (VBM) levels that align with the H+/H2 and O2/H2O redox potentials. An alternative and increasingly popular method is two-step photoexcitation, which combines two different photocatalysts that separately generate H2 and O2. This technique, inspired by natural photosynthesis, is known as Z-scheme water splitting. In this process, photogenerated electrons in the hydrogen-evolving photocatalyst (HEP) reduce H+ to H2, while holes in the oxygen-evolving photocatalyst (OEP) oxidize H2O to O2. The photoexcited electrons and holes remain in the OEP and HEP, respectively, and their recombination, facilitated by either an aqueous redox mediator or a solid-state electron mediator, completes the photocatalytic cycle [3]. An exemplary representation of a possible electron–hole recombination can be seen in Figure 3. Theoretical models, such as the density functional theory (DFT) model, illuminate the electronic configurations of these ceramics, affording predictive insights into their catalytic behavior and the need for nanoscale control over the catalyst composition and morphology [34,35,36,37,38,39]. Crystal structure, intimately intertwined with surface properties, controls the reactivity of functional ceramics. Theoretical simulations, employing methodologies like Monte Carlo simulation and molecular dynamics, unravel the dynamic behavior of these materials under the rigors of water-splitting conditions, shedding light on the intricate relationship between crystal facets, defects, and catalytic activity [40]. Central to the theoretical framework is the identification and characterization of catalytically active sites on the ceramic surface. Quantum mechanical calculations and ab initio simulations navigate the energetic landscapes of reaction pathways, aiding in the strategic design of catalysts with heightened activity and selectivity [41]. Efficient ion and charge transport within functional ceramics form the bedrock for high-performance water-splitting devices. Theoretical models, including the Nernst–Planck equation and kinetic Monte Carlo simulations, unveil insights into the diffusion and migration of ions and electrons, paving the way for materials with improved conductivity and stability [40,42]. In summation, the theoretical landscape of functional ceramics for water splitting is expansive, traversing electronic structure, crystallography, and electrochemical processes. The symbiosis of theoretical models with experimental validation emerges as a linchpin for advancing our comprehension and steering the rational design of functional ceramics, poised to catalyze sustainable hydrogen production.
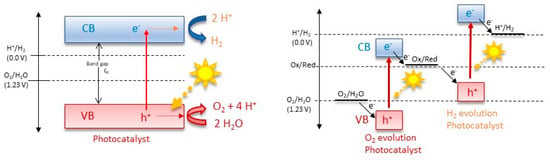
Figure 2.
Mechanisms of one-step and two-step photocatalysis towards water splitting.
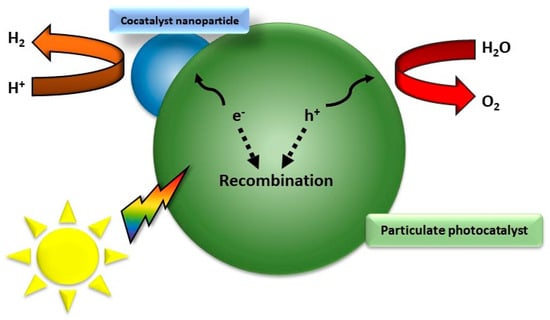
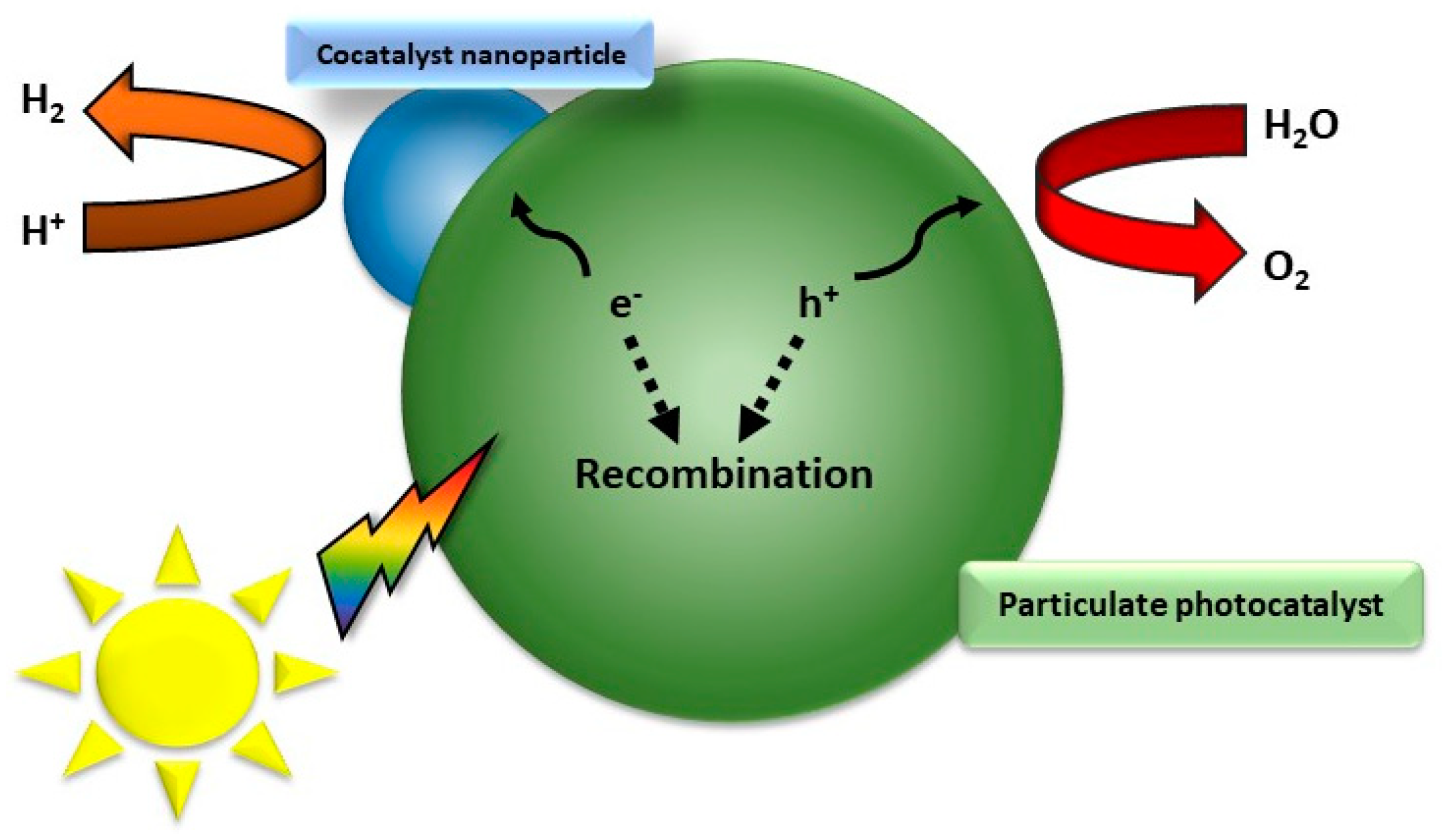
Figure 3.
Recombination process of a particulate photocatalyst.
The influence of ceramics on the mechanical properties of materials, particularly dislocation-based mechanics, is an area that requires further exploration, particularly in understanding the effect of point defects such as vacancies and interstitials on the mechanical properties of ceramics [43]. Ceramic materials are characteristically brittle, with low tensile strength, poor plasticity, and limited toughness [44]. Additionally, conventional single-structure ceramics suffer from high density, limited specific surface area, and poor functionality. These drawbacks have compromised their reliability in engineering applications and significantly restricted their use in the field [45,46]. As a typical inorganic nonmetallic material, ceramics foster advantages such as a high melting point, high hardness, excellent wear resistance, and corrosion resistance [47,48]. Moreover, functional ceramics have found utility in biomimetic modified ceramics and ceramic composites, leveraging the diverse range of ceramic raw materials and their excellent thermal, magnetic, and optical properties to prepare porous ceramics with a wide range of functionalities for various applications [49].
3. The OER Process
The OER process is the key half-reaction in overall water splitting, as it involves a four-electron transfer. The process requires a high overpotential in comparison to the HER process [50]. Ru- and Ir-based materials are the current state of the art. RuO2 and IrO2 are widely recognized for their high efficiency, stability, and durability under harsh electrochemical conditions [51,52]. However, due to their high cost and limited availability, extensive research has focused on developing alternative materials with comparable performance. Strategies such as nanostructuring, alloying, and surface modification have been explored to enhance the catalytic properties of these materials while reducing the usage of precious metals.
4. Photoanodes for Water Splitting
Hematite (α-Fe2O3) has emerged as a leading candidate for photoelectrochemical water splitting due to its stability and potential for solar-driven hydrogen production. The development of efficient hematite photoanodes is crucial for advancing renewable energy technologies. Hematite, as a semiconductor material, possesses favorable properties for photoelectrochemical water splitting. However, its practical application has been hindered by challenges such as limited charge carrier diffusion lengths, sluggish charge transfer kinetics, and poor conductivity.
To address these limitations, extensive research has been conducted to enhance the performance of hematite photoanodes through various strategies. One approach involves the modification of hematite photoanodes with heterojunction structures. For instance, the in situ synthesis of α-Fe2O3/Fe3O4 heterojunction photoanodes via fast flame annealing has been shown to enhance charge separation and water oxidation efficiency [53]. Additionally, the formation of n-Fe2O3-TiO2 heterojunctions has been demonstrated to suppress charge recombination, leading to improved photoelectrochemical behavior [54]. Furthermore, the integration of cocatalysts onto hematite surfaces has been explored to facilitate water oxidation. For example, surface-oxidized titanium diboride (TiB2) has been utilized as a cocatalyst on hematite photoanodes, aiming to accelerate the kinetics of oxidative water splitting and inhibit electron-hole recombination [55].
Similarly, the modification of hematite photoanodes with cobalt-based oxygen evolution catalysts has been investigated to enhance water-splitting efficiency [56]. In addition to structural modifications, the influence of surface passivation and nanoparticle decoration on hematite photoanodes has been a subject of interest. Ternary hematite nanocomposite structures with fullerene and 2D-electrochemical reduced graphene oxide have been developed to achieve superior photoelectrochemical performance [57]. Moreover, the influence of the magnetic field and nanoparticle concentration on the thin film colloidal deposition process of magnetic nanoparticles has been studied to optimize the efficiency of hematite photoanodes [58]. The development of efficient hematite photoanodes also involves understanding the influence of external factors such as halides and co-catalysts. The influence of sodium halides on the photocatalytic performance of hydrothermally synthesized hematite photoanodes has been investigated, emphasizing the critical role of surface ion loading at the electrolyte-hematite interface [59]. Additionally, the stable and efficient photoelectrochemical water splitting of GaN nanowire photoanodes coated with Au nanoparticles has been explored, highlighting the potential of hot-electron-assisted transport in enhancing water splitting efficiency [60].
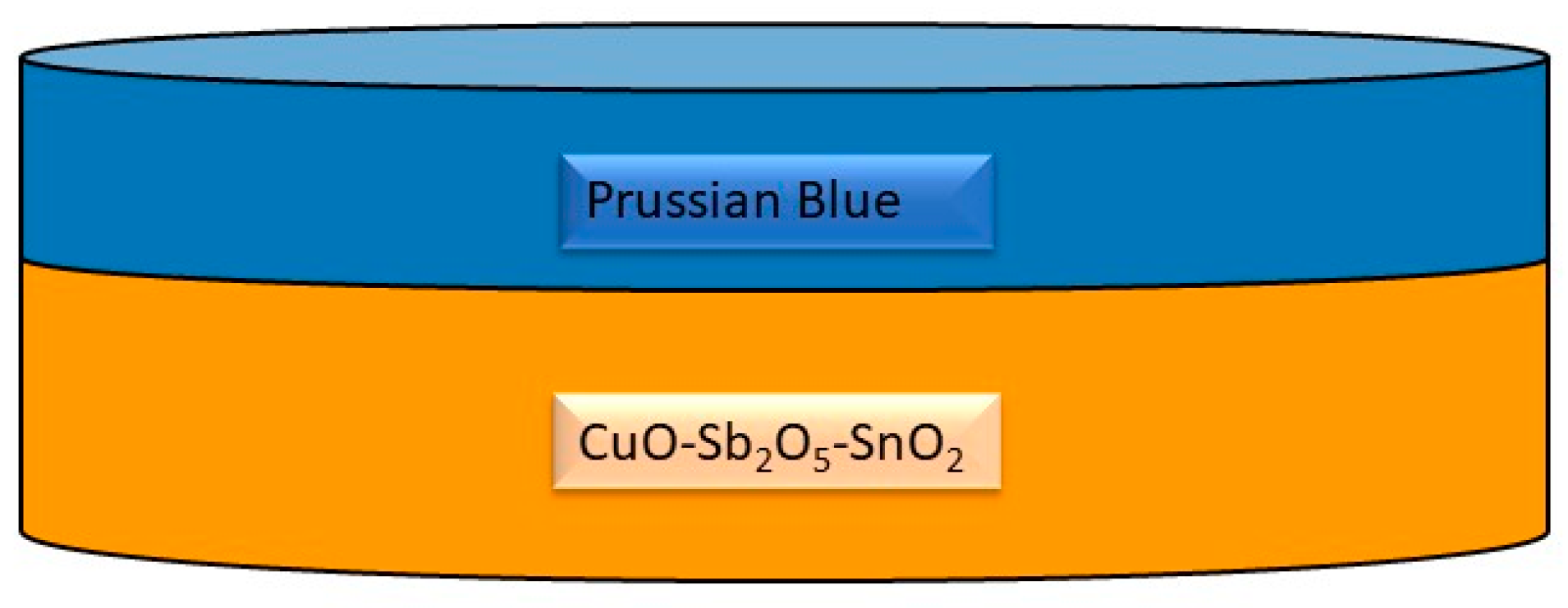
Despite significant progress, challenges remain in achieving high-performance hematite photoanodes for water splitting. The co-dependency of TiO2 underlayers and ZrO2 top layers in sandwiched microwave-assisted Zr-Fe2O3 photoanodes has been studied to address electron–hole recombination pathways that limit the photoelectrochemical capabilities of hematite photoanodes [61]. Furthermore, the origin of photocurrent enhancement in hematite photoanodes decorated with gold nanoparticles has been investigated, shedding light on microstructural characterizations and performance improvements [62]. The study “Hematite Photoanodes for Water Splitting from Directed Assembly of Prussian Blue onto CuO–Sb2O5–SnO2 Ceramics” outlines the development of hematite photoanodes on porous CuO–Sb2O5–SnO2 ceramics for photoelectrochemical water splitting (Figure 4) [63]. This research demonstrates the controlled layer-by-layer growth of Prussian blue to create a functional hematite coating on the grain surfaces of the ceramics, which enhances the efficiency of water splitting [64]. Such an approach is significant, as it presents a potential solution for solar-driven water splitting, a critical process for renewable energy generation [65]. The use of Prussian blue in this context is notable due to its unique properties. Prussian blue is a three-dimensional cubic polymeric porous network consisting of alternating ferric and ferrous ions, allowing for facile assembly and precise interaction with active sites at functional interfaces [66]. Additionally, Prussian blue analogues have been utilized in photocatalytic water oxidation, demonstrating their potential as earth-abundant and efficient water oxidation catalysts [67]. Furthermore, the incorporation of Prussian blue structures for sensitization of TiO2 and water oxidation catalysis has been established, highlighting its versatility in photoelectrode applications [68]. The combination of CuO–Sb2O5–SnO2 ceramics with Prussian blue for the fabrication of hematite photoanodes represents a significant advancement in the field of photoelectrochemical water splitting. The controlled growth of hematite on these ceramics, facilitated by the directed assembly of Prussian blue, offers a promising approach for the development of efficient and stable photoanodes for renewable energy generation.
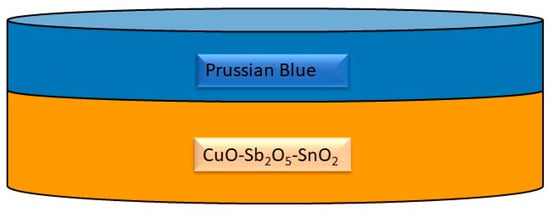
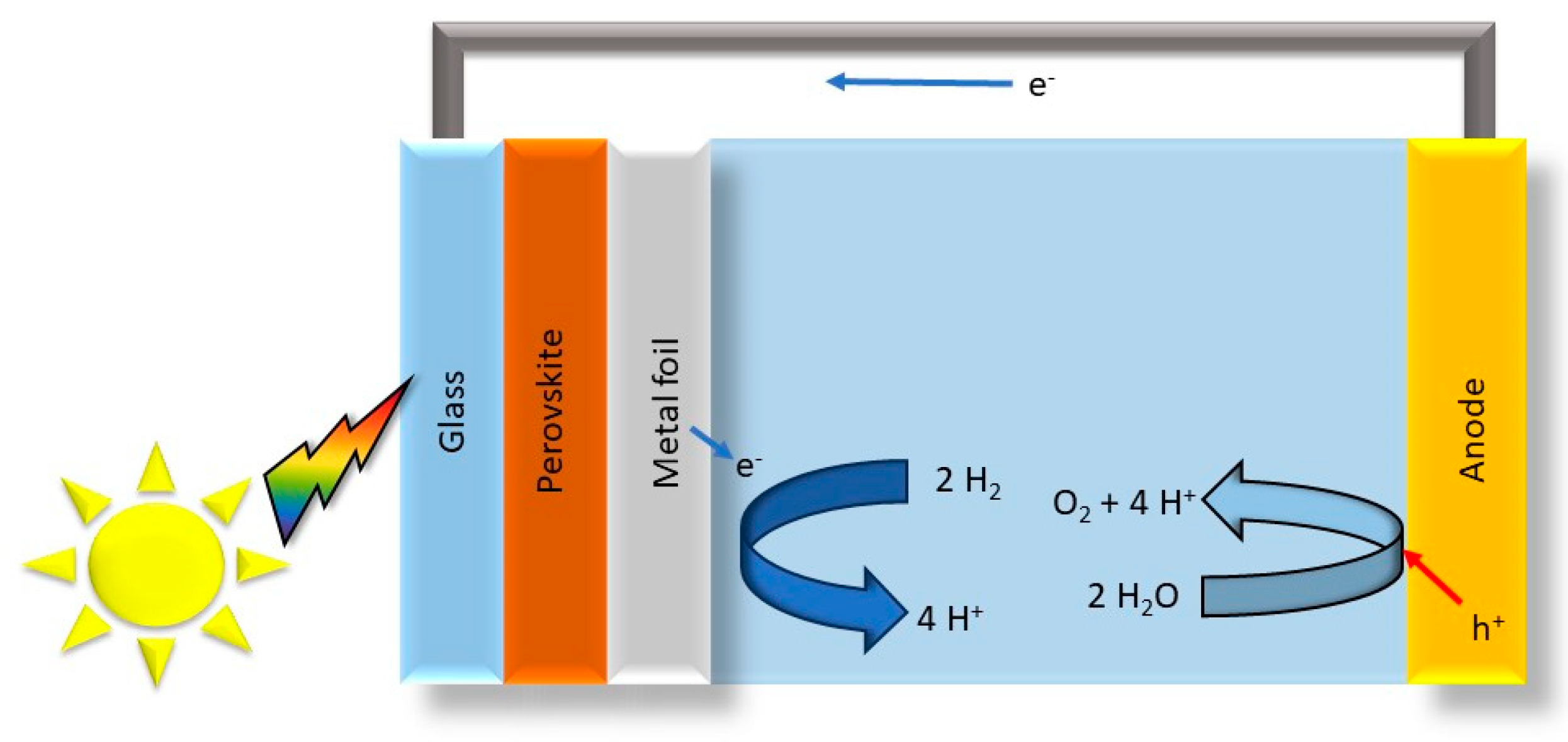
Figure 4.
Sketch of a Prussian blue-coated OER ceramic [63].
Figure 4.
Sketch of a Prussian blue-coated OER ceramic [63].
5. Compositionally Complex Perovskite Oxides
The quest for a sustainable and efficient solar-driven water splitting process has led to significant research regarding the development of compositionally complex perovskite oxides. Compositionally complex perovskite oxides have garnered attention due to their potential in solar-driven water splitting applications. The study by Zhang et al. explores a new class of compositionally complex perovskite oxides, (La0.8Sr0.2)(Mn(1−x)/3Fe(1−x)/3CoxAl(1−x)/3)O3, with non-equimolar designs for solar thermochemical water splitting [69]. This research expands upon the nascent high-entropy ceramics field, demonstrating the potential of compositionally complex perovskite oxides in advancing solar-driven water splitting technologies. The non-equimolar designs of the perovskite oxides offer a novel approach to enhancing solar thermochemical water splitting, presenting opportunities for the development of efficient and sustainable energy conversion systems. The bifunctional nature of perovskite oxides is highlighted in the work of Pornrungroj et al., where a bifunctional perovskite-BiVO4 tandem device achieved efficient solar-to-hydrogen conversion under simulated solar irradiation [70]. This demonstrates the potential of compositionally complex perovskite oxides for enabling uninterrupted solar and electrocatalytic water-splitting cycles, paving the way for around-the-clock hydrogen fuel production.
Furthermore, the surface defect engineering of perovskite oxides has been investigated as an efficient bifunctional electrocatalyst for water splitting [71]. The study emphasizes the significance of defect engineering through element doping or deficiency during the synthesis process, offering insights into enhancing the catalytic activity of compositionally complex perovskite oxides for solar water-splitting applications. The integration of compositionally complex perovskite oxides with solar cells has also been explored. Song et al. employed all-perovskite tandem photoelectrodes wired to an iridium oxide anode, achieving a high solar-to-hydrogen conversion efficiency of 15 % under simulated solar illumination (Figure 5) [72]. This highlights the potential of compositionally complex perovskite oxides in tandem photoelectrodes for efficient solar hydrogen production.
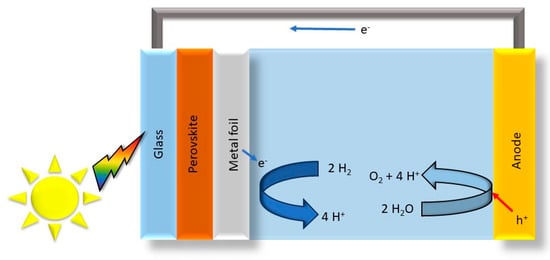
Figure 5.
Perovskite photoelectrode wired to an iridium oxide anode, modified from the work of Ref. [72].
Moreover, the computational analysis of oxide ion conduction in orthorhombic perovskite structured La0.9A0.1InO2.95 has shed light on the behavior of compositionally complex perovskite oxides as electrolytes, providing insights into their potential in solar water-splitting applications [73]. In addition, the adsorption of copper ions onto poly(1,8-diaminonaphthalene)/graphene film for the voltammetric determination of pyridoxine provides insights into the utilization of advanced electrode materials, highlighting the relevance of exploring innovative material compositions for enhancing electrochemical processes. This parallels the exploration of compositionally complex perovskite oxides as potential electrode materials for efficient solar water-splitting applications, emphasizing the importance of advanced material design in achieving high-performance photoelectrochemical systems [74].
Despite the promising advancements, challenges remain in the development of compositionally complex perovskite oxides for solar water splitting. The redox property of perovskite-type oxides, closely related to the nature of B-site or A-site cations, presents a challenge in optimizing the catalytic performance of compositionally complex perovskite oxides [75]. Additionally, issues surrounding the efficiency and stability of photoelectrode materials impose restrictions on the large-scale implementation of solar-driven water splitting, necessitating further research and development in this area [76].
6. Superfunctional High Entropy Alloys and Ceramics for Water Splitting
High-entropy alloys and ceramics have garnered significant attention due to their potential applications in various fields, including water splitting. The versatile application of high-entropy materials has been highlighted in integrating high-entropy metal phosphides into electrocatalytic water splitting, demonstrating their potential in this area [77]. While extensive research has been conducted on high-entropy alloys, comparably little has been performed for high-entropy ceramics, especially high-entropy diborides [78]. However, the functional properties of high-entropy ceramics span a wide range, including water splitting and catalysis, indicating their potential in these applications [79].
Coatings composed of high-entropy alloys and ceramics have attracted global attention due to their outstanding properties, including their potential for water-splitting applications [80]. Additionally, the combination of metal oxynitrides and high-entropy ceramics has shown promise in significant CO2 photoreduction, indicating the potential of high-entropy ceramics in addressing environmental challenges [81].
Furthermore, the rapid fabrication of high-entropy ceramic nanomaterials for catalytic reactions has been explored, emphasizing the importance of finding efficient fabrication methods for high-entropy ceramics to overcome synthesis challenges [82]. The synthesis of high-entropy ceramics has been a subject of interest, with studies focusing on the development of defective high-entropy oxide photocatalysts with high activity for CO2 conversion, indicating their potential in addressing environmental concerns through catalytic processes [83].
Moreover, the reliable brazing of ceramics to high-entropy alloys has been achieved, laying the foundation for further research on ceramic high-entropy alloy dissimilar joining, which could have implications for water-splitting applications [84]. The effect of ceramic phases on high-entropy alloys has also been investigated, indicating the potential for the development of high-entropy alloy–ceramic composites with enhanced properties for water-splitting applications [85]. High-entropy ceramics have rapidly developed as a class of materials based on high-entropy alloys, attracting increasing interest due to their unique structure and potential applications, including water splitting [86].
7. Further Inorganic Materials for Water Splitting
In the pursuit of efficient water splitting, the interaction of water with inorganic clusters, such as manganese oxide, has been investigated due to their potential as versatile and earth-abundant catalysts [87]. Additionally, the development of inorganic catalysts, like nanoporous carbon-coated bimetallic phosphides, highlights the importance of synthesizing transition metal compounds to meet the cost-effective and high-efficiency requirements for water-splitting applications [88]. The significance of inorganic materials in water splitting is further emphasized by the discovery of over 130 inorganic catalysts since the pioneering work on TiO2 and Pt-based systems in 1972 [89]. While precious metals like platinum, ruthenium, and iridium have traditionally been used in water-splitting catalysts, there is a growing interest in exploring earth-abundant materials like copper for catalytic hydrogen production and water oxidation [90]. Studies have shown that mixed metal oxides exhibit enhanced catalytic activity for both HER and the OER. Combinations of metals such as nickel, iron, cobalt, and other transition metals have demonstrated high efficiency as water oxidation electrocatalysts [91]. The utilization of binary and ternary metal oxide compositions in amorphous phases has paved the way for the development of advanced catalysts for water oxidation, offering improved performance and stability [92].
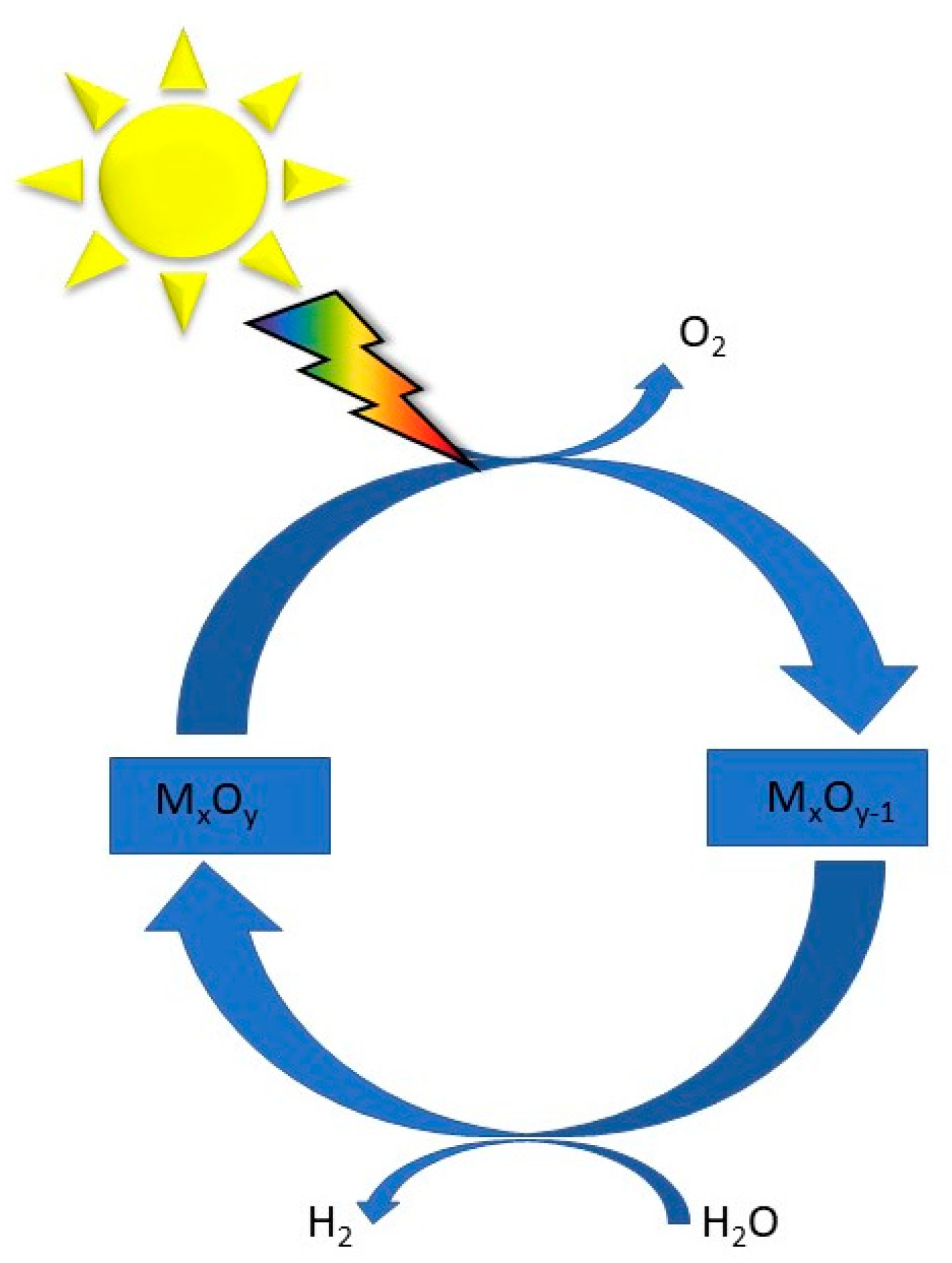
Mixed metal anion compounds, including oxynitrides, oxysulphides, and oxyhalides, have emerged as promising photocatalysts for water splitting due to their unique properties, such as a negative valence band maximum (VBM) compared to that of conventional oxides [92]. Common processes typically involve two-step redox cycles: first, the solar thermal reduction of a metal oxide to release oxygen, and second, the exothermic oxidation of the reduced oxide with water to generate hydrogen, as displayed in Figure 6. Developing redox-active and thermally stable oxide materials is crucial for achieving high fuel productivities and conversion rates. The main relevant two-step metal oxide systems include both volatile (e.g., ZnO/Zn, SnO2/SnO) and non-volatile redox pairs (e.g., Fe3O4/FeO, ferrites, CeO2/CeO2−δ, perovskites) [93]. Additionally, the design of hierarchical structures, such as hierarchical NiCo2O4 hollow microcuboids, has shown bifunctional electrocatalytic activity for overall water splitting, highlighting the potential of mixed metal oxides in achieving efficient electrolysis processes [94]. Metal–organic frameworks (MOFs) possess the highest surface area and the lowest densities among known materials. These properties brand MOFs as particularly advantageous for numerous technological applications, especially in photocatalysis [95].
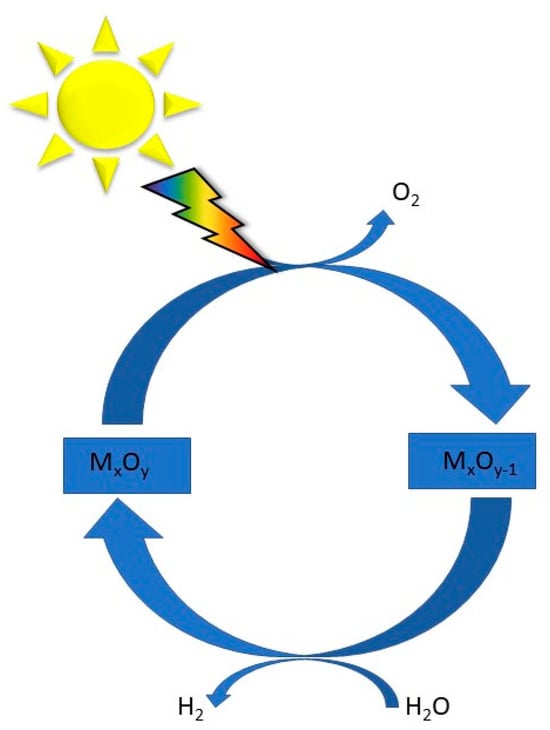
Figure 6.
Water splitting cycle based on a MxOy system, modified from the work of Ref. [93].
The development of yolk–shell nanostructures has garnered significant attention due to their numerous advantageous features, including a large surface area, efficient light harvesting, a uniform catalytic environment, and improved molecular diffusion kinetics [96]. The development of mixed metal oxide catalysts for water splitting also extends to photoelectrochemical applications. Nanoscale porosity in mixed metal phosphonates, such as Ni-W mixed metal phosphonate, has been found to be responsible for efficient photoelectrochemical OER in alkaline conditions, showcasing the versatility of these materials in harnessing solar energy for water splitting [97]. Furthermore, the combinatorial synthesis and screening of ternary NiFeCoOx libraries have provided insights into the optimization of mixed metal oxide combinations for the oxygen evolution reaction [98].
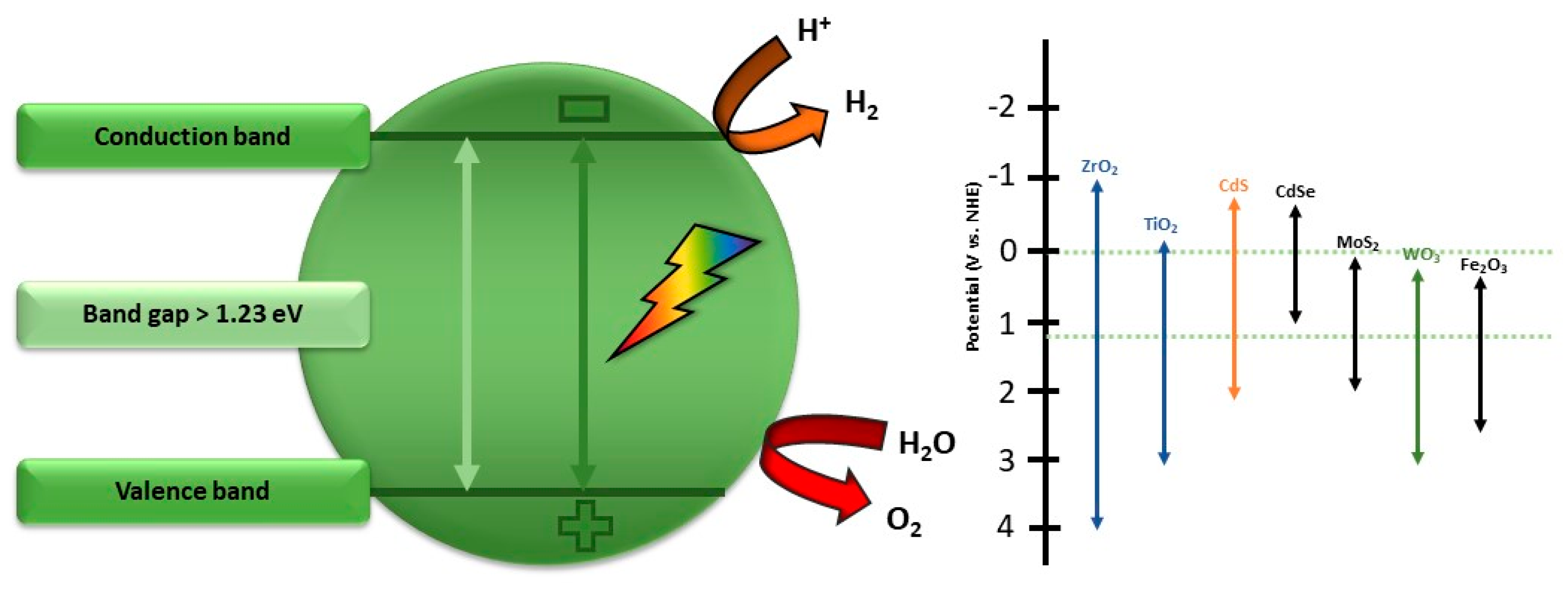
Some boundary conditions of inorganic materials which are applicable as a water-splitting photocatalyst are depicted in Figure 7. Molybdates have emerged as promising materials for water-splitting applications due to their unique properties and catalytic capabilities. The utilization of molybdates in water-splitting processes offers a pathway towards developing efficient and sustainable catalysts for electrolysis. Various studies have highlighted the potential of molybdates in enhancing the efficiency of both the HER and OER in water-splitting systems [99]. One notable example is the use of nickel-based molybdates, which have been reported as multifunctional catalysts for photocatalytic reactions, water splitting, and electrochemical applications [100]. The incorporation of molybdates into transition metal hydroxides has been shown to enhance the electrocatalytic activity for overall water splitting, providing insights into the role of molybdates in improving the bifunctional electrocatalytic activity of low-cost catalysts [101].
Recent advancements have focused on the synthesis of ultrathin bimetallic molybdate nanosheets coated on CuOx nanotubes to form hierarchical heterostructures for efficient overall water splitting [102]. This approach demonstrates the synergistic optimization of active sites through the combination of different metal oxides, showcasing the potential of molybdates in enhancing the catalytic activity of water-splitting electrocatalysts.
Moreover, the use of molybdates in photoelectrochemical water splitting has shown promising results, with molybdate-based microcrystals exhibiting efficient water splitting under blue-light excitation [103]. The energy conversion applications of molybdates as photocatalysts and anodic materials for batteries underscore their versatility in harnessing solar energy for water-splitting processes. Studies have shown that rare earth molybdates can play a crucial role in enhancing the efficiency of water splitting systems. The incorporation of rare earth elements into molybdates has been found to influence the structural and energetic properties of these compounds, providing insights into their potential as water-splitting catalysts.
The introduction of rare earth elements into synthetic [Mn4CaO4]n+ clusters, mimicking the oxygen-evolving center in photosynthesis, has shed light on the design of new water-splitting catalysts for artificial photosynthesis [104]. Furthermore, the adjustable electronic structure of transition metal molybdates between molybdenum, oxygen, and transition metals renders them active electrocatalysts for both the HER and OER [105]. The tunability of molybdates offers opportunities for optimizing the catalytic performance of water-splitting systems, contributing to the development of efficient and cost-effective electrolysis technologies.
Recent advancements have focused on band gap engineering in rare earth high-entropy oxides with fluorite structures for photocatalytic water-splitting applications [106]. The tunability of the band gap in these materials offers opportunities for optimizing their photocatalytic behavior, contributing to the development of efficient water-splitting technologies. Efforts to develop non-noble metal catalysts, such as V4P6.98/VO(PO3)2, have been expended to address the challenge of achieving high activity for water splitting in alkaline media [107]. Furthermore, the design of hybrid bioinorganic systems presents a unique approach where the biological component utilizes reducing equivalents generated from water splitting for CO2 fixation, leveraging the near-thermodynamic potential of biological catalysts [108].
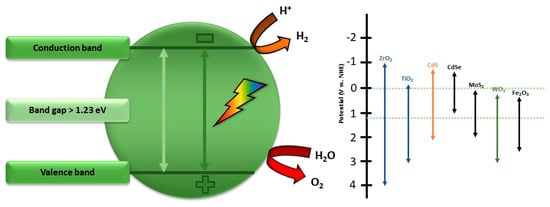
Figure 7.
Boundary conditions for inorganic materials applicable as a water-splitting photocatalyst, modified from the work of Ref. [109].
Figure 7.
Boundary conditions for inorganic materials applicable as a water-splitting photocatalyst, modified from the work of Ref. [109].
8. Conclusions
The development of efficient photoanodes for water splitting, e.g., based on hematite, is a multidisciplinary endeavor that encompasses materials science, chemistry, physics, and engineering. The advancements in heterojunction structures, cocatalyst integration, surface passivation, and nanoparticle decoration have significantly contributed to improving the performance of hematite photoanodes. Compositionally complex oxidic perovskites hold great promise for solar water-splitting applications. The advancements in defect engineering, tandem devices, and computational analysis have significantly contributed to understanding and harnessing the potential of these materials. High-entropy alloys and ceramics have shown promise in various applications, including water splitting. Their unique properties and potential for integration into electrocatalytic and photocatalytic processes brand them as a subject of significant research interest, with the potential to contribute to advancements in sustainable energy technologies. Many other inorganic ceramic materials show promising results towards water splitting, even though they have not yet been tested as functional ceramic bodies. Therefore, much more research is essential to address the remaining challenges and unlock the full potential for sustainable and efficient solar-driven water splitting by photocatalytic ceramics.
9. Outlook
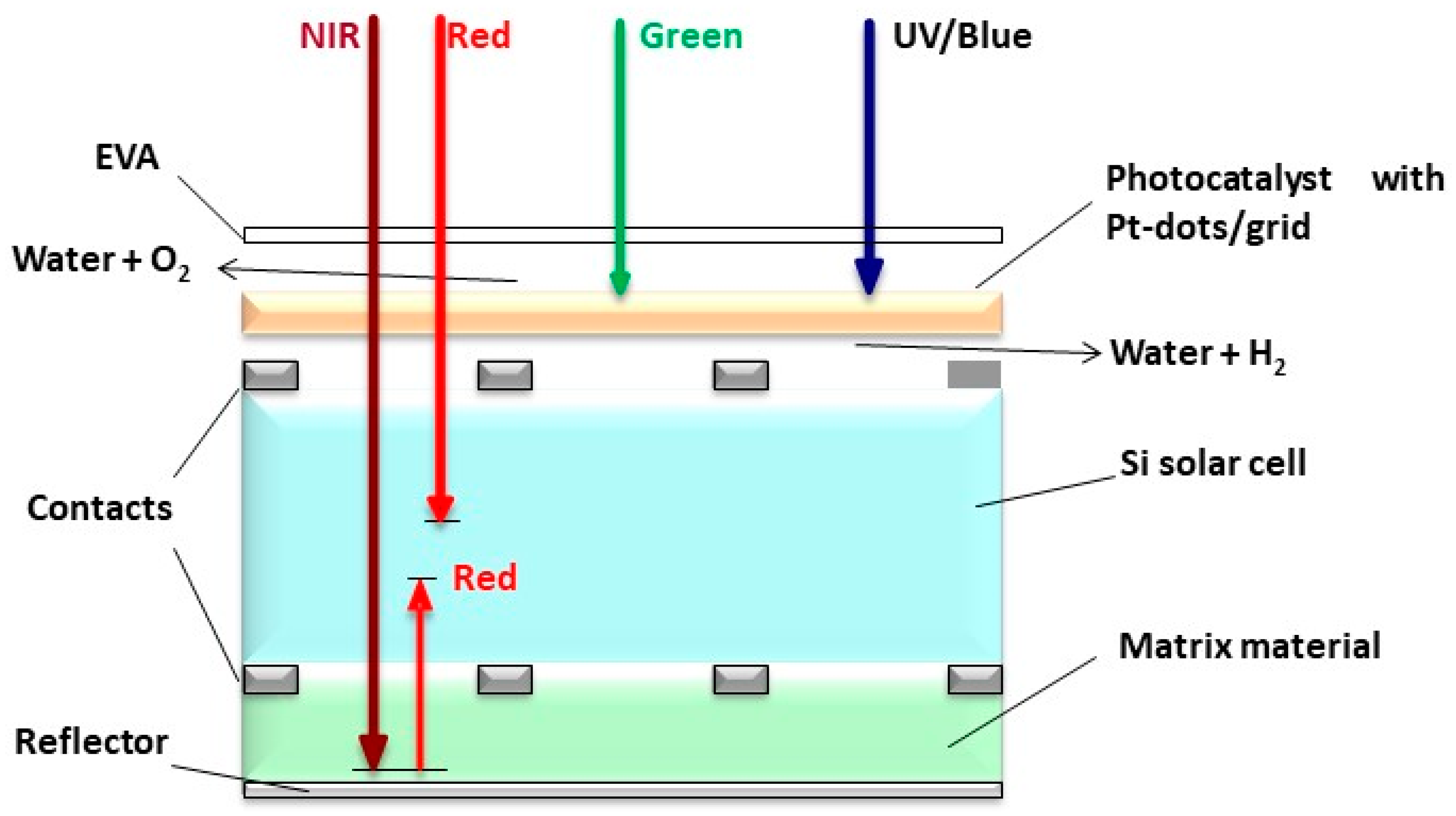
A novel proposal to address the future energy demand also concerns a solar light-splitting tandem cell, as illustrated below. A water-splitting flatbed reactor with an embedded photocatalytic (PC) ceramic layer may be combined with a conventional photovoltaic (PV) unit, which is typically mounted onto roofs (Figure 8). Such a tandem device comprising a PV unit and a PC cell offers some advantages, i.e., the PV cell will be cooled by the water film, which leads to less thermalization and thus, a higher yield of the PV unit. Moreover, the UV to green solar radiation will cause less harm to the PV unit, as this spectral fraction is absorbed by the photocatalyst to cleave water for hydrogen generation. Since the PV unit is the most efficient from the yellow to the NIR range, the removal of the UV to green spectral range will scarcely diminish the efficiency of the PV unit. Such a tandem device delivers hydrogen by the high energy fraction of the solar spectrum, which is storable on site, while the remaining solar visible light and NIR is well utilized by the conventional PV unit to produce instant electricity [110].
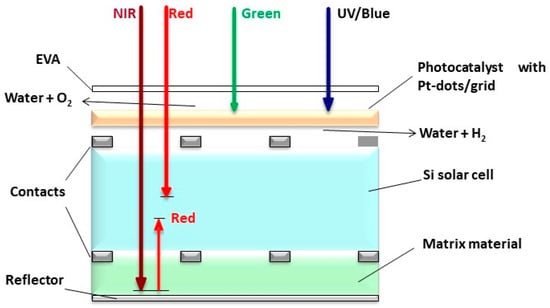
Figure 8.
Proposed light-splitting tandem cell: PV unit topped by a photocatalytic unit, modified from the work of Ref. [110].
Author Contributions
It is not easy to allocate the individual areas of responsibility in the preparation of this work, as many tasks went hand in hand. Nevertheless, we have made a classification as follows: J.E.: investigation, writing—original draft preparation, visualization, and project administration; T.J.: conceptualization, resources, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the state of North Rhine Westphalia, Germany.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are grateful to the state of North Rhine Westphalia, Germany, for its generous financial support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Beswick, R.R.; Oliveira, A.M.; Yan, Y. Does the Green Hydrogen Economy Have a Water Problem? ACS Energy Lett. 2021, 6, 3167–3169. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Overall water splitting: What’s next? Next Energy 2023, 1, 100006. [Google Scholar] [CrossRef]
- Ivon, A.; Glot, A.; Lavrov, R.; Lu, Z.-Y. Grain resistivity in zinc oxide and tin dioxide varistor ceramics. J. Alloys Compd. 2014, 616, 372–377. [Google Scholar] [CrossRef]
- Koga, E.; Higashi, Y.; Matsuoka, M. Latest Trend of ZnO Multilayer Ceramic Varistors. In Encyclopedia of Materials: Technical Ceramics and Glasses; Elsevier: Amsterdam, The Netherlands, 2021; pp. 272–280. [Google Scholar]
- Matsuoka, M. Nonohmic Properties of Zinc Oxide Ceramics. Jpn. J. Appl. Phys. 1971, 10, 736. [Google Scholar] [CrossRef]
- Reimann, T.; Töpfer, J. Low-temperature sintered Ni–Zn–Co–Mn–O spinel oxide ceramics for multilayer NTC thermistors. J. Mater. Sci. Mater. Electron. 2021, 32, 10761–10768. [Google Scholar] [CrossRef]
- Whatmore, R.W. Pyroelectric Crystals, Ceramics, and Thin Films for IR Sensors. In Encyclopedia of Materials: Technical Ceramics and Glasses; Elsevier: Amsterdam, The Netherlands, 2021; pp. 139–150. [Google Scholar]
- Bondarchuk, A.N.; Corrales-Mendoza, I.; Aguilar-Martínez, J.A.; García-Pérez, U.M.; Marken, F. Porous and conductive SnO2 ceramics as a promising nanostructured substrate to host photocatalytic hematite coatings: Towards low cost solar-driven water splitting. Catal. Commun. 2023, 174, 106593. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Bolton, J.R. Solar photoproduction of hydrogen: A review. Sol. Energy 1996, 57, 37–50. [Google Scholar] [CrossRef]
- Domen, K.; Kondo, J.N.; Hara, M.; Takata, T. Photo- and Mechano-Catalytic Overall Water Splitting Reactions to Form Hydrogen and Oxygen on Heterogeneous Catalysts. Bull. Chem. Soc. Jpn. 2000, 73, 1307–1331. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2008, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Nande, A.; Kalyani, N.T.; Tiwari, A.; Dhoble, S.J. Exploring the world of functional materials. In Functional Materials from Carbon, Inorganic, and Organic Sources; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–19. [Google Scholar]
- Keane, M.A. Ceramics for catalysis. J. Mater. Sci. 2003, 38, 4661–4675. [Google Scholar] [CrossRef]
- Labhsetwar, N.; Doggali, P.; Rayalu, S.; Yadav, R.; Mistuhashi, T.; Haneda, H. Ceramics in Environmental Catalysis: Applications and Possibilities. Chin. J. Catal. 2012, 33, 1611–1621. [Google Scholar] [CrossRef]
- Bie, C.; Wang, L.; Yu, J. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2016, 16, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. [Google Scholar] [CrossRef]
- Fabian, D.M.; Hu, S.; Singh, N.; Houle, F.A.; Hisatomi, T.; Domen, K.; Osterloh, F.E.; Ardo, S. Particle suspension reactors and materials for solar-driven water splitting. Energy Environ. Sci. 2015, 8, 2825–2850. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Visible-Light-Response and Photocatalytic Activities of TiO2 and SrTiO3 Photocatalysts Codoped with Antimony and Chromium. J. Phys. Chem. B 2002, 106, 5029–5034. [Google Scholar] [CrossRef]
- Ishii, T.; Kato, H.; Kudo, A. H2 evolution from an aqueous methanol solution on SrTiO3 photocatalysts codoped with chromium and tantalum ions under visible light irradiation. J. Photochem. Photobiol. A Chem. 2004, 163, 181–186. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Niishiro, R.; Kato, H.; Kudo, A. Nickel and either tantalum or niobium-codoped TiO2 and SrTiO3 photocatalysts with visible-light response for H2 or O2 evolution from aqueous solutions. Phys. Chem. Chem. Phys. 2005, 7, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Niishiro, R.; Konta, R.; Kato, H.; Chun, W.-J.; Asakura, K.; Kudo, A. Photocatalytic O2 Evolution of Rhodium and Antimony-Codoped Rutile-Type TiO2 under Visible Light Irradiation. J. Phys. Chem. C 2007, 111, 17420–17426. [Google Scholar] [CrossRef]
- Shimodaira, Y.; Kato, H.; Kobayashi, H.; Kudo, A. Investigations of Electronic Structures and Photocatalytic Activities under Visible Light Irradiation of Lead Molybdate Replaced with Chromium(VI). Bull. Chem. Soc. Jpn. 2007, 80, 885–893. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Hosogi, Y.; Kato, H.; Kudo, A. Photocatalytic Activities of Layered Titanates and Niobates Ion-Exchanged with Sn2+ under Visible Light Irradiation. J. Phys. Chem. C 2008, 112, 17678–17682. [Google Scholar] [CrossRef]
- Kim, H.G.; Hwang, D.W.; Lee, J.S. An undoped, single-phase oxide photocatalyst working under visible light. J. Am. Chem. Soc. 2004, 126, 8912–8913. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Nunoshige, J.; Takata, T.; Kondo, J.N.; Domen, K. Unusual enhancement of H2 evolution by Ru on TaON photocatalyst under visible light irradiation. Chem. Commun. 2003, 24, 3000–3001. [Google Scholar] [CrossRef]
- Liu, M.; You, W.; Lei, Z.; Zhou, G.; Yang, J.; Wu, G.; Ma, G.; Luan, G.; Takata, T.; Hara, M.; et al. Water reduction and oxidation on Pt–Ru/Y2Ta2O5N2 catalyst under visible light irradiation. Chem. Commun. 2004, 36, 2192–2193. [Google Scholar] [CrossRef]
- Ogisu, K.; Ishikawa, A.; Teramura, K.; Toda, K.; Hara, M.; Domen, K. Lanthanum–Indium Oxysulfide as a Visible Light Driven Photocatalyst for Water Splitting. Chem. Lett. 2007, 36, 854–855. [Google Scholar] [CrossRef]
- Liao, P.; Keith, J.A.; Carter, E.A. Water oxidation on pure and doped hematite (0001) surfaces: Prediction of Co and Ni as effective dopants for electrocatalysis. J. Am. Chem. Soc. 2012, 134, 13296–13309. [Google Scholar] [CrossRef]
- Rao, R.R.; Kolb, M.J.; Halck, N.B.; Pedersen, A.F.; Mehta, A.; You, H.; Stoerzinger, K.A.; Feng, Z.; Hansen, H.A.; Zhou, H.; et al. Towards identifying the active sites on RuO2(110) in catalyzing oxygen evolution. Energy Environ. Sci. 2017, 10, 2626–2637. [Google Scholar] [CrossRef]
- Mefford, J.T.; Rong, X.; Abakumov, A.M.; Hardin, W.G.; Dai, S.; Kolpak, A.M.; Johnston, K.P.; Stevenson, K.J. Water electrolysis on La1−xSrxCoO3−δ perovskite electrocatalysts. Nat. Commun. 2016, 7, 11053. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef]
- Kudernatsch, W.; Peng, G.; Zeuthen, H.; Bai, Y.; Merte, L.R.; Lammich, L.; Besenbacher, F.; Mavrikakis, M.; Wendt, S. Direct Visualization of Catalytically Active Sites at the FeO–Pt(111) Interface. ACS Nano 2015, 9, 7804–7814. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Pahlevanpour, G.; Bashiri, H. Kinetic Monte Carlo simulation of hydrogen production from photocatalytic water splitting in the presence of methanol by 1 wt% Au/TiO2. Int. J. Hydrogen Energy 2022, 47, 12975–12987. [Google Scholar] [CrossRef]
- Sproviero, E.M.; Gascón, J.A.; McEvoy, J.P.; Brudvig, G.W.; Batista, V.S. Quantum mechanics/molecular mechanics study of the catalytic cycle of water splitting in photosystem II. J. Am. Chem. Soc. 2008, 130, 3428–3442. [Google Scholar] [CrossRef] [PubMed]
- Nikonenko, V.; Urtenov, M.; Mareev, S.; Pourcelly, G. Mathematical Modeling of the Effect of Water Splitting on Ion Transfer in the Depleted Diffusion Layer Near an Ion-Exchange Membrane. Membranes 2020, 10, 22. [Google Scholar] [CrossRef]
- Stich, S.; Ding, K.; Muhammad, Q.K.; Porz, L.; Minnert, C.; Rheinheimer, W.; Durst, K.; Rödel, J.; Frömling, T.; Fang, X. Room-temperature dislocation plasticity in SrTiO3 tuned by defect chemistry. J. Am. Ceram. Soc. 2022, 105, 1318–1329. [Google Scholar] [CrossRef]
- Chen, F.; Yan, K.; Zhou, J.; Zhu, Y.; Hong, J. High toughness Si3N4 ceramic composites synergistically toughened by multilayer graphene/β-Si3N4 whisker: Preparation and toughening mechanism investigation. J. Alloys Compd. 2022, 921, 166183. [Google Scholar] [CrossRef]
- Andraskar, N.D.; Tiwari, G.; Goel, M.D. Impact response of ceramic structures—A review. Ceram. Int. 2022, 48, 27262–27279. [Google Scholar] [CrossRef]
- Yi, W.; Hu, X.; Ichim, P.; Sun, X. Processing and properties of pressable ceramic with non-uniform reinforcement for selective-toughening. Mater. Sci. Eng. A 2012, 558, 543–549. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Ye, F.; Cheng, L.; Zhang, Y. High-temperature atomically laminated materials: The toughening components of ceramic matrix composites. Ceram. Int. 2022, 48, 32628–32648. [Google Scholar] [CrossRef]
- Rahimizadeh, A.; Sarvestani, H.Y.; Li, L.; Robles, J.B.; Backman, D.; Lessard, L.; Ashrafi, B. Engineering toughening mechanisms in architectured ceramic-based bioinspired materials. Mater. Des. 2021, 198, 109375. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; Huang, Q.; Yuan, C. Research and application of biomimetic modified ceramics and ceramic composites: A review. J. Am. Ceram. Soc. 2024, 107, 663–697. [Google Scholar] [CrossRef]
- Nong, H.N.; Falling, L.J.; Bergmann, A.; Klingenhof, M.; Tran, H.P.; Spöri, C.; Mom, R.; Timoshenko, J.; Zichittella, G.; Knop-Gericke, A.; et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 2020, 587, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Lei, B.; Xu, D.; Wei, B.; Xie, T.; Xiao, C.; Jin, W.; Xu, L. In Situ Synthesis of α-Fe2O3/Fe3O4 Heterojunction Photoanode via Fast Flame Annealing for Enhanced Charge Separation and Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 4785–4795. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lin, W.-H.; Lin, C.-Y.; Wang, B.-S.; Wu, J.-J. n-Fe2O3 to N+-TiO2 Heterojunction Photoanode for Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 13314–13321. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, X.; Chen, H.; Yang, L.; Xie, T.; Zou, X. Surface-oxidized titanium diboride as cocatalyst on hematite photoanode for solar water splitting. CrystEngComm 2022, 24, 2251–2257. [Google Scholar] [CrossRef]
- Ahmed, A.Y.; Ahmed, M.G.; Kandiel, T.A. Modification of Hematite Photoanode with Cobalt Based Oxygen Evolution Catalyst via Bifunctional Linker Approach for Efficient Water Splitting. J. Phys. Chem. C 2016, 120, 23415–23420. [Google Scholar] [CrossRef]
- Phuan, Y.W.; Chong, M.N.; Satokhee, O.; De Souza, A.B.; Zhu, T.; Chan, E.S. Synthesis and Characterization of a Novel Ternary Hematite Nanocomposites Structure with Fullerene and 2D-Electrochemical Reduced Graphene Oxide for Superior Photoelectrochemical Performance. Part. Part. Syst. Charact. 2016, 34, 1600216. [Google Scholar] [CrossRef]
- Rodrigues, M.H.d.M.; Junior, J.B.S.; Leite, E.R. The Influence of Magnetic Field and Nanoparticle Concentration on the Thin Film Colloidal Deposition Process of Magnetic Nanoparticles: The Search for High-Efficiency Hematite Photoanodes. Nanomaterials 2022, 12, 1636. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, M.-C.; Hsieh, Y.-K.; Chang, W.-S.; Lin, J.-C.; Lee, C.-H.; Wang, C.-F. Influence of sodium halides (NaF, NaCl, NaBr, NaI) on the Photocatalytic performance of hydrothermally synthesized hematite photoanodes. ACS Appl. Mater. Interfaces 2013, 5, 7937–7949. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Waseem, A.; Bagal, I.V.; Johar, M.A.; Kulkarni, M.A.; Lee, J.K.; Ryu, S.-W. Stable and Efficient Photoelectrochemical Water Splitting of GaN Nanowire Photoanode Coated with Au Nanoparticles by Hot-Electron-Assisted Transport. ACS Appl. Energy Mater. 2021, 4, 13759–13765. [Google Scholar] [CrossRef]
- Hwang, J.B.; Mahadik, M.A.; Anushkkaran, P.; Choi, S.H.; Chae, W.-S.; Kumar, M.; Pathan, H.M.; Lee, H.H.; Jang, J.S. Co-dependency of TiO2 underlayer and ZrO2 top layer in sandwiched microwave-assisted Zr-Fe2O3 photoanodes for photoelectrochemical water splitting. Sustain. Energy Fuels 2023, 7, 4914–4921. [Google Scholar] [CrossRef]
- Koren, M.G.; Dotan, H.; Rothschild, A. Nano Gold Rush: On the Origin of the Photocurrent Enhancement in Hematite Photoanodes Decorated with Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 15042–15051. [Google Scholar] [CrossRef]
- Bondarchuk, A.N.; Corrales-Mendoza, I.; Marken, F.; Arellanes-Mendoza, L.; Aguilar-Martínez, J.A.; Silva-Vidaurri, L.; Curiel-Olivares, G.; Montejo-Alvaro, F. Hematite photoelectrodes grown on porous CuO–Sb2O5–SnO2 ceramics for photoelectrochemical water splitting. Sol. Energy Mater. Sol. Cells 2021, 221, 110886. [Google Scholar] [CrossRef]
- Bondarchuk, A.N.; Marken, F. Hematite photoanodes for water splitting from directed assembly of Prussian blue onto CuO–Sb2O5–SnO2 ceramics. Phys. Chem. Chem. Phys. 2023, 25, 25681–25688. [Google Scholar] [CrossRef] [PubMed]
- Bondarchuk, A.N.; Corrales-Mendoza, I.; Tomás, S.A.; Marken, F. A hematite photoelectrode grown on porous and conductive SnO2 ceramics for solar-driven water splitting. Int. J. Hydrogen Energy 2019, 44, 19667–19675. [Google Scholar] [CrossRef]
- Kong, B.; Selomulya, C.; Zheng, G.; Zhao, D. New faces of porous Prussian blue: Interfacial assembly of integrated hetero-structures for sensing applications. Chem. Soc. Rev. 2015, 44, 7997–8018. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.S.; Unal, U.; Karadas, F. Photocatalytic Water Oxidation with a CoFe Prussian Blue Analogue–Layered Niobate Hybrid Material. ACS Appl. Energy Mater. 2021, 4, 12383–12390. [Google Scholar] [CrossRef]
- Ghobadi, T.G.U.; Ghobadi, A.; Buyuktemiz, M.; Yildiz, E.A.; Yildiz, D.B.; Yaglioglu, H.G.; Dede, Y.; Ozbay, E.; Karadas, F. A Robust, Precious-Metal-Free Dye-Sensitized Photoanode for Water Oxidation: A Nanosecond-Long Excited State Lifetime through a Prussian Blue Analogue. Angew. Chem. Int. Ed. 2020, 59, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; De Santiago, H.A.; Xu, B.; Liu, C.; Trindell, J.A.; Li, W.; Park, J.; Rodriguez, M.A.; Coker, E.N.; Sugar, J.D.; et al. Compositionally Complex Perovskite Oxides for Solar Thermochemical Water Splitting. Chem. Mater. 2023, 35, 1901–1915. [Google Scholar] [CrossRef]
- Pornrungroj, C.; Andrei, V.; Rahaman, M.; Uswachoke, C.; Joyce, H.J.; Wright, D.S.; Reisner, E. Bifunctional Perovskite-BiVO4 Tandem Devices for Uninterrupted Solar and Electrocatalytic Water Splitting Cycles. Adv. Funct. Mater. 2021, 31, 2008182. [Google Scholar] [CrossRef]
- Zong, R.; Fang, Y.; Zhu, C.; Zhang, X.; Wu, L.; Hou, X.; Tao, Y.; Shao, J. Surface Defect Engineering on Perovskite Oxides as Efficient Bifunctional Electrocatalysts for Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 42852–42860. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, C.; Chen, L.; Dolia, K.; Fu, S.; Sun, N.; Li, Y.; Wyatt, K.; Young, J.L.; Deutsch, T.G.; et al. All-Perovskite Tandem Photoelectrodes for Unassisted Solar Hydrogen Production. ACS Energy Lett. 2023, 8, 2611–2619. [Google Scholar] [CrossRef]
- Yoon, M.; Hwang, K.; Byeon, D.; Kim, J.; Hwang, H.; Jeong, S. Computational Analysis of Oxide Ion Conduction in Orthorhombic Perovskite Structured La0.9A0.1InO2.95 (A = Ca, Sr and Ba). J. Am. Ceram. Soc. 2015, 98, 515–519. [Google Scholar] [CrossRef]
- Vu, T.V.; Nguyen, M.T.T.; Do, T.T.; Nguyen, H.L.; Nguyen, V.; Nguyen, D.T. Adsorption of Copper Ions onto Poly(1,8-diaminonaphthalene)/Graphene Film for Voltammetric Determination of Pyridoxine. Electroanalysis 2022, 34, 1478–1486. [Google Scholar] [CrossRef]
- Mishra, A.; Prasad, R. Synthesis and Performance of Transition Metal Based Perovskite Catalysts for Diesel Soot Oxidation. Bull. Chem. React. Eng. Catal. 2017, 12, 469–477. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.; Kwon, S.-R.; Chang, W.J.; Nam, K.T.; Chung, T.D. Reverse Electrodialysis-Assisted Solar Water Splitting. Sci. Rep. 2017, 7, 12281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xue, Z.; Chen, W.; Wang, Y.; Mu, T. Eutectic Synthesis of High-Entropy Metal Phosphides for Electrocatalytic Water Splitting. ChemSusChem 2020, 13, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, P.; Kirnbauer, A.; Ertelthaler, P.; Koller, C. High-entropy ceramic thin films; A case study on transition metal diborides. Scr. Mater. 2018, 149, 93–97. [Google Scholar] [CrossRef]
- Inui, H.; Kishida, K.; Chen, Z. Recent Progress in Our Understanding of Phase Stability, Atomic Structures and Mechanical and Functional Properties of High-Entropy Alloys. Mater. Trans. 2022, 63, 394–401. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A Review on High Entropy Alloys Coatings: Fabrication Processes and Property Assessment. Adv. Eng. Mater. 2019, 21, 1900343. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Shundo, Y.; Watanabe, M.; Ishihara, T.; Fuji, M.; Edalati, K. Significant CO2 photoreduction on a high-entropy oxynitride. Chem. Eng. J. 2022, 449, 137800. [Google Scholar] [CrossRef]
- Yang, J.X.; Dai, B.-H.; Chiang, C.-Y.; Chiu, I.-C.; Pao, C.-W.; Lu, S.-Y.; Tsao, I.-Y.; Lin, S.-T.; Chiu, C.-T.; Yeh, J.-W.; et al. Rapid Fabrication of High-Entropy Ceramic Nanomaterials for Catalytic Reactions. ACS Nano 2021, 15, 12324–12333. [Google Scholar] [CrossRef]
- Akrami, S.; Murakami, Y.; Watanabe, M.; Ishihara, T.; Arita, M.; Fuji, M.; Edalati, K. Defective high-entropy oxide photocatalyst with high activity for CO2 conversion. Appl. Catal. B Environ. 2022, 303, 120896. [Google Scholar] [CrossRef]
- Sun, J.; Lei, Y.Y.; Fu, W.; Lin, D.; Hu, S.; Song, X.; Cao, J.; Yang, M. Brazing SiC ceramic to Al0.3CoCrFeNi high-entropy alloy using Ag-Cu filler metal. J. Am. Ceram. Soc. 2022, 105, 6570–6580. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, H.; Qu, Y.; Li, C.; Lv, Q.; Li, Z.; Li, R.; Tan, B.; Tian, C.; Nie, S. The effect of TiC addition on the microstructure and mechanical properties of Al0.6CrFe2Ni2 high entropy alloys. SN Appl. Sci. 2020, 2, 493. [Google Scholar] [CrossRef]
- Shu, R. Nonstoichiometric Multicomponent Nitride Thin Films; Linkoping University Electronic Press: Linkoping, Sweden, 2020. [Google Scholar]
- Lang, S.M.; Bernhardt, T.M.; Kiawi, D.M.; Bakker, J.M.; Barnett, R.N.; Landman, U. The Interaction of Water with Free Mn4O4+ Clusters: Deprotonation and Adsorption-Induced Structural Transformations. Angew. Chem. Int. Ed. 2015, 54, 15113–15117. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Wu, L.; Long, Y.; Li, J.; Song, S.; Zhang, H. Nanoporous Carbon-Coated Bimetallic Phosphides for Efficient Electrochemical Water Splitting. Cryst. Growth Des. 2018, 18, 3404–3410. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic Materials as Catalysts for Photochemical Splitting of Water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, H.; Sun, Z.; Han, A.; Du, P. Earth-Abundant Copper-Based Bifunctional Electrocatalyst for Both Catalytic Hydrogen Production and Water Oxidation. ACS Catal. 2015, 5, 1530–1538. [Google Scholar] [CrossRef]
- Roohi, P.; Alizadeh, R.; Fatehifar, E. Thermodynamic Study of Transformation of Methane to Synthesis Gas Over Metal Oxides. Int. J. Thermophys. 2015, 36, 88–103. [Google Scholar] [CrossRef]
- Chatterjee, K.; Bueno, S.; Skrabalak, S.; Dravid, V.; dos Reis, R. Nanoscale Investigation of Layered Oxychloride Intergrowth Photocatalysts for Visible Light Driven Water Splitting. Microsc. Microanal. 2020, 26, 376–379. [Google Scholar] [CrossRef]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. Chemengineering 2019, 3, 63. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Li, Q.; Yu, X.; Hong, Z.; Zhang, X.; Liang, C.; Lin, Z. Hierarchical NiCo2O4 Hollow Microcuboids as Bifunctional Electrocatalysts for Overall Water-Splitting. Angew. Chem. 2016, 128, 6398–6402. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chang, H.-E.; Wang, C.-Y.; Kurioka, T.; Chen, C.-Y.; Chang, T.-F.M.; Sone, M.; Hsu, Y.-J. Manipulation of interfacial charge dynamics for metal–organic frameworks toward advanced photocatalytic applications. Nanoscale Adv. 2023, 6, 1039–1058. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Wang, Y.-T.; Moon, H.S.; Yong, K.; Hsu, Y.-J. Yolk–shell nanostructures: Synthesis, photocatalysis and interfacial charge dynamics. RSC Adv. 2021, 11, 12288–12305. [Google Scholar] [CrossRef]
- Chakraborty, D.; Shyamal, S.; Bhaumik, A. A New Porous Ni-W Mixed Metal Phosphonate Open Framework Material for Efficient Photoelectrochemical OER. ChemCatChem 2020, 12, 1504–1511. [Google Scholar] [CrossRef]
- Vijayakumar, A.U.; Aloni, N.; Veettil, V.T.; Rahamim, G.; Hardisty, S.S.; Zysler, M.; Tirosh, S.; Zitoun, D. Combinatorial Synthesis and Screening of a Ternary NiFeCoOx Library for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 4017–4024. [Google Scholar] [CrossRef]
- Ni, B.; Shi, Y.; Wang, X. The Sub-Nanometer Scale as a New Focus in Nanoscience. Adv. Mater. 2018, 30, e1802031. [Google Scholar] [CrossRef]
- Ahmed, J.; Ahamad, T.; Alshehri, S.M. rGO supported CuMoO4 nanoparticles: Synthesis, characterization, and electrocatalytic oxygen evolution reaction. New J. Chem. 2023, 47, 13903–13910. [Google Scholar] [CrossRef]
- Ganguli, S.; Ghosh, S.; Das, S.; Mahalingam, V. Inception of molybdate as a “pore forming additive” to enhance the bifunctional electrocatalytic activity of nickel and cobalt based mixed hydroxides for overall water splitting. Nanoscale 2019, 11, 16896–16906. [Google Scholar] [CrossRef]
- Gu, M.; Deng, X.; Lin, M.; Wang, H.; Gao, A.; Huang, X.; Zhang, X. Ultrathin NiCo Bimetallic Molybdate Nanosheets Coated CuOx Nanotubes: Heterostructure and Bimetallic Synergistic Optimization of the Active Site for Highly Efficient Overall Water Splitting. Adv. Energy Mater. 2021, 11, 2102361. [Google Scholar] [CrossRef]
- Moura, J.V.B.; de Souza, A.A.G.; de Tarso Cavalcante Freire, P.; Da Luz Lima, C.; Oliveira, T.M.B.F. Blue-light-excited NaCe(MoO4)2 microcrystals for photoelectrochemical water splitting. Int. J. Appl. Ceram. Technol. 2021, 18, 615–621. [Google Scholar] [CrossRef]
- Yao, R.; Li, Y.; Chen, Y.; Xu, B.; Chen, C.; Zhang, C. Rare-Earth Elements Can Structurally and Energetically Replace the Calcium in a Synthetic Mn4CaO4-Cluster Mimicking the Oxygen-Evolving Center in Photosynthesis. J. Am. Chem. Soc. 2021, 143, 17360–17365. [Google Scholar] [CrossRef] [PubMed]
- Dalai, N.; Dash, B.; Jena, B. Bifunctional Activity of PVP K-30 Assisted Cobalt Molybdate for Electrocatalytic Water Splitting**. ChemistrySelect 2022, 7, e202202270. [Google Scholar] [CrossRef]
- Nundy, S.; Tatar, D.; Kojcinovic, J.; Ullah, H.; Ghosh, A.; Mallick, T.K.; Meinusch, R.; Smarsly, B.M.; Tahir, A.A.; Djerdj, I. Bandgap Engineering in Novel Fluorite-Type Rare Earth High-Entropy Oxides (RE-HEOs) with Computational and Experi-mental Validation for Photocatalytic Water Splitting Applications. Adv. Sustain. Syst. 2022, 6, 2200067. [Google Scholar] [CrossRef]
- Zhang, L.; Cong, M.; Wang, Y.; Ding, X.; Liu, A.; Gao, Y. V4P6.98/VO(PO3)2 as an Efficient Non-Noble Metal Catalyst for Electrochemical Hydrogen Evolution in Alkaline Electrolyte. ChemElectroChem 2019, 6, 1329–1332. [Google Scholar] [CrossRef]
- Nichols, E.M.; Gallagher, J.J.; Liu, C.; Su, Y.; Resasco, J.; Yu, Y.; Sun, Y.; Yang, P.; Chang, M.C.Y.; Chang, C.J. Hybrid bioinorganic approach to solar-to-chemical conversion. Proc. Natl. Acad. Sci. USA 2015, 112, 11461–11466. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef]
- Jüstel, T. Energy Conversion System. DE102014107268 A1, WO2015177216 A1, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).