Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges
Abstract
:1. Introduction
2. The History of Light Therapies
3. Principles of PDT
4. Photosensitizers
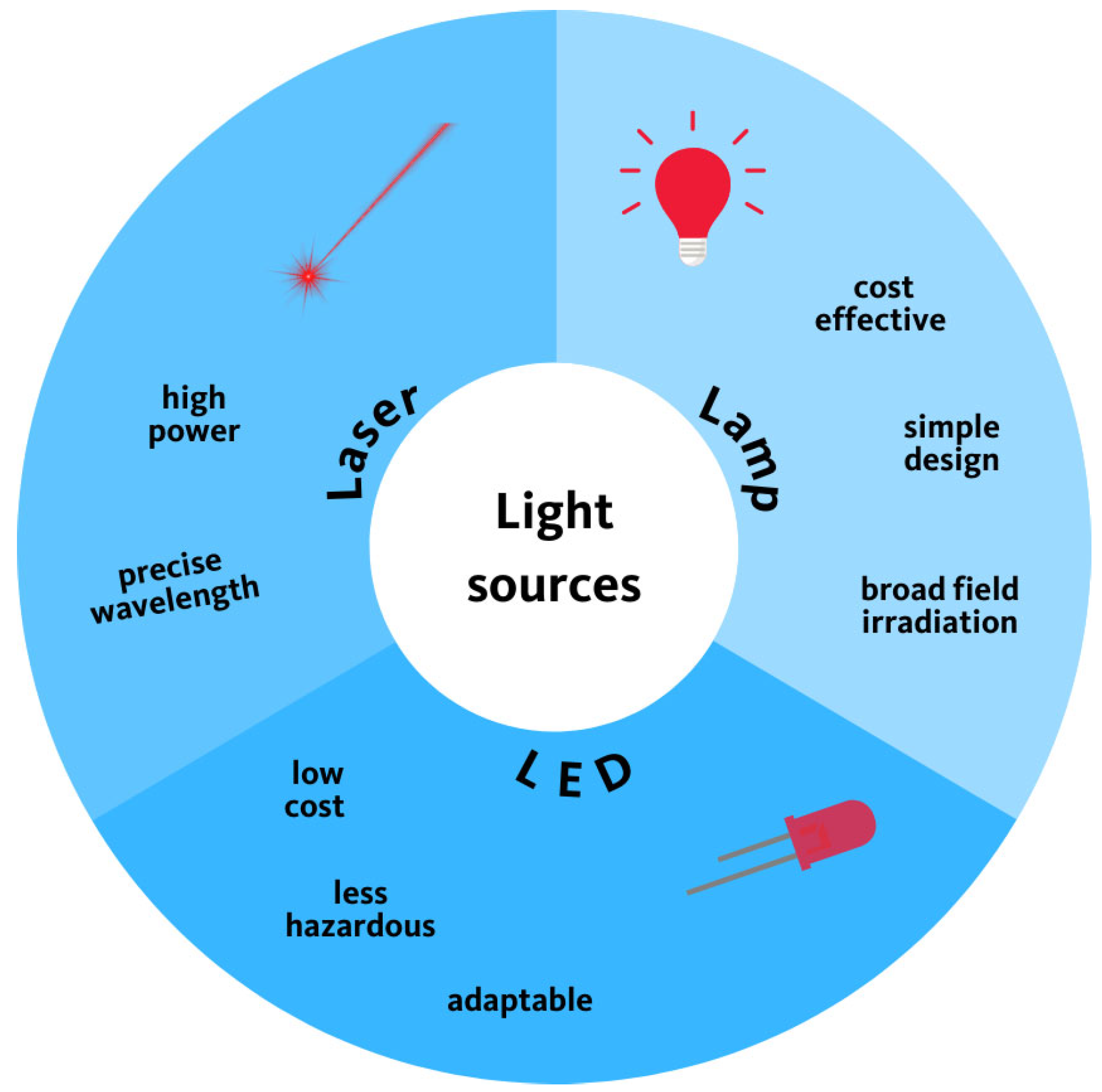
5. Light
6. Oxygen
7. Mechanism of PDT
8. Limitations of PDT
9. Adjuvants in Photodynamic Therapy
10. Gold Nanoparticles
11. Silver Nanoparticles
12. Silica Nanoparticles
13. Magnetic Nanoparticles
14. Graphene Derivatives
15. Organic Adjuvants
16. Inorganic Adjuvants
17. Antibiotics
18. PDT in Fungi Treatment
19. Novel Strategies
20. Modulation of Environment
21. Macrophages in PDT
22. PDT Tolerance Development
23. Endogenous Photosensitizers
24. The Future, Opportunities, and Challenges of Photodynamic Therapy
25. Conclusions
Funding
Conflicts of Interest
References
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Pérez, M.E.; Santamarina, S.C.; Durantini, J.E.; Milanesio, M.E.; Durantini, E.N. Photodynamic Polymers Constituted by Porphyrin Units as Antibacterial Materials. Photochem 2022, 2, 891–904. [Google Scholar] [CrossRef]
- Berezin, D.B.; Kruchin, S.O.; Kukushkina, N.V.; Venediktov, E.A.; Koifman, M.O.; Kustov, A.V. Water-Soluble Dicationic Deuteroporphyrin Derivative for Antimicrobial PDT: Singlet Oxygen Generation, Passive Carrier Interaction and Nosocomial Bacterial Strains Photoinactivation. Photochem 2023, 3, 171–186. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y. Photodynamic Therapy for the Treatment of Fungal Infections. IDR 2022, 15, 3251–3266. [Google Scholar] [CrossRef]
- Dos Santos Vitorio, G.; Godoi, B.H.; Pinto, J.G.; Ferreira, I.; Soares, C.P.; Ferreira-Strixino, J. Action of Photodynamic Therapy at Low Fluence in 9 L/lacZ Cells after Interaction with Chlorins. Photochem 2023, 3, 82–97. [Google Scholar] [CrossRef]
- Fontana, L.C.; Pinto, J.G.; Magalhães, J.A.; Tada, D.B.; De Almeida, R.M.S.; Pacheco-Soares, C.; Ferreira-Strixino, J. Comparison of the Photodynamic Effect of Two Chlorins, Photodithazine and Fotoenticine, in Gliosarcoma Cells. Photochem 2022, 2, 165–180. [Google Scholar] [CrossRef]
- Dingiswayo, S.; Babu, B.; Burgess, K.; Mack, J.; Nyokong, T. Photodynamic Anticancer and Antibacterial Activities of Sn(IV) N-Confused Meso-Tetra(Methylthiophenyl)Porphyrin. Photochem 2023, 3, 313–326. [Google Scholar] [CrossRef]
- Fornaciari, B.; Juvenal, M.S.; Martins, W.K.; Junqueira, H.C.; Baptista, M.S. Photodynamic Activity of Acridine Orange in Keratinocytes under Blue Light Irradiation. Photochem 2023, 3, 209–226. [Google Scholar] [CrossRef]
- Richy, N.; Gam, S.; Messaoudi, S.; Triadon, A.; Mongin, O.; Blanchard-Desce, M.; Latouche, C.; Humphrey, M.G.; Boucekkine, A.; Halet, J.-F.; et al. Linear and Nonlinear Optical Properties of Quadrupolar Bithiophenes and Cyclopentadithiophenes as Fluorescent Oxygen Photosensitizers. Photochem 2023, 3, 127–154. [Google Scholar] [CrossRef]
- Dias, L.D.; Buzzá, H.H.; Stringasci, M.D.; Bagnato, V.S. Recent Advances in Combined Photothermal and Photodynamic Therapies against Cancer Using Carbon Nanomaterial Platforms for In Vivo Studies. Photochem 2021, 1, 434–447. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. JCM 2019, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Hönigsmann, H. History of Phototherapy in Dermatology. Photochem. Photobiol. Sci. 2012, 12, 16–21. [Google Scholar] [CrossRef]
- Gauthier, Y.; Benzekri, L. Historical Aspects of Vitiligo. In Vitiligo; Picardo, M., Taïeb, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–10. ISBN 978-3-319-62958-2. [Google Scholar]
- Boddupalli, R.S. Skin Disorders (Twak Rogas) Revealed in the Atharvaveda: Descriptions of Medicinal Plants and Utilization. Indian J. Hist. Sci. 2022, 57, 63–77. [Google Scholar] [CrossRef]
- Koo, J.; Nakamura, M. Heliotherapy. In Clinical Cases in Phototherapy; Springer International Publishing: Cham, Switzerland, 2017; pp. 77–80. ISBN 978-3-319-51598-4. [Google Scholar]
- Downes, A.; Thos, P.B. Researches on the Effect of Light upon Bacteria and Other Organisms. Proc. R. Soc. Lond. 1877, 26, 488–500. [Google Scholar]
- Ekpe, J. The Chemistry of Light: The Life and Work of Theobald Adrian Palm (1848–1928). J. Med. Biogr. 2009, 17, 155–160. [Google Scholar] [CrossRef]
- Grzybowski, A.; Pietrzak, K. From Patient to Discoverer—Niels Ryberg Finsen (1860–1904)—The Founder of Phototherapy in Dermatology. Clin. Dermatol. 2012, 30, 451–455. [Google Scholar] [CrossRef]
- Samia, A.C.S.; Chen, X.; Burda, C. Semiconductor Quantum Dots for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef]
- Bakalova, R.; Ohba, H.; Zhelev, Z.; Ishikawa, M.; Baba, Y. Quantum Dots as Photosensitizers? Nat. Biotechnol. 2004, 22, 1360–1361. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.-T.; Law, W.-C.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity Assessment of Quantum Dots: From Cellular to Primate Studies. Chem. Soc. Rev. 2013, 42, 1236–1250. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Huang, Y.-Y. (Eds.) Handbook of Photomedicine; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4398-8469-0. [Google Scholar]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A Genetically Encoded Photosensitizer. Nat. Biotechnol. 2006, 24, 95–99. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A Graphene Quantum Dot Photodynamic Therapy Agent with High Singlet Oxygen Generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Ristic, B.Z.; Arsikin, K.M.; Klisic, D.G.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepic, D.P.; Kravic-Stevovic, T.K.; Jovanovic, S.P.; Milenkovic, M.M.; et al. Graphene Quantum Dots as Autophagy-Inducing Photodynamic Agents. Biomaterials 2012, 33, 7084–7092. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic Antimicrobial Chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Zeina, B.; Greenman, J.; Purcell, W.M.; Das, B. Killing of Cutaneous Microbial Species by Photodynamic Therapy. Br. J. Dermatol. 2001, 144, 274–278. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic Therapy for Localized Infections—State of the Art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Boyle, R.W.; Dolphin, D. Structure and Biodistribution Relationships of Photodynamic Sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921. [Google Scholar] [CrossRef]
- Müller-Breitkreutz, K.; Mohr, H.; Briviba, K.; Sies, H. Inactivation of Viruses by Chemically and Photochemically Generated Singlet Molecular Oxygen. J. Photochem. Photobiol. B Biol. 1995, 30, 63–70. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Chow, Y.W.; Pietranico, R.; Mukerji, A. Studies of Oxygen Binding Energy to Hemoglobin Molecule. Biochem. Biophys. Res. Commun. 1975, 66, 1424–1431. [Google Scholar] [CrossRef]
- Spikes, J.D.; Jori, G. Photodynamic Therapy of Tumours and Other Diseases Using Porphyrins. Laser Med. Sci. 1987, 2, 3–15. [Google Scholar] [CrossRef]
- Moreira, L.M.; Vieira Dos Santos, F.; Lyon, J.P.; Maftoum-Costa, M.; Pacheco-Soares, C.; Soares Da Silva, N. Photodynamic Therapy: Porphyrins and Phthalocyanines as Photosensitizers. Aust. J. Chem. 2008, 61, 741. [Google Scholar] [CrossRef]
- Blume, J.E.; Oseroff, A.R. Aminolevulinic Acid Photodynamic Therapy for Skin Cancers. Dermatol. Clin. 2007, 25, 5–14. [Google Scholar] [CrossRef]
- Ong, Y.H.; Kim, M.M.; Finlay, J.C.; Dimofte, A.; Singhal, S.; Glatstein, E.; Cengel, K.A.; Zhu, T.C. PDT Dose Dosimetry for Photofrin-Mediated Pleural Photodynamic Therapy (pPDT). Phys. Med. Biol. 2017, 63, 015031. [Google Scholar] [CrossRef]
- Moghissi, K.; Dixon, K.; Stringer, M.; Thorpe, J.A.C. Photofrin PDT for Early Stage Oesophageal Cancer: Long Term Results in 40 Patients and Literature Review. Photodiagnosis Photodyn. Ther. 2009, 6, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.B.; Da Silva, C.L.; Davanzo, N.N.; Da Silva Souza, R.; Correa, R.J.; Tedesco, A.C.; Riemma Pierre, M.B. Protoporphyrin IX (PpIX) Loaded PLGA Nanoparticles for Topical Photodynamic Therapy of Melanoma Cells. Photodiagnosis Photodyn. Ther. 2021, 35, 102317. [Google Scholar] [CrossRef]
- Tsuchida, T.; Matsumoto, Y.; Imabayashi, T.; Uchimura, K.; Sasada, S. Photodynamic Therapy Can Be Safely Performed with Talaporfin Sodium as a Day Treatment for Central-Type Early-Stage Lung Cancer. Photodiagnosis Photodyn. Ther. 2022, 38, 102836. [Google Scholar] [CrossRef]
- Tanaka, M.; Kataoka, H.; Yano, S.; Sawada, T.; Akashi, H.; Inoue, M.; Suzuki, S.; Inagaki, Y.; Hayashi, N.; Nishie, H.; et al. Immunogenic Cell Death Due to a New Photodynamic Therapy (PDT) with Glycoconjugated Chlorin (G-Chlorin). Oncotarget 2016, 7, 47242–47251. [Google Scholar] [CrossRef] [PubMed]
- Zenkevich, E.; Sagun, E.; Knyukshto, V.; Shulga, A.; Mironov, A.; Efremova, O.; Bonnett, R.; Songca, S.P.; Kassem, M. Photophysical and Photochemical Properties of Potential Porphyrin and Chlorin Photosensitizers for PDT. J. Photochem. Photobiol. B Biol. 1996, 33, 171–180. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New Photodynamic Therapy with Next-Generation Photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef] [PubMed]
- Duchi, S.; Ramos-Romero, S.; Dozza, B.; Guerra-Rebollo, M.; Cattini, L.; Ballestri, M.; Dambruoso, P.; Guerrini, A.; Sotgiu, G.; Varchi, G.; et al. Development of Near-Infrared Photoactivable Phthalocyanine-Loaded Nanoparticles to Kill Tumor Cells: An Improved Tool for Photodynamic Therapy of Solid Cancers. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Muli, D.K.; Carpenter, B.L.; Mayukh, M.; Ghiladi, R.A.; McGrath, D.V. Dendritic Near-IR Absorbing Zinc Phthalocyanines for Antimicrobial Photodynamic Therapy. Tetrahedron Lett. 2015, 56, 3541–3545. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; De Sousa Júnior, W.T.; Mundim, T.; Vale, C.L.C.; De Oliveira, J.V.; Ganassin, R.; Pacheco, T.J.A.; Vasconcelos Morais, J.A.; Longo, J.P.F.; Azevedo, R.B.; et al. Induction of Immunogenic Cell Death by Photodynamic Therapy Mediated by Aluminum-Phthalocyanine in Nanoemulsion. Pharmaceutics 2022, 14, 196. [Google Scholar] [CrossRef]
- Bonelli, J.; Ortega-Forte, E.; Vigueras, G.; Follana-Berná, J.; Ashoo, P.; Abad-Montero, D.; Isidro, N.; López-Corrales, M.; Hernández, A.; Ortiz, J.; et al. A Nanoencapsulated Ir(III)-Phthalocyanine Conjugate as a Promising Photodynamic Therapy Anticancer Agent. ACS Appl. Mater. Interfaces 2024, 16, 38916–38930. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, K.; Wang, S.; Qiu, L.; Liu, Q.; Xie, M.; Lin, J. Targeted Photodynamic Therapy (PDT) of Lung Cancer with Biotinylated Silicon (IV) Phthalocyanine. Curr. Parmaceutical Biotechnol. 2021, 22, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and Their Metal Complexes as NIR-Absorbing Photosensitizers: Properties, Mechanisms, and Applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Pratavieira, S.; Uliana, M.P.; Dos Santos Lopes, N.S.; Donatoni, M.C.; Linares, D.R.; De Freitas Anibal, F.; De Oliveira, K.T.; Kurachi, C.; De Souza, C.W.O. Photodynamic Therapy with a New Bacteriochlorin Derivative: Characterization and in Vitro Studies. Photodiagnosis Photodyn. Ther. 2021, 34, 102251. [Google Scholar] [CrossRef]
- Amos-Tautua, B.; Fakayode, O.; Songca, S.; Oluwafemi, S. Synthesis, Spectroscopic Characterization and Singlet Oxygen Generation of 5,10,15,20-Tetrakis(3,5-Dimethoxyphenyl) Porphyrin as a Potential Photosensitizer for Photodynamic Therapy. Chem. Int. 2021, 7, 30–38. [Google Scholar] [CrossRef]
- Szurko, A.; Rams-Baron, M.; Montforts, F.-P.; Bauer, D.; Kozub, P.; Gubernator, J.; Altmann, S.; Stanek, A.; Sieroń, A.; Ratuszna, A. Photodynamic Performance of Amphiphilic Chlorin E6 Derivatives with Appropriate Properties: A Comparison between Different-Type Liposomes as Delivery Systems. Photodiagnosis Photodyn. Ther. 2020, 30, 101799. [Google Scholar] [CrossRef] [PubMed]
- Vilsinski, B.H.; Gonçalves, R.S.; Caetano, W.; De Souza, P.R.; De Oliveira, A.C.; Gomes, Y.S.; Gerola, A.P.; Martins, A.F.; Valente, A.J.M.; Muniz, E.C. Photodynamic Therapy: Use of Nanocarrier Systems to Improve Its Effectiveness. In Functional Properties of Advanced Engineering Materials and Biomolecules; La Porta, F.A., Taft, C.A., Eds.; Engineering Materials; Springer International Publishing: Cham, Switzerland, 2021; pp. 289–316. ISBN 978-3-030-62225-1. [Google Scholar]
- Bistaffa, M.J.; Camacho, S.A.; Melo, C.F.O.R.; Catharino, R.R.; Toledo, K.A.; Aoki, P.H.B. Plasma Membrane Permeabilization to Explain Erythrosine B Phototoxicity on in Vitro Breast Cancer Cell Models. J. Photochem. Photobiol. B Biol. 2021, 223, 112297. [Google Scholar] [CrossRef]
- Karaman, O.; Alkan, G.A.; Kizilenis, C.; Akgul, C.C.; Gunbas, G. Xanthene Dyes for Cancer Imaging and Treatment: A Material Odyssey. Coord. Chem. Rev. 2023, 475, 214841. [Google Scholar] [CrossRef]
- Ebaston, T.M.; Nakonechny, F.; Talalai, E.; Gellerman, G.; Patsenker, L. Iodinated Xanthene-Cyanine NIR Dyes as Potential Photosensitizers for Antimicrobial Photodynamic Therapy. Dye. Pigment. 2021, 184, 108854. [Google Scholar] [CrossRef]
- Dhaini, B.; Wagner, L.; Moinard, M.; Daouk, J.; Arnoux, P.; Schohn, H.; Schneller, P.; Acherar, S.; Hamieh, T.; Frochot, C. Importance of Rose Bengal Loaded with Nanoparticles for Anti-Cancer Photodynamic Therapy. Pharmaceuticals 2022, 15, 1093. [Google Scholar] [CrossRef]
- Hirose, M.; Yoshida, Y.; Horii, K.; Hasegawa, Y.; Shibuya, Y. Efficacy of Antimicrobial Photodynamic Therapy with Rose Bengal and Blue Light against Cariogenic Bacteria. Arch. Oral Biol. 2021, 122, 105024. [Google Scholar] [CrossRef]
- Cho, G.; Ha, J. Erythrosine B (Red Dye No. 3): A Potential Photosensitizer for the Photodynamic Inactivation of Foodborne Pathogens in Tomato Juice. J. Food Saf. 2020, 40, e12813. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Ohkubo, K.; Sato, K.; Bunno, A.; Mori, T.; Osakada, Y.; Fujitsuka, M.; Kida, T. Fluorescein-Based Type I Supramolecular Photosensitizer via Induction of Charge Separation by Self-Assembly. JACS Au 2022, 2, 1472–1478. [Google Scholar] [CrossRef]
- Garg, P.; Kaur, G.; Sharma, B.; Chaudhary, G.R. Fluorescein–Metal Hybrid Surfactant Conjugates as a Smart Material for Antimicrobial Photodynamic Therapy against Staphylococcus Aureus. ACS Appl. Bio Mater. 2020, 3, 4674–4683. [Google Scholar] [CrossRef]
- Yadav, A.K.; Upadhyay, A.; Bera, A.; Kushwaha, R.; Mandal, A.A.; Acharjee, S.; Kunwar, A.; Banerjee, S. Anticancer Profile of Coumarin 6-Based Ir(iii) Photocatalysts under Normoxia and Hypoxia by ROS Generation and NADH Oxidation. Inorg. Chem. Front. 2024, 11, 5435–5448. [Google Scholar] [CrossRef]
- Ortega-Forte, E.; Rovira, A.; Gandioso, A.; Bonelli, J.; Bosch, M.; Ruiz, J.; Marchán, V. COUPY Coumarins as Novel Mitochondria-Targeted Photodynamic Therapy Anticancer Agents. J. Med. Chem. 2021, 64, 17209–17220. [Google Scholar] [CrossRef] [PubMed]
- Ratkaj, I.; Mušković, M.; Malatesti, N. Targeting Microenvironment of Melanoma and Head and Neck Cancersin Photodynamic Therapy. CMC 2022, 29, 3261–3299. [Google Scholar] [CrossRef]
- Hanada, Y.; Pereira, S.P.; Pogue, B.; Maytin, E.V.; Hasan, T.; Linn, B.; Mangels-Dick, T.; Wang, K.K. EUS-Guided Verteporfin Photodynamic Therapy for Pancreatic Cancer. Gastrointest. Endosc. 2021, 94, 179–186. [Google Scholar] [CrossRef]
- Mae, Y.; Kanda, T.; Sugihara, T.; Takata, T.; Kinoshita, H.; Sakaguchi, T.; Hasegawa, T.; Tarumoto, R.; Edano, M.; Kurumi, H.; et al. Verteporfin-photodynamic Therapy Is Effective on Gastric Cancer Cells. Mol. Clin. Onc. 2020. [Google Scholar] [CrossRef]
- Jeising, S.; Geerling, G.; Guthoff, R.; Hänggi, D.; Sabel, M.; Rapp, M.; Nickel, A.-C. In-Vitro Use of Verteporfin for Photodynamic Therapy in Glioblastoma. Photodiagnosis Photodyn. Ther. 2022, 40, 103049. [Google Scholar] [CrossRef] [PubMed]
- Sheng, T.; Ong, Y.; Guo, W.; Zhu, T.C. Reactive Oxygen Species Explicit Dosimetry to Predict Tumor Growth for Benzoporphyrin Derivative-Mediated Vascular Photodynamic Therapy. J. Biomed. Opt. 2020, 25, 1. [Google Scholar] [CrossRef]
- Yassine, A.-A.; Lo, W.C.Y.; Saeidi, T.; Ferguson, D.; Whyne, C.M.; Akens, M.K.; Betz, V.; Lilge, L. Photodynamic Therapy Outcome Modelling for Patients with Spinal Metastases: A Simulation-Based Study. Sci. Rep. 2021, 11, 17871. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K. Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Verteporfin Therapy of Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration: 5-Year Results of Two Randomized Clinical Trials with an Open-Label Extension: TAP Report No. 8. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 1132–1142. [Google Scholar] [CrossRef]
- Photodynamic Therapy of Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration with Verteporfin: One-Year Results of 2 Randomized Clinical Trials—TAP Report 1. Arch. Ophthalmol. 1999, 117, 1329. [CrossRef]
- Macdonald, I.J.; Dougherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 05, 105–129. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Broadwater, D.M. Tunable Fluorescent Organic Salts for Imaging and Therapy (Order No. 29062411). Available from ProQuest Dissertations & Theses Global. (2644425694). 2022. Available online: https://Wku.Idm.Oclc.Org/Login?Url=https://Www.Proquest.Com/Dissertations-Theses/Tunable-Fluorescent-Organic-Salts-Imaging-Therapy/Docview/2644425694/Se-2 (accessed on 8 August 2024).
- Kylychbekov, S.; Allamyradov, Y.; Khuzhakulov, Z.; Majidov, I.; Banga, S.; Ben Yosef, J.; Duta, L.; Er, A.O. Bioactivity and Mechanical Properties of Hydroxyapatite on Ti6Al4V and Si(100) Surfaces by Pulsed Laser Deposition. Coatings 2023, 13, 1681. [Google Scholar] [CrossRef]
- Khuzhakulov, Z.; Kylychbekov, S.; Allamyradov, Y.; Majidov, I.; Ben Yosef, J.; Er, A.Y.; Kitchens, C.; Banga, S.; Badarudeen, S.; Er, A.O. Formation of Picosecond Laser-Induced Periodic Surface Structures on Steel for Knee Arthroplasty Prosthetics. Front. Met. Alloy 2023, 1, 1090104. [Google Scholar] [CrossRef]
- Ilhom, S.; Kholikov, K.; Li, P.; Ottman, C.; Sanford, D.; Thomas, Z. Scalable Patterning Using Laser-Induced Shock Waves. Opt. Eng. 2018, 57, 1. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Algorri, J.F.; López-Higuera, J.M.; Rodríguez-Cobo, L.; Cobo, A. Advanced Light Source Technologies for Photodynamic Therapy of Skin Cancer Lesions. Pharmaceutics 2023, 15, 2075. [Google Scholar] [CrossRef]
- Hammond, E.M.; Asselin, M.-C.; Forster, D.; O’Connor, J.P.B.; Senra, J.M.; Williams, K.J. The Meaning, Measurement and Modification of Hypoxia in the Laboratory and the Clinic. Clin. Oncol. 2014, 26, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Han, J.; Jin, F.; Du, Y. Recent Strategies to Address Hypoxic Tumor Environments in Photodynamic Therapy. Pharmaceutics 2022, 14, 1763. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sinha, S.; Shrivastava, N. Therapeutic Targeting Hypoxia-Inducible Factor (HIF-1) in Cancer: Cutting Gordian Knot of Cancer Cell Metabolism. Front. Genet. 2022, 13, 849040. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; O’Hara, J.A.; Goodwin, I.A.; Wilmot, C.J.; Fournier, G.P.; Akay, A.R.; Swartz, H. Tumor Po2 Changes during Photodynamic Therapy Depend upon Photosensitizer Type and Time after Injection. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 132, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Szeimies, R.-M.; Basset-Séguin, N.; Calzavara-Pinton, P.G.; Gilaberte, Y.; Hædersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum Guidelines on Topical Photodynamic Therapy 2019 Part 2: Emerging IndicationsField Cancerization, Photorejuvenation and Inflammatory/Infective Dermatoses. Acad. Dermatol. Venereol. 2020, 34, 17–29. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic Therapy with Fullerenes In Vivo: Reality or a Dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef]
- Ronzani, F.; Trivella, A.; Arzoumanian, E.; Blanc, S.; Sarakha, M.; Richard, C.; Oliveros, E.; Lacombe, S. Comparison of the Photophysical Properties of Three Phenothiazine Derivatives: Transient Detection and Singlet Oxygen Production. Photochem. Photobiol. Sci. 2013, 12, 2160–2169. [Google Scholar] [CrossRef]
- Chen, J.; Cesario, T.C.; Rentzepis, P.M. Time Resolved Spectroscopic Studies of Methylene Blue and Phenothiazine Derivatives Used for Bacteria Inactivation. Chem. Phys. Lett. 2010, 498, 81–85. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The Photodegradation of Porphyrins in Cells Can Be Used to Estimate the Lifetime of Singlet Oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Pryor, W.A. Oxy-Radicals and Related Species: Their Formation, Lifetimes, and Reactions. Annu. Rev. Physiol. 1986, 48, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Barbora, A.; Bohar, O.; Sivan, A.A.; Magory, E.; Nause, A.; Minnes, R. Higher Pulse Frequency of Near-Infrared Laser Irradiation Increases Penetration Depth for Novel Biomedical Applications. PLoS ONE 2021, 16, e0245350. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 12556. [Google Scholar] [CrossRef]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.-C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef]
- Udomsak, W.; Kucinska, M.; Pospieszna, J.; Dams-Kozlowska, H.; Chatuphonprasert, W.; Murias, M. Antioxidant Enzymes in Cancer Cells: Their Role in Photodynamic Therapy Resistance and Potential as Targets for Improved Treatment Outcomes. Int. J. Mol. Sci. 2024, 25, 3164. [Google Scholar] [CrossRef] [PubMed]
- Aniogo, E.C.; George, B.P.; Abrahamse, H. Molecular Effectors of Photodynamic Therapy-Mediated Resistance to Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13182. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; De Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; De Fátima Turchiello, R.; Baptista, M.S. Methylene Blue in Photodynamic Therapy: From Basic Mechanisms to Clinical Applications. Photodiagnosis Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Almeida, J.; Silva, A.M.G.; Rangel, M. Application of Photosensitizers in Photodynamic Diagnosis and Therapy of Cancer. In Interdisciplinary Cancer Research; Springer International Publishing: Cham, Switzerland, 2024. [Google Scholar]
- Dabrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Millenbaugh, N.; Baskin, J.; DeSilva, M.; Elliott, W.R.; Glickman, R. Photothermal Killing of Staphylococcus aureus Using Antibody-Targeted Gold Nanoparticles. Int. J. Nanomed. 2015, 1953. [Google Scholar] [CrossRef]
- Ge, P.; Zhang, J.; Ding, T.; Xianyu, Y. Surface Chemistry of Gold Nanoparticles for Bacterial Detection and Antimicrobial Applications. ACS Mater. Lett. 2023, 5, 638–655. [Google Scholar] [CrossRef]
- Akhtar, F.; Khan, A.U.; Qazi, B.; Kulanthaivel, S.; Mishra, P.; Akhtar, K.; Ali, A. A Nano Phototheranostic Approach of Toluidine Blue Conjugated Gold Silver Core Shells Mediated Photodynamic Therapy to Treat Diabetic Foot Ulcer. Sci. Rep. 2021, 11, 24464. [Google Scholar] [CrossRef]
- Chi, Y.; Qin, J.; Li, Z.; Ge, Q.; Zeng, W. Enhanced Anti-Tumor Efficacy of 5-Aminolevulinic Acid-Gold Nanoparticles-Mediated Photodynamic Therapy in Cutaneous Squamous Cell Carcinoma Cells. Braz. J. Med. Biol. Res. 2020, 53, e8457. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, D.; George, B.P.; Abrahamse, H. Conjugation of Hypericin to Gold Nanoparticles for Enhancement of Photodynamic Therapy in MCF-7 Breast Cancer Cells. Pharmaceutics 2022, 14, 2212. [Google Scholar] [CrossRef] [PubMed]
- Hakimov, S.; Kylychbekov, S.; Harness, B.; Neupane, S.; Hurley, J.; Brooks, A.; Banga, S.; Er, A.O. Evaluation of Silver Nanoparticles Attached to Methylene Blue as an Antimicrobial Agent and Its Cytotoxicity. Photodiagnosis Photodyn. Ther. 2022, 39, 102904. [Google Scholar] [CrossRef]
- Belekov, E.; Kholikov, K.; Cooper, L.; Banga, S.; Er, A.O. Improved Antimicrobial Properties of Methylene Blue Attached to Silver Nanoparticles. Photodiagnosis Photodyn. Ther. 2020, 32, 102012. [Google Scholar] [CrossRef] [PubMed]
- Allamyradov, Y.; Yosef, J.B.; Kylychbekov, S.; Majidov, I.; Khuzhakulov, Z.; Er, A.Y.; Kitchens, C.; Banga, S.; Er, A.O. The Role of Efflux Pump Inhibitor in Enhancing Antimicrobial Efficiency of Ag NPs and MB as an Effective Photodynamic Therapy Agent. Photodiagnosis Photodyn. Ther. 2024, 47, 104212. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, T.; Yang, N.; Niu, X.; Zhou, Z.; Wang, J.; Yang, D.; Chen, P.; Zhong, L.; Dong, X.; et al. POD Nanozyme Optimized by Charge Separation Engineering for Light/pH Activated Bacteria Catalytic/Photodynamic Therapy. Sig. Transduct. Target Ther. 2022, 7, 86. [Google Scholar] [CrossRef]
- Habiba, K.; Encarnacion-Rosado, J.; Garcia-Pabon, K.; Villalobos-Santos, J.C.; Makarov, V.I.; Avalos, J.A.; Weiner, B.R.; Morell, G. Improving Cytotoxicity against Cancer Cells by Chemo-Photodynamic Combined Modalities Using Silver-Graphene Quantum Dots Nanocomposites. Int. J. Nanomed. 2015, 107. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Z.; Zhang, L.; Zhao, J.; Hou, C.; Zhao, S. A near Infrared Dye-Coated Silver Nanoparticle/Carbon Dot Nanocomposite for Targeted Tumor Imaging and Enhanced Photodynamic Therapy. Nanoscale Adv. 2020, 2, 489–494. [Google Scholar] [CrossRef]
- Paramanantham, P.; Siddhardha, B.; Lal Sb, S.; Sharan, A.; Alyousef, A.A.; Al Dosary, M.S.; Arshad, M.; Syed, A. Antimicrobial Photodynamic Therapy on Staphylococcus Aureus and Escherichia Coli Using Malachite Green Encapsulated Mesoporous Silica Nanoparticles: An in Vitro Study. PeerJ 2019, 7, e7454. [Google Scholar] [CrossRef]
- Mirzahosseinipour, M.; Khorsandi, K.; Hosseinzadeh, R.; Ghazaeian, M.; Shahidi, F.K. Antimicrobial Photodynamic and Wound Healing Activity of Curcumin Encapsulated in Silica Nanoparticles. Photodiagnosis Photodyn. Ther. 2020, 29, 101639. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chu, B.; Wang, J.; Song, B.; Su, Y.; Wang, H.; He, Y. Multifunctional Nanoagents for Ultrasensitive Imaging and Photoactive Killing of Gram-Negative and Gram-Positive Bacteria. Nat. Commun. 2019, 10, 4057. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.P.; Agarrabeitia, A.R.; Ortiz, M.J.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Xia, Y.; Lan, Y.; Mokeem, L.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Magnetic-Responsive Photosensitizer Nanoplatform for Optimized Inactivation of Dental Caries-Related Biofilms: Technology Development and Proof of Principle. ACS Nano 2021, 15, 19888–19904. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Mokeem, L.; Alsahafi, R.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Magnetic-based Photosensitizer to Improve the Efficiency of Antimicrobial Photodynamic Therapy against Mature Dental Caries-related Biofilms. MedComm Biomater. Appl. 2023, 2, e67. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene Quantum Dots: Emergent Nanolights for Bioimaging, Sensors, Catalysis and Photovoltaic Devices. Chem. Commun. 2012, 48, 3686. [Google Scholar] [CrossRef]
- Thakur, M.; Kumawat, M.K.; Srivastava, R. Multifunctional Graphene Quantum Dots for Combined Photothermal and Photodynamic Therapy Coupled with Cancer Cell Tracking Applications. RSC Adv. 2017, 7, 5251–5261. [Google Scholar] [CrossRef]
- Du, D.; Wang, K.; Wen, Y.; Li, Y.; Li, Y.Y. Photodynamic Graphene Quantum Dot: Reduction Condition Regulated Photoactivity and Size Dependent Efficacy. ACS Appl. Mater. Interfaces 2016, 8, 3287–3294. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Nguyen, P.; Alshogeathri, A.; Hibbeler, L.; Martinez, F.; McNeil, N.; Berry, V. Graphene Quantum Dots Interfaced with Single Bacterial Spore for Bio-Electromechanical Devices: A Graphene Cytobot. Sci. Rep. 2015, 5, 9138. [Google Scholar] [CrossRef]
- Christensen, I.L.; Sun, Y.-P.; Juzenas, P. Carbon Dots as Antioxidants and Prooxidants. J. Biomed. Nanotechnol 2011, 7, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene Quantum Dots-Band-Aids Used for Wound Disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef] [PubMed]
- Kholikov, K. Enhanced Singlet Oxygen Generation and Antimicrobial Activity of Methylene Blue Coupled with Graphene Quantum Dots as an Effective Photodynamic Therapy Agent. Master’s Thesis, Western Kentucky University, Bowling Green, KY, USA, 2018. Available online: https://digitalcommons.wku.edu/theses/3059/ (accessed on 8 August 2024).
- Pourhajibagher, M.; Etemad-Moghadam, S.; Alaeddini, M.; Miri Mousavi, R.S.; Bahador, A. DNA-Aptamer-Nanographene Oxide as a Targeted Bio-Theragnostic System in Antimicrobial Photodynamic Therapy against Porphyromonas Gingivalis. Sci. Rep. 2022, 12, 12161. [Google Scholar] [CrossRef]
- Mir, I.A.; Radhakrishanan, V.S.; Rawat, K.; Prasad, T.; Bohidar, H.B. Bandgap Tunable AgInS Based Quantum Dots for High Contrast Cell Imaging with Enhanced Photodynamic and Antifungal Applications. Sci. Rep. 2018, 8, 9322. [Google Scholar] [CrossRef]
- Mohapatra, P.; Singh, D.; Sahoo, S.K. PEGylated Nanoparticles as a Versatile Drug Delivery System. In Nanoengineering of Biomaterials; Jana, S., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 309–341. ISBN 978-3-527-34904-3. [Google Scholar]
- Lee, H.M.; Jeong, Y.-I.; Kim, D.H.; Kwak, T.W.; Chung, C.-W.; Kim, C.H.; Kang, D.H. Ursodeoxycholic Acid-Conjugated Chitosan for Photodynamic Treatment of HuCC-T1 Human Cholangiocarcinoma Cells. Int. J. Pharm. 2013, 454, 74–81. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Polycationic Chitosan-Conjugated Photosensitizer for Antibacterial Photodynamic Therapy. Photochem. Photobiol. 2012, 88, 577–583. [Google Scholar] [CrossRef]
- Pietra, R.C.C.D.S.; Cruz, R.C.; Melo, C.N.; Rodrigues, L.B.; Santos, P.C.; Bretz, G.P.M.; Soares, B.M.; Sousa, G.R.D.; Ferreira, M.V.L.; Cisalpino, P.S.; et al. Evaluation of Polymeric PLGA Nanoparticles Conjugated to Curcumin for Use in aPDT. Braz. J. Pharm. Sci. 2017, 53. [Google Scholar] [CrossRef]
- Dereje, D.M.; Pontremoli, C.; García, A.; Galliano, S.; Colilla, M.; González, B.; Vallet-Regí, M.; Izquierdo-Barba, I.; Barbero, N. Poly Lactic-Co-Glycolic Acid (PLGA) Loaded with a Squaraine Dye as Photosensitizer for Antimicrobial Photodynamic Therapy. Polymers 2024, 16, 1962. [Google Scholar] [CrossRef]
- Perni, S.; Preedy, E.C.; Prokopovich, P. Amplify Antimicrobial Photo Dynamic Therapy Efficacy with Poly-Beta-Amino Esters (PBAEs). Sci. Rep. 2021, 11, 7275. [Google Scholar] [CrossRef]
- De Freitas, L.M.; Lorenzón, E.N.; Santos-Filho, N.A.; Zago, L.H.D.P.; Uliana, M.P.; De Oliveira, K.T.; Cilli, E.M.; Fontana, C.R. Antimicrobial Photodynamic Therapy Enhanced by the Peptide Aurein 1.2. Sci. Rep. 2018, 8, 4212. [Google Scholar] [CrossRef] [PubMed]
- Cimino, F.; Saija, A.; Speciale, A. Applications of Plant-Derived Products in Photodynamic Therapy. In Modulation of Oxidative Stress; Elsevier: Amsterda, The Neterlands, 2023; pp. 175–197. ISBN 978-0-443-19247-0. [Google Scholar]
- Rout, B.; Liu, C.-H.; Wu, W.-C. Photosensitizer in Lipid Nanoparticle: A Nano-Scaled Approach to Antibacterial Function. Sci. Rep. 2017, 7, 7892. [Google Scholar] [CrossRef] [PubMed]
- Silva, Z.S.; Huang, Y.-Y.; De Freitas, L.F.; França, C.M.; Botta, S.B.; Ana, P.A.; Mesquita-Ferrari, R.A.; Santos Fernandes, K.P.; Deana, A.; Lima Leal, C.R.; et al. Papain Gel Containing Methylene Blue for Simultaneous Caries Removal and Antimicrobial Photoinactivation against Streptococcus Mutans Biofilms. Sci. Rep. 2016, 6, 33270. [Google Scholar] [CrossRef] [PubMed]
- Costa-Santos, L.; Da Mota, A.C.C.; Prates, R.A.; Motta, L.J.; Horliana, A.C.R.T.; Silva-Junior, Z.S.; Mesquita-Ferrari, R.A.; Santos Fernandes, K.P.; Bussadori, S.K. The Effect of Antimicrobial Photodynamic Therapy Mediated by Papain Gel on Infected Dentin in Primary Teeth: A Clinical Trial with Microbiological Evaluation. Laser Dent. Sci. 2019, 3, 275–281. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Guo, Q.; Tao, L.; Ding, Y.; Ding, X.; Shen, X. Cyclodextrin-Based Nanoplatforms for Tumor Phototherapy: An Update. Pharmaceutics 2022, 14, 1375. [Google Scholar] [CrossRef]
- Rout, S.R.; Bandaru, R.; Kenguva, G.; Hasan, N.; Alam, M.S.; Shukla, R.; Almalki, W.H.; Kesharwani, P.; Dandela, R. Dendrimers in Photodynamic Therapy. In Nanomaterials for Photodynamic Therapy; Elsevier: Amsterda, The Neterlands, 2023; pp. 281–305. ISBN 978-0-323-85595-2. [Google Scholar]
- Mikulich, A.V.; Plavskii, V.Y.; Tretyakova, A.I.; Nahorny, R.K.; Sobchuk, A.N.; Dudchik, N.V.; Emeliyanova, O.A.; Zhabrouskaya, A.I.; Plavskaya, L.G.; Ananich, T.S.; et al. Potential of Using Medicinal Plant Extracts as Photosensitizers for Antimicrobial Photodynamic Therapy. Photochem. Photobiol. 2024. [Google Scholar] [CrossRef]
- Erdogan Eliuz, E.A.; Ayas, D.; Goksen, G. In Vitro Phototoxicity and Antimicrobial Activity of Volatile Oil Obtained from Some Aromatic Plants. J. Essent. Oil Bear. Plants 2017, 20, 758–768. [Google Scholar] [CrossRef]
- Sajjad, F.; Sun, N.; Chen, T.; Yan, Y.; Margetić, D.; Chen, Z. Evaluation of Antimicrobial Photodynamic Activities of 5-aminolevulinic Acid Derivatives. Photoderm. Photoimm. Photomed. 2021, 37, 296–305. [Google Scholar] [CrossRef]
- Li, X.; Guo, H.; Tian, Q.; Zheng, G.; Hu, Y.; Fu, Y.; Tan, H. Effects of 5-Aminolevulinic Acid–Mediated Photodynamic Therapy on Antibiotic-Resistant Staphylococcal Biofilm: An in Vitro Study. J. Surg. Res. 2013, 184, 1013–1021. [Google Scholar] [CrossRef]
- Tan, Y.; Cheng, Q.; Yang, H.; Li, H.; Gong, N.; Liu, D.; Wu, J.; Lei, X. Effects of ALA-PDT on Biofilm Structure, Virulence Factor Secretion, and QS in Pseudomonas aeruginosa. Photodiagnosis Photodyn. Ther. 2018, 24, 88–94. [Google Scholar] [CrossRef]
- Yu, T.-T.; Peng, X.-C.; Wang, M.-F.; Han, N.; Xu, H.-Z.; Li, Q.-R.; Li, L.-G.; Xu, X.; Ma, Q.-L.; Liu, B.; et al. Harnessing Chlorin E6 Loaded by Functionalized Iron Oxide Nanoparticles Linked with Glucose for Target Photodynamic Therapy and Improving of the Immunogenicity of Lung Cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Review: Organic Nanoparticle Based Active Targeting for Photodynamic Therapy Treatment of Breast Cancer Cells. Oncotarget 2020, 11, 2120–2136. [Google Scholar] [CrossRef] [PubMed]
- Faid, A.H.; Shouman, S.A.; Badr, Y.A.; Sharaky, M. Enhanced Cytotoxic Effect of Doxorubicin Conjugated Gold Nanoparticles on Breast Cancer Model. BMC Chem. 2022, 16, 90. [Google Scholar] [CrossRef]
- Awuah, S.G.; You, Y. Boron Dipyrromethene (BODIPY)-Based Photosensitizers for Photodynamic Therapy. RSC Adv. 2012, 2, 11169. [Google Scholar] [CrossRef]
- Li, G.; Yang, M.; Sha, Q.; Li, L.; Luo, X.; Wu, F. Self-Assembled BODIPY Derivative with A-D-A Structure as Organic Nanoparticles for Photodynamic/Photothermal Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 14473. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Z.; Shuai, Q.; Zhu, F.; Xu, J.; Gao, X.; Sun, X. Tumor-Targeting Peptide Functionalized PEG-PLA Micelles for Efficient Drug Delivery. Biomater. Sci. 2020, 8, 2274–2282. [Google Scholar] [CrossRef]
- Marasini, R.; Nguyen, T.D.T.; Rayamajhi, S.; Aryal, S. Synthesis and Characterization of a Tumor-Seeking LyP-1 Peptide Integrated Lipid–Polymer Composite Nanoparticle. Mater. Adv. 2020, 1, 469–480. [Google Scholar] [CrossRef]
- Shuai, Q.; Cai, Y.; Zhao, G.; Sun, X. Cell-Penetrating Peptide Modified PEG-PLA Micelles for Efficient PTX Delivery. Int. J. Mol. Sci. 2020, 21, 1856. [Google Scholar] [CrossRef]
- Lei, L.; Dai, W.; Man, J.; Hu, H.; Jin, Q.; Zhang, B.; Tang, Z. Lonidamine Liposomes to Enhance Photodynamic and Photothermal Therapy of Hepatocellular Carcinoma by Inhibiting Glycolysis. J. Nanobiotechnol. 2023, 21, 482. [Google Scholar] [CrossRef]
- De Leo, V.; Marras, E.; Maurelli, A.M.; Catucci, L.; Milano, F.; Gariboldi, M.B. Polydopamine-Coated Liposomes for Methylene Blue Delivery in Anticancer Photodynamic Therapy: Effects in 2D and 3D Cellular Models. Int. J. Mol. Sci. 2024, 25, 3392. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; Vazquez-Duhalt, R.; Hernández, L.R.; Sánchez-Arreola, E.; Bach, H. Virus-like Particles: Fundamentals and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 8579. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jia, D.; Yuan, D.; Yin, X.; Yuan, F.; Wang, F.; Shi, W.; Li, H.; Zhu, L.-M.; Fan, Q. Dimeric Her2-Specific Affibody Mediated Cisplatin-Loaded Nanoparticles for Tumor Enhanced Chemo-Radiotherapy. J. Nanobiotechnol. 2021, 19, 138. [Google Scholar] [CrossRef]
- White, B.E.; White, M.K.; Adhvaryu, H.; Makhoul, I.; Nima, Z.A.; Biris, A.S.; Ali, N. Nanotechnology Approaches to Addressing HER2-Positive Breast Cancer. Cancer Nano 2020, 11, 12. [Google Scholar] [CrossRef]
- Gong, H.; He, H.; Cai, Q.; Su, Z.; Wang, X.; Zhu, H. Precise Tuning of Porphyrin Self-Assembly and Photo-Activated Antimicrobial Activity via Metal Ion Coordination. Colloids Surf. A Physicochem. Eng. Asp. 2024, 134954. [Google Scholar] [CrossRef]
- Pucelik, B.; Barzowska, A.; Sułek, A.; Werłos, M.; Dąbrowski, J.M. Refining Antimicrobial Photodynamic Therapy: Effect of Charge Distribution and Central Metal Ion in Fluorinated Porphyrins on Effective Control of Planktonic and Biofilm Bacterial Forms. Photochem. Photobiol. Sci. 2024, 23, 539–560. [Google Scholar] [CrossRef]
- Tunçel, A.; Öztürk, İ.; Ince, M.; Ocakoglu, K.; Hoşgör-Limoncu, M.; Yurt, F. Antimicrobial Photodynamic Therapy against Staphylococcus Aureus Using Zinc Phthalocyanine and Zinc Phthalocyanine-Integrated TiO2 Nanoparticles. J. Porphyr. Phthalocyanines 2019, 23, 206–212. [Google Scholar] [CrossRef]
- Pujari, A.K.; Kaur, R.; Reddy, Y.N.; Paul, S.; Gogde, K.; Bhaumik, J. Design and Synthesis of Metalloporphyrin Nanoconjugates for Dual Light-Responsive Antimicrobial Photodynamic Therapy. J. Med. Chem. 2024, 67, 2004–2018. [Google Scholar] [CrossRef] [PubMed]
- Skwor, T.A.; Klemm, S.; Zhang, H.; Schardt, B.; Blaszczyk, S.; Bork, M.A. Photodynamic Inactivation of Methicillin-Resistant Staphylococcus aureus and Escherichia Coli: A Metalloporphyrin Comparison. J. Photochem. Photobiol. B Biol. 2016, 165, 51–57. [Google Scholar] [CrossRef]
- Gholibegloo, E.; Karbasi, A.; Pourhajibagher, M.; Chiniforush, N.; Ramazani, A.; Akbari, T.; Bahador, A.; Khoobi, M. Carnosine-Graphene Oxide Conjugates Decorated with Hydroxyapatite as Promising Nanocarrier for ICG Loading with Enhanced Antibacterial Effects in Photodynamic Therapy against Streptococcus Mutans. J. Photochem. Photobiol. B Biol. 2018, 181, 14–22. [Google Scholar] [CrossRef]
- Guo, W.; Li, Y.; Wang, S.; Wang, Y.; Li, C.; Jin, Y.; Li, Y.; Chen, X.; Miao, W. Photodynamic Nano Hydroxyapatite with Biofilm Penetration Capability for Dental Plaque Eradication and Prevention of Demineralization. Colloids Surf. B Biointerfaces 2023, 225, 113242. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jia, C.; Zheng, N.; Liu, S.; Qi, Z.; Wang, R.; Zhang, L.; Niu, Y.; Pan, S. Effects of Photodynamic Therapy on Streptococcus Mutans and Enamel Remineralization of Multifunctional TiO2-HAP Composite Nanomaterials. Photodiagnosis Photodyn. Ther. 2023, 42, 103141. [Google Scholar] [CrossRef] [PubMed]
- Komine, C.; Takahashi, C.; Omori, H.; Ogura, Y.; Konishi, Y.; Suzuki, H.; Otsuka, I.; Fuchigami, M.; Tsuzukibashi, O.; Fukatsu, A.; et al. Bactericidal Effect of Antimicrobial Photodynamic Therapy Using Visible Light-Responsive Titanium Dioxide -the First Report. IJOMS 2020, 18, 155–163. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Wintner, A.; Seed, P.C.; Brauns, T.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial Photodynamic Therapy Mediated by Methylene Blue and Potassium Iodide to Treat Urinary Tract Infection in a Female Rat Model. Sci. Rep. 2018, 8, 7257. [Google Scholar] [CrossRef]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, X.; Szewczyk, G.; El-Hussein, A.; Huang, Y.-Y.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Antimicrobial Photodynamic Inactivation Mediated by Rose Bengal in In Vitro and In Vivo Studies. Antimicrob. Agents Chemother. 2017, 61, e00467-17. [Google Scholar] [CrossRef]
- Kashef, N.; Hamblin, M.R. In Vitro Potentiation of Antimicrobial Photodynamic Inactivation by Addition of Potassium Iodide. In Photodynamic Therapy; Broekgaarden, M., Zhang, H., Korbelik, M., Hamblin, M.R., Heger, M., Eds.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2022; Volume 2451, pp. 607–619. ISBN 978-1-07-162098-4. [Google Scholar]
- Benine-Warlet, J.; Brenes-Alvarado, A.; Steiner-Oliveira, C. Potassium Iodide Enhances Inactivation of Streptococcus Mutans Biofilm in Antimicrobial Photodynamic Therapy with Red Laser. Photodiagnosis Photodyn. Ther. 2022, 37, 102622. [Google Scholar] [CrossRef]
- Xuan, W.; He, Y.; Huang, L.; Huang, Y.-Y.; Bhayana, B.; Xi, L.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial Photodynamic Inactivation Mediated by Tetracyclines in Vitro and in Vivo: Photochemical Mechanisms and Potentiation by Potassium Iodide. Sci. Rep. 2018, 8, 17130. [Google Scholar] [CrossRef]
- Huang, L.; St. Denis, T.G.; Xuan, Y.; Huang, Y.-Y.; Tanaka, M.; Zadlo, A.; Sarna, T.; Hamblin, M.R. Paradoxical Potentiation of Methylene Blue-Mediated Antimicrobial Photodynamic Inactivation by Sodium Azide: Role of Ambient Oxygen and Azide Radicals. Free Radic. Biol. Med. 2012, 53, 2062–2071. [Google Scholar] [CrossRef]
- Hamblin, M.R. Potentiation of Antimicrobial Photodynamic Inactivation by Inorganic Salts. Expert Rev. Anti-Infect. Ther. 2017, 15, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Lee, D.W.; Park, H.J.; Kwak, M.H.; Park, J.M.; Choi, M. Hydrogen Peroxide Enhances the Antibacterial Effect of Methylene Blue-based Photodynamic Therapy on Biofilm-forming Bacteria. Photochem. Photobiol. 2019, 95, 833–838. [Google Scholar] [CrossRef]
- Sammarro Silva, K.J.; Lima, A.R.; Dias, L.D.; De Souza, M.; Nunes Lima, T.H.; Bagnato, V.S. Hydrogen Peroxide Preoxidation as a Strategy for Enhanced Antimicrobial Photodynamic Action against Methicillin-Resistant Staphylococcus Aureus. J. Water Health 2023, 21, 1922–1932. [Google Scholar] [CrossRef]
- Viana De Sousa, T.; Carolina Jordão, C.; Augusto Abreu-Pereira, C.; Gorayb Pereira, A.L.; Barbugli, P.A.; Klein, M.I.; Pavarina, A.C. Hydrogen Peroxide Enhances the Efficacy of Photodynamic Therapy against Candida Albicans Biofilms. Biofouling 2023, 39, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Rajda, P.J.; Szewczyk, G.; Bhayana, B.; Chiang, L.Y.; Sarna, T.; Hamblin, M.R. Sodium Nitrite Potentiates Antimicrobial Photodynamic Inactivation: Possible Involvement of Peroxynitrate. Photochem. Photobiol. Sci. 2019, 18, 505–515. [Google Scholar] [CrossRef]
- Miller, M.; James, G.; Bell, D.; Schultz, G. Antimicrobial Effects of an Acidified Nitrite Foam on Drip Flow Reactor Biofilm. JOWM 2024. [Google Scholar] [CrossRef]
- Sarda, R.A.; Shetty, R.M.; Tamrakar, A.; Shetty, S.Y. Antimicrobial Efficacy of Photodynamic Therapy, Diode Laser, and Sodium Hypochlorite and Their Combinations on Endodontic Pathogens. Photodiagnosis Photodyn. Ther. 2019, 28, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Oh, H.Y.; Kim, E.J.; Lee, H.K.; Kim, H.K.; Choi, H.H.; Kim, S.W.; Chae, H.S. In Vitro Bactericidal Effects of Photodynamic Therapy Combined with Four Tetracyclines against Clostridioides Difficile KCTC5009 in Planktonic Cultures. Pathogens 2020, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Branco, T.M.; Valério, N.C.; Jesus, V.I.R.; Dias, C.J.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Single and Combined Effects of Photodynamic Therapy and Antibiotics to Inactivate Staphylococcus aureus on Skin. Photodiagnosis Photodyn. Ther. 2018, 21, 285–293. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Partoazar, A.; Chiniforush, N. In Vitro Study of Nanoliposomes Containing Curcumin and Doxycycline for Enhanced Antimicrobial Photodynamic Therapy against Aggregatibacter Actinomycetemcomitans. Sci. Rep. 2023, 13, 11552. [Google Scholar] [CrossRef]
- Openda, Y.I.; Nyokong, T. Combination of Photodynamic Antimicrobial Chemotherapy and Ciprofloxacin to Combat S. Aureus and E. Coli Resistant Biofilms. Photodiagnosis Photodyn. Ther. 2023, 42, 103142. [Google Scholar] [CrossRef] [PubMed]
- Tichaczek-Goska, D.; Gleńsk, M.; Wojnicz, D. The Enhancement of the Photodynamic Therapy and Ciprofloxacin Activity against Uropathogenic Escherichia Coli Strains by Polypodium Vulgare Rhizome Aqueous Extract. Pathogens 2021, 10, 1544. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Dias, L.D.; Blanco, K.C.; Soares, J.M.; Alves, F.; Da Silva, G.J.; Arnaut, L.G.; Bagnato, V.S.; Pereira, M.M. Synergic Dual Phototherapy: Cationic Imidazolyl Photosensitizers and Ciprofloxacin for Eradication of in Vitro and in Vivo E. Coli Infections. J. Photochem. Photobiol. B Biol. 2022, 233, 112499. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Lee, J.; Im, B.N.; Park, H.; Na, K. Combined Photodynamic and Antibiotic Therapy for Skin Disorder via Lipase-Sensitive Liposomes with Enhanced Antimicrobial Performance. Biomaterials 2017, 141, 243–250. [Google Scholar] [CrossRef]
- Chibebe Junior, J.; Fuchs, B.B.; Sabino, C.P.; Junqueira, J.C.; Jorge, A.O.C.; Ribeiro, M.S.; Gilmore, M.S.; Rice, L.B.; Tegos, G.P.; Hamblin, M.R.; et al. Photodynamic and Antibiotic Therapy Impair the Pathogenesis of Enterococcus Faecium in a Whole Animal Insect Model. PLoS ONE 2013, 8, e55926. [Google Scholar] [CrossRef]
- Collins, T.L.; Markus, E.A.; Hassett, D.J.; Robinson, J.B. The Effect of a Cationic Porphyrin on Pseudomonas aeruginosa Biofilms. Curr Microbiol 2010, 61, 411–416. [Google Scholar] [CrossRef]
- Gong, N.; Tan, Y.; Li, M.; Lu, W.; Lei, X. ALA-PDT Combined with Antibiotics for the Treatment of Multiple Skin Abscesses Caused by Mycobacterium fortuitum. Photodiagnosis Photodyn. Ther. 2016, 15, 70–72. [Google Scholar] [CrossRef]
- Sun, K.; Yang, H.; Huang, X.; Gong, N.; Qin, Q.; Lu, W.; Lei, X. ALA-PDT Combined with Antibiotics for the Treatment of Atypical Mycobacterial Skin Infections: Outcomes and Safety. Photodiagnosis Photodyn. Ther. 2017, 19, 274–277. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R. A Comprehensive Overview of Photodynamic Therapy in the Treatment of Superficial Fungal Infections of the Skin. J. Photochem. Photobiol. B Biol. 2005, 78, 1–6. [Google Scholar] [CrossRef]
- Roomaney, I.A.; Holmes, H.K.; Engel, M.E. Treatment of Oral Fungal Infections Using Photodynamic Therapy: Systematic Review and Meta-analysis. Clin. Exp. Dent. Res. 2021, 7, 354–364. [Google Scholar] [CrossRef]
- Shen, J.J.; Jemec, G.B.E.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic Therapy Treatment of Superficial Fungal Infections: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 31, 101774. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Hamblin, M.R. Visible Blue Light Is Capable of Inactivating Candida Albicans and Other Fungal Species. Photomed. Laser Surg. 2017, 35, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.O.; Chau, M.; Stack, C. In Vitro Combination Therapy Using Low Dose Clotrimazole and Photodynamic Therapy Leads to Enhanced Killing of the Dermatophyte Trichophyton Rubrum. BMC Microbiol. 2014, 14, 261. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Zhang, J.; Chen, R.; Zhong, X.; Wu, X.; Zheng, L.; Zhao, J. In Vitro Evaluation of Photodynamic Effects Against Biofilms of Dermatophytes Involved in Onychomycosis. Front. Microbiol. 2019, 10, 1228. [Google Scholar] [CrossRef]
- Huang, X.; Han, K.; Wang, L.; Peng, X.; Zeng, K.; Li, L. Successful Treatment of Chromoblastomycosis Using ALA-PDT in a Patient with Leukopenia. Photodiagnosis Photodyn. Ther. 2019, 26, 13–14. [Google Scholar] [CrossRef]
- Xiu, W.; Wan, L.; Yang, K.; Li, X.; Yuwen, L.; Dong, H.; Mou, Y.; Yang, D.; Wang, L. Potentiating Hypoxic Microenvironment for Antibiotic Activation by Photodynamic Therapy to Combat Bacterial Biofilm Infections. Nat. Commun. 2022, 13, 3875. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Cheng, W.; Li, Y.; Li, D.; Wang, L.; Zhang, X.; Xiao, Y. Adoptive Macrophage Directed Photodynamic Therapy of Multidrug-Resistant Bacterial Infection. Nat. Commun. 2023, 14, 7251. [Google Scholar] [CrossRef] [PubMed]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Pieranski, M.; Woziwodzka, A.; Bielawski, K.P.; Grinholc, M. Development of Staphylococcus aureus Tolerance to Antimicrobial Photodynamic Inactivation and Antimicrobial Blue Light upon Sub-Lethal Treatment. Sci. Rep. 2019, 9, 9423. [Google Scholar] [CrossRef]
- Hui, J.; Moon, W.; Dong, P.-T.; Anjos, C.D.; Negri, L.; Yan, H.; Wang, Y.; Tam, J.; Dai, T.; Anderson, R.R.; et al. Antimicrobial Blue Light-Bathing Therapy for Wound Infection Control. bioRxiv 2024. [Google Scholar]


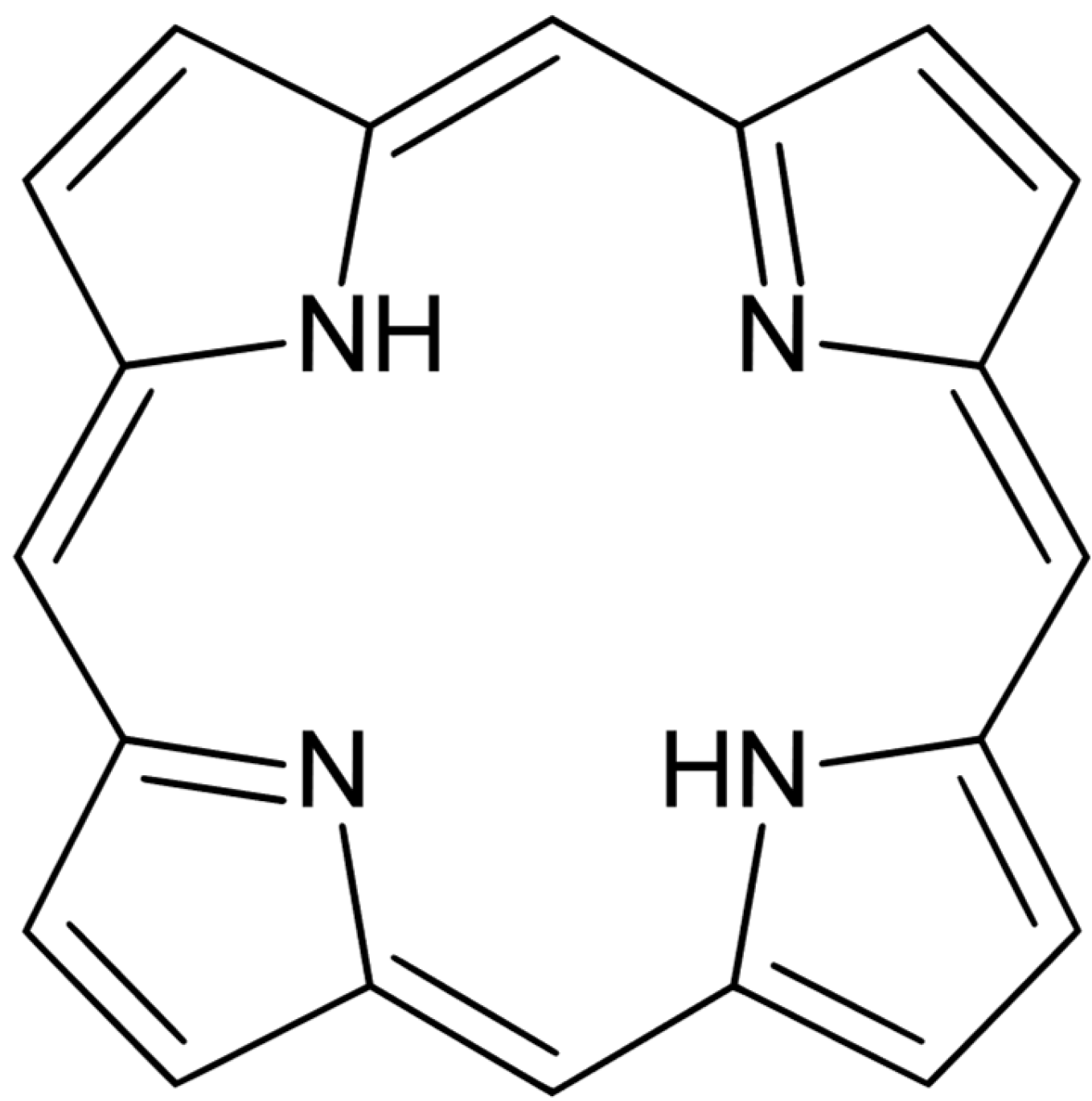
| Name (Commercially Available) | Structure | Wavelength | Application |
|---|---|---|---|
| Allumera (Hexyl Aminolevulinate) | C11H21NO3 | 560 nm | Facial photodamage |
| Photofrin | C68H74N8O11 (for n = 0) | 630 nm | Esophageal cancer, bladder cancer, non-small cell lung carcinoma |
| Visudyne | C41H42N4O8 | 689 nm | Retinopathy |
| Levulan (Aminolevulinic acid) | C5H9NO3 | 635 nm | Cancer diagnosis and experimental brain cancer treatment |
| Foscan (Temoporfin) | C44H32N4O4 | 652 nm | Squamous cell carcinoma of head and neck |
| Metvix (Methyl aminolevulinate) | C6H11NO3 | 630 nm | Non-melanoma skin cancer, basal cell carcinoma |
| Hevvix (Hexaminolevulinate) | C11H21NO3 | 360–450 nm | Bladder cancer |
| Laserphyrin | C38H41N5O9 | 665 nm | Lung cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allamyradov, Y.; ben Yosef, J.; Annamuradov, B.; Ateyeh, M.; Street, C.; Whipple, H.; Er, A.O. Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem 2024, 4, 434-461. https://doi.org/10.3390/photochem4040027
Allamyradov Y, ben Yosef J, Annamuradov B, Ateyeh M, Street C, Whipple H, Er AO. Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem. 2024; 4(4):434-461. https://doi.org/10.3390/photochem4040027
Chicago/Turabian StyleAllamyradov, Yaran, Justice ben Yosef, Berdimyrat Annamuradov, Mahmood Ateyeh, Carli Street, Hadley Whipple, and Ali Oguz Er. 2024. "Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges" Photochem 4, no. 4: 434-461. https://doi.org/10.3390/photochem4040027
APA StyleAllamyradov, Y., ben Yosef, J., Annamuradov, B., Ateyeh, M., Street, C., Whipple, H., & Er, A. O. (2024). Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem, 4(4), 434-461. https://doi.org/10.3390/photochem4040027





