Excitation–Emission Fluorescence Mapping Analysis of Microplastics That Are Typically Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Scanning Electron Microscopy
2.3. X-Ray Photoelectron Spectroscopy (XPS)
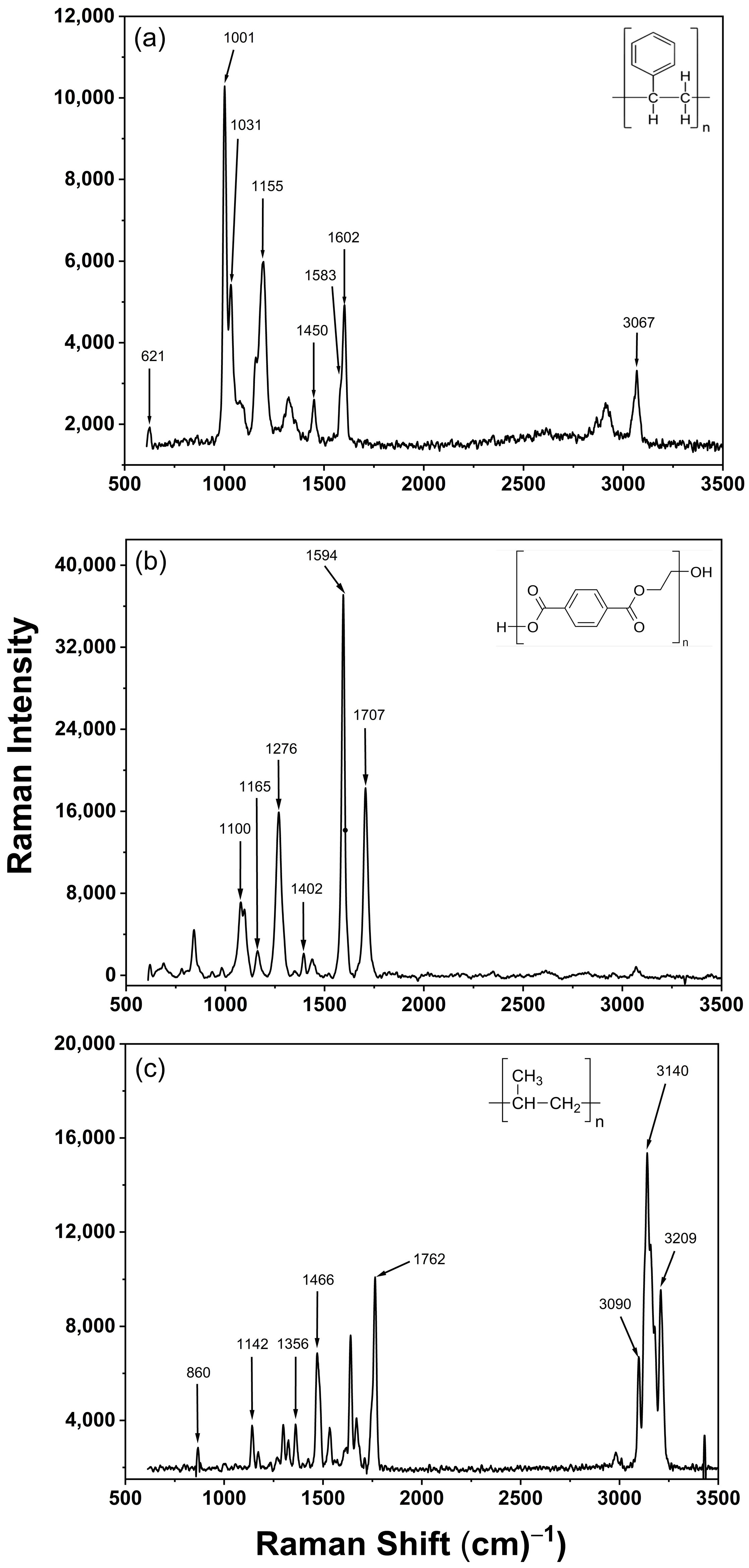
2.4. Raman Spectroscopy
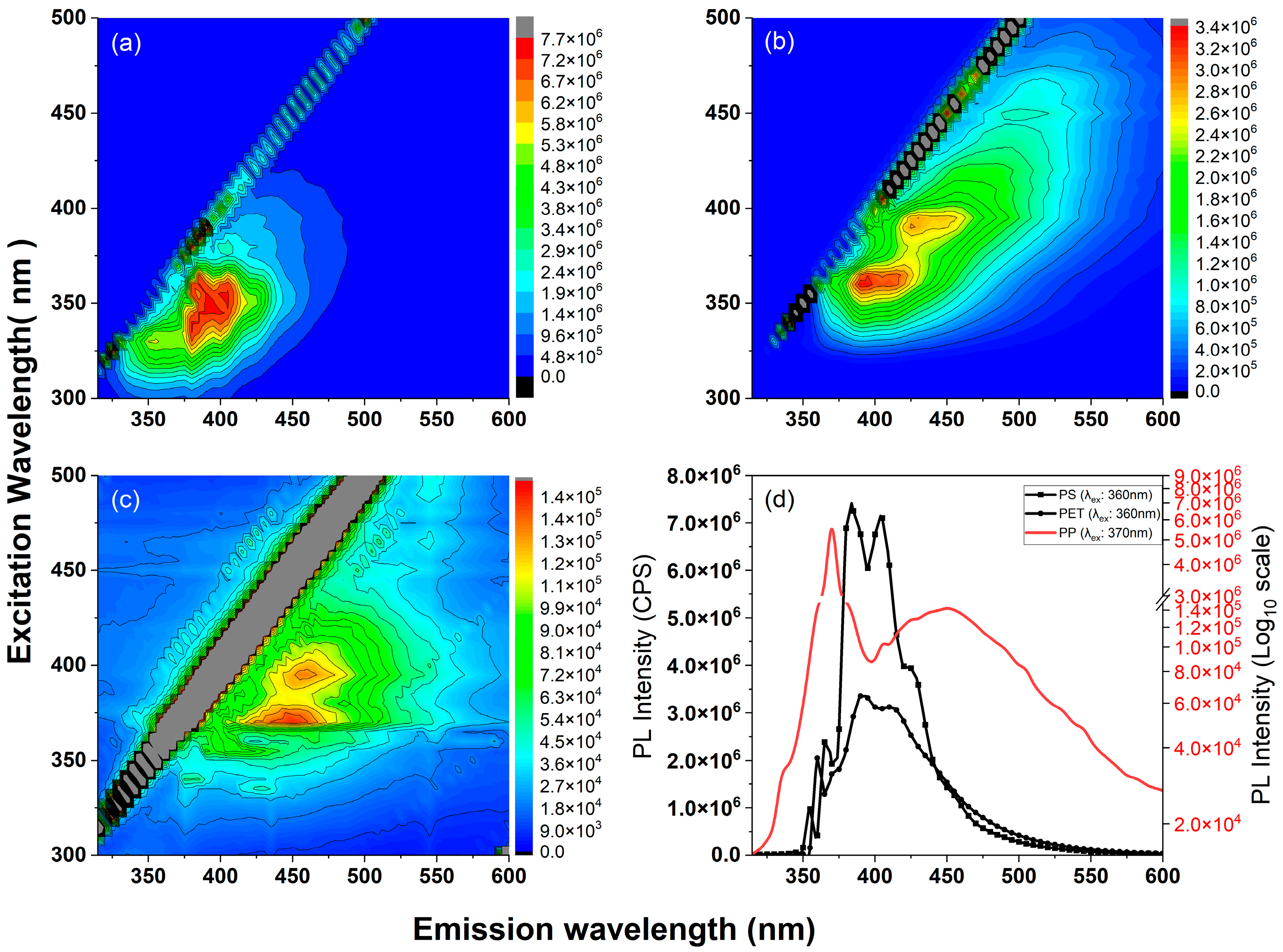
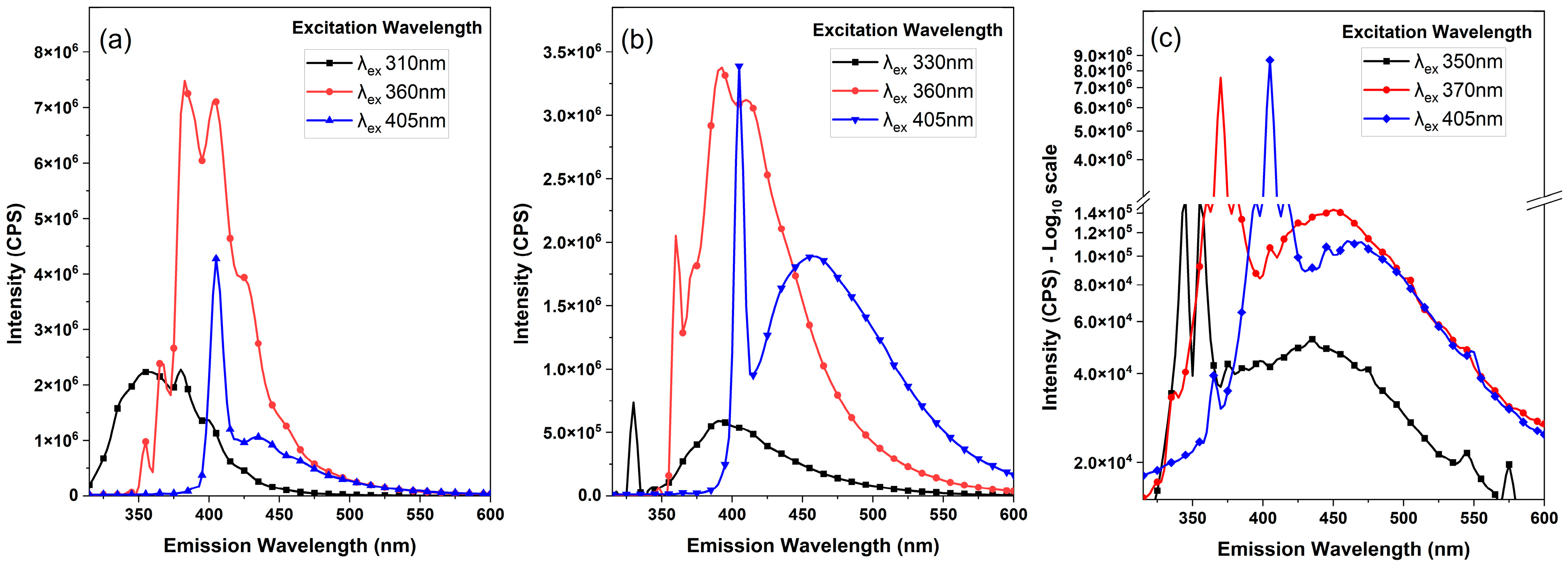
2.5. Fluorescence Spectroscopy
3. Results and Discussion
4. Conclusions
- For polystyrene, peak FL emissions occurred at 380 nm and 405 nm when excited at 360 nm.
- Polyethylene terephthalate exhibited its most intense FL emissions at 390 nm when excited at 360 nm.
- Polypropylene displayed its dominant FL emissions at 455 nm when excited at 370 nm.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogunola, O.S.; Onada, O.A.; Falaye, A.E. Mitigation measures to avert the impacts of plastics and microplastics in the marine environment (a review). Environ. Sci. Pollut. Res. 2018, 25, 9293–9310. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, K.; Rangasamy, G.; Ramya, M.; Shankar, V.U.; Rajesh, G. A critical review on recent research progress on microplastic pollutants in drinking water. Environ. Res. 2023, 222, 115312. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Al Harraq, A.; Brahana, P.J.; Arcemont, O.; Zhang, D.; Valsaraj, K.T.; Bharti, B. Effects of weathering on microplastic dispersibility and pollutant uptake capacity. ACS Environ. Au 2022, 2, 549–555. [Google Scholar] [CrossRef]
- Zhou, C.; Bi, R.; Su, C.; Liu, W.; Wang, T. The emerging issue of microplastics in marine environment: A bibliometric analysis from 2004 to 2020. Mar. Pollut. Bull. 2022, 179, 113712. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Feng, A.; Shi, X.; Feng, Y.; Yang, Y.; Wang, Y.; Wu, Z.; Zou, Z.; Ma, W. Study on detection method of microplastics in farmland soil based on hyperspectral imaging technology. Environ. Res. 2023, 232, 116389. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Chase, Z.; Zhang, J.; Holl, M.M.B.; Willis, K.; Williams, A.; Hardesty, B.D.; Wilcox, C. Microplastic pollution in deep-sea sediments from the Great Australian Bight. Front. Mar. Sci. 2020, 7, 576170. [Google Scholar] [CrossRef]
- Wesch, C.; Bredimus, K.; Paulus, M.; Klein, R. Towards the suitable monitoring of ingestion of microplastics by marine biota: A review. Environ. Pollut. 2016, 218, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Xu, J.-L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Elert, A.M.; Becker, R.; Duemichen, E.; Eisentraut, P.; Falkenhagen, J.; Sturm, H.; Braun, U. Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 2017, 231, 1256–1264. [Google Scholar] [CrossRef]
- Phan, S.; Padilla-Gamiño, J.L.; Luscombe, C.K. The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: Part I. polyethylene and polypropylene. Polym. Test. 2022, 116, 107752. [Google Scholar] [CrossRef]
- Dey, T. Microplastic pollutant detection by Surface Enhanced Raman Spectroscopy (SERS): A mini-review. Nanotechnol. Environ. Eng. 2023, 8, 41–48. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman microspectroscopy as a tool for microplastic particle analysis. TrAC Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Mogha, N.K.; Shin, D. Nanoplastic detection with surface enhanced Raman spectroscopy: Present and future. TrAC Trends Anal. Chem. 2023, 158, 116885. [Google Scholar] [CrossRef]
- Choi, D.S.; Lim, S.; Park, J.-S.; Kim, C.-H.; Rhee, H.; Cho, M. Label-free live-cell imaging of internalized microplastics and cytoplasmic organelles with multicolor CARS microscopy. Environ. Sci. Technol. 2022, 56, 3045–3055. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S.; Song, B.; Burbage, C. Quantifying and identifying microplastics in the effluent of advanced wastewater treatment systems using Raman microspectroscopy. Mar. Pollut. Bull. 2019, 149, 110579. [Google Scholar] [CrossRef] [PubMed]
- Käppler, A.; Windrich, F.; Löder, M.G.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm−1 for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, D.; Pei, J.; Fei, Y.; Ouyang, D.; Zhang, H.; Luo, Y. Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: Current status and future prospects. Curr. Opin. Environ. Sci. Health 2020, 18, 14–19. [Google Scholar] [CrossRef]
- Nahorniak, M.L.; Booksh, K.S. Excitation-emission matrix fluorescence spectroscopy in conjunction with multiway analysis for PAH detection in complex matrices. Analyst 2006, 131, 1308–1315. [Google Scholar] [CrossRef]
- Strasburg, G.M.; Ludescher, R.D. Theory and applications of fluorescence spectroscopy in food research. Trends Food Sci. Technol. 1995, 6, 69–75. [Google Scholar] [CrossRef]
- Baibarac, M.; Baltog, I.; Lefrant, S.; Mevellec, J.; Bucur, C. Vibrational and photoluminescence properties of the polystyrene functionalized single-walled carbon nanotubes. Diam. Relat. Mater. 2008, 17, 1380–1388. [Google Scholar] [CrossRef]
- Healy, M.S.; Hanson, J.E. Fluorescence excitation spectroscopy of polystyrene near the critical concentration c. J. Appl. Polym. Sci. 2007, 104, 360–364. [Google Scholar] [CrossRef]
- Torkelson, J.M.; Lipsky, S.; Tirrell, M.; Tirrell, D.A. Fluorescence and absorbance of polystyrene in dilute and semidilute solutions. Macromolecules 1983, 16, 326–330. [Google Scholar] [CrossRef]
- Fechine, G.; Souto-Maior, R.; Rabello, M. Photodegradation of multilayer films based on PET copolymers. J. Appl. Polym. Sci. 2007, 104, 51–57. [Google Scholar] [CrossRef]
- Charlesby, A.; Partridge, R. Thermoluminescence and phosphorescence in polyethylene under ultra-violet irradiation. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1965, 283, 329–342. [Google Scholar]
- Osawa, Z.; Kuroda, H. Luminescence emission of high-density polyethylene. J. Polym. Sci. Polym. Lett. Ed. 1982, 20, 577–581. [Google Scholar] [CrossRef]
- Douminge, L.; Mallarino, S.; Cohendoz, S.; Feaugas, X.; Bernard, J. Extrinsic fluorescence as a sensitive method for studying photo-degradation of high density polyethylene part I. Curr. Appl. Phys. 2010, 10, 1211–1215. [Google Scholar] [CrossRef]
- Allen, N.; Homer, J.; McKellar, J.; Wood, D. Identification of the luminescent species in low-density polyethylene. J. Appl. Polym. Sci. 1977, 21, 3147–3152. [Google Scholar] [CrossRef]
- Allen, N.; Homer, J.; McKellar, J. Origin and role of the luminescent species in the photo-oxidation of commercial polypropylene. J. Appl. Polym. Sci. 1977, 21, 2261–2267. [Google Scholar] [CrossRef]
- Konde, S.; Ornik, J.; Prume, J.A.; Taiber, J.; Koch, M. Exploring the potential of photoluminescence spectroscopy in combination with Nile Red staining for microplastic detection. Mar. Pollut. Bull. 2020, 159, 111475. [Google Scholar] [CrossRef]
- Ornik, J.; Sommer, S.; Gies, S.; Weber, M.; Lott, C.; Balzer, J.C.; Koch, M. Could photoluminescence spectroscopy be an alternative technique for the detection of microplastics? First experiments using a 405 nm laser for excitation. Appl. Phys. B 2020, 126, 1–7. [Google Scholar] [CrossRef]
- Gies, S.; Schömann, E.-M.; Anna Prume, J.; Koch, M. Exploring the potential of time-resolved photoluminescence spectroscopy for the detection of plastics. Appl. Spectrosc. 2020, 74, 1161–1166. [Google Scholar] [CrossRef]
- Bartis, E.A.J.; Luan, P.; Knoll, A.J.; Hart, C.; Seog, J.; Oehrlein, G.S. Polystyrene as a model system to probe the impact of ambient gas chemistry on polymer surface modifications using remote atmospheric pressure plasma under well-controlled conditions. Biointerphases 2015, 10, 029512. [Google Scholar] [CrossRef]
- Du, H.; Komuro, A.; Ono, R. Quantitative and selective study of the effect of O radicals on polypropylene surface treatment. Plasma Sources Sci. Technol. 2023, 32, 075013. [Google Scholar] [CrossRef]
- Adalsteinsson, V.; Parajuli, O.; Kepics, S.; Gupta, A.; Reeves, W.B.; Hahm, J.-I. Ultrasensitive detection of cytokines enabled by nanoscale ZnO arrays. Anal. Chem. 2008, 80, 6594–6601. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, A.; Kumar, N.; Hahm, J.-I. Highly sensitive biomolecular fluorescence detection using nanoscale ZnO platforms. Langmuir 2006, 22, 4890–4895. [Google Scholar] [CrossRef]
- Dorfman, A.; Parajuli, O.; Kumar, N.; Hahm, J.-I. Novel Telomeric Repeat Elongation Assay Performed on Zinc Oxide Nanorod Array Supports. J. Nanosci. Nanotechnol. 2008, 8, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hu, Z.; Feng, S. Nonconventional Luminescence Enhanced by Silicone-Induced Aggregation. Chem. Asian J. 2017, 12, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Gaft, M.; Nagli, L.; Panczer, G.; Waychunas, G.; Porat, N. The nature of unusual luminescence in natural calcite CaCO3. Am. Mineral. 2008, 93, 158–167. [Google Scholar] [CrossRef]
- Padwa, A. Photochemistry of the carbon-nitrogen double bond. Chem. Rev. 1977, 77, 37–68. [Google Scholar] [CrossRef]
- Kaszowska, Z.; Malek, K.; Staniszewska-Slezak, E.; Niedzielska, K. Raman scattering or fluorescence emission? Raman spectroscopy study on lime-based building and conservation materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 169, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mazilu, M.; Luca, A.C.D.; Riches, A.; Herrington, C.S.; Dholakia, K. Optimal algorithm for fluorescence suppression of modulated Raman spectroscopy. Opt. Express 2010, 18, 11382–11395. [Google Scholar] [CrossRef]
- Rebollar, E.; Pérez, S.; Hernández, M.; Domingo, C.; Martín, M.; Ezquerra, T.A.; García-Ruiz, J.P.; Castillejo, M. Physicochemical modifications accompanying UV laser induced surface structures on poly(ethylene terephthalate) and their effect on adhesion of mesenchymal cells. Phys. Chem. Chem. Phys. 2014, 16, 17551–17559. [Google Scholar] [CrossRef]
- Meng, M.; Feng, Y.; Liu, Y.; Dai, X.; Pan, J.; Yan, Y. Fabrication of submicrosized imprinted spheres attached polypropylene membrane using “two-dimensional” molecular imprinting method for targeted separation. Adsorpt. Sci. Technol. 2017, 35, 162–177. [Google Scholar] [CrossRef]
- Guo, X.; Lin, Z.; Wang, Y.; He, Z.; Wang, M.; Jin, G. In-line monitoring the degradation of polypropylene under multiple extrusions based on Raman spectroscopy. Polymers 2019, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Pichat, L.; Pesteil, P.; Clément, J. Solides fluorescents non cristallins pour mesures de radiactivité. J. De Chim. Phys. 1953, 50, 26–41. [Google Scholar] [CrossRef]
- Allen, N.S.; McKellar, J.F. Luminescent species in poly (ethylene terephthalate). Die Makromol. Chem. 1978, 179, 523–526. [Google Scholar] [CrossRef]
- Saudrais, F.; Schvartz, M.; Renault, J.-P.; Vieira, J.; Devineau, S.; Leroy, J.; Taché, O.; Boulard, Y.; Pin, S. The Impact of Virgin and Aged Microstructured Plastics on Proteins: The Case of Hemoglobin Adsorption and Oxygenation. Int. J. Mol. Sci. 2024, 25, 7047. [Google Scholar] [CrossRef] [PubMed]
- Basile, L.J. Effect of styrene monomer on the fluorescence properties of polystyrene. J. Chem. Phys. 1962, 36, 2204–2210. [Google Scholar] [CrossRef]
- Nurmukhametov, R.; Volkova, L.; Kabanov, S. Fluorescence and absorption of polystyrene exposed to UV laser radiation. J. Appl. Spectrosc. 2006, 73, 55–60. [Google Scholar] [CrossRef]
- Swank, R.K.; Buck, W.L. The scintillation process in plastic solid solutions. Phys. Rev. 1953, 91, 927. [Google Scholar] [CrossRef]
- Chen, L.; Jin, X.; Du, J.; Qian, R. On the origin of the fluorescence emission of poly (ethylene terephthalate) by excitation at 340 nm. Die Makromol. Chem. Macromol. Chem. Phys. 1991, 192, 1399–1408. [Google Scholar] [CrossRef]
- Day, M.; Wiles, D. Photochemical degradation of poly (ethylene terephthalate). III. Determination of decomposition products and reaction mechanism. J. Appl. Polym. Sci. 1972, 16, 203–215. [Google Scholar] [CrossRef]
- Hemker, D.J.; Frank, C.W.; Thomas, J.W. Photophysical studies of amorphous orientation in poly (ethylene terephthalate) films. Polymer 1988, 29, 437–447. [Google Scholar] [CrossRef]
- LaFemina, J.P.; Carter, D.R.; Bass, M.B. Photophysical properties, intermolecular interactions, and molecular dynamics of poly (ethylene terephthalate). J. Phys. Chem. 1992, 96, 2767–2772. [Google Scholar] [CrossRef]
- Merrill, R.G.; Roberts, C.W. Photophysical processes and interactions between poly (ethylene terephthalate) and 1-amino-2-(2-methoxyethoxy)-4-hydroxy-9, 10-anthraquinone. J. Appl. Polym. Sci. 1977, 21, 2745–2768. [Google Scholar] [CrossRef]
- Sonnenschein, M.; Roland, C. Absorption and fluorescence spectra of poly (ethylene terephthalate) dimers. Polymer 1990, 31, 2023–2026. [Google Scholar] [CrossRef]
- Massines, F.; Tiemblo, P.; Teyssedre, G.; Laurent, C. On the nature of the luminescence emitted by a polypropylene film after interaction with a cold plasma at low temperature. J. Appl. Phys. 1997, 81, 937–943. [Google Scholar] [CrossRef]
- Ho, D.; Liu, S.; Wei, H.; Karthikeyan, K.G. The glowing potential of Nile red for microplastics Identification: Science and mechanism of fluorescence staining. Microchem. J. 2024, 197, 109708. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hong, S.; Hur, J. A fluorescence indicator for source discrimination between microplastic-derived dissolved organic matter and aquatic natural organic matter. Water Res. 2021, 207, 117833. [Google Scholar] [CrossRef]
- Motalebizadeh, A.; Fardindoost, S.; Hoorfar, M. Selective on-site detection and quantification of polystyrene microplastics in water using fluorescence-tagged peptides and electrochemical impedance spectroscopy. J. Hazard. Mater. 2024, 480, 136004. [Google Scholar] [CrossRef]
- Hu, B.; Dai, Y.; Zhou, H.; Sun, Y.; Yu, H.; Dai, Y.; Wang, M.; Ergu, D.; Zhou, P. Using artificial intelligence to rapidly identify microplastics pollution and predict microplastics environmental behaviors. J. Hazard. Mater. 2024, 474, 134865. [Google Scholar] [CrossRef]
- Merlemis, N.; Drakaki, E.; Zekou, E.; Ninos, G.; Kesidis, A.L. Laser induced fluorescence and machine learning: A novel approach to microplastic identification. Appl. Phys. B 2024, 130, 29. [Google Scholar] [CrossRef]
- Childress, E.S.; Roberts, C.A.; Sherwood, D.Y.; LeGuyader, C.L.; Harbron, E.J. Ratiometric fluorescence detection of mercury ions in water by conjugated polymer nanoparticles. Anal. Chem. 2012, 84, 1235–1239. [Google Scholar] [CrossRef]
- Oki, Y.; Furukawa, K.; Maeda, M. Extremely sensitive Na detection in pure water by laser ablation atomic fluorescence spectroscopy. Opt. Commun. 1997, 133, 123–128. [Google Scholar] [CrossRef]
- Costa, C.Q.; Cruz, J.; Martins, J.; Teodósio, M.A.A.; Jockusch, S.; Ramamurthy, V.; Da Silva, J.P. Fluorescence sensing of microplastics on surfaces. Environ. Chem. Lett. 2021, 19, 1797–1802. [Google Scholar] [CrossRef]
- Peinador, R.I.; HP, P.T.; Calvo, J.I. Innovative application of Nile Red (NR)-based dye for direct detection of micro and nanoplastics (MNPs) in diverse aquatic environments. Chemosphere 2024, 362, 142609. [Google Scholar] [CrossRef] [PubMed]
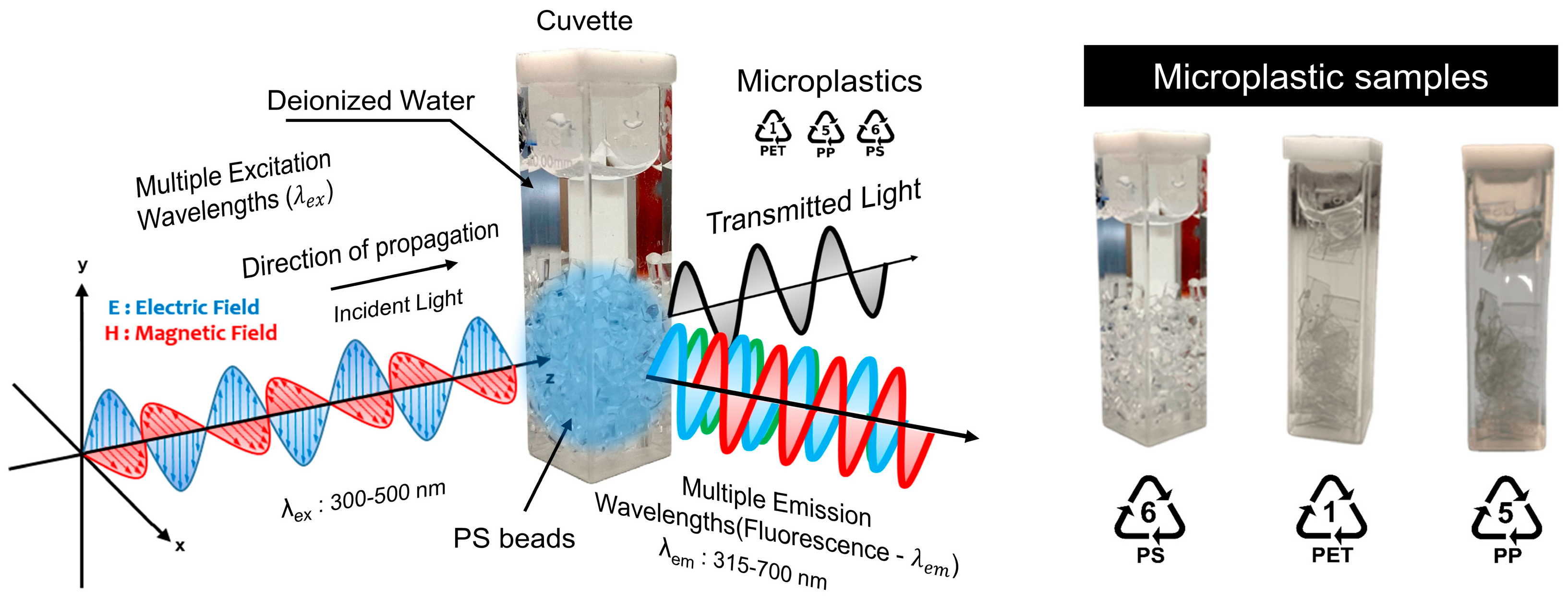
| Elements | Relative Atomic Concentration | ||
|---|---|---|---|
| Polystyrene (PS) | Polyethylene Terephthalate (PET) | Polypropylene (PP) | |
| C-C/C-H | 91.2 | 74.5 | 83.8 |
| C-O | 7.0 | 22.5 | 11.2 |
| Si | - | 2.2 | 3.9 |
| Zn | 1.8 | 0.1 | 0.1 |
| N | - | 0.4 | 0.7 |
| Ca | - | 0.2 | 0.2 |
| S | - | 0.2 | 0.1 |
| Microplastic | Raman Peaks (cm−1) | Corresponding Vibrational Band |
|---|---|---|
| Polystyrene | 621 | Ring deformation mode |
| 1001 | Ring breathing mode | |
| 1031 | C-H in-plane deformation | |
| 1155 | C-C stretch | |
| 1450 | CH2 scissoring | |
| 1583 | C=C stretch | |
| 1602 | Ring skeletal stretch | |
| Polyethylene Terephthalate | 1100 | Ester C(O)-O and C-C bond |
| 1165 | Ring in-plane C-H & C-C stretch | |
| 1276 | C(O)-O stretching | |
| 1402 | CCH bending and OCH bending | |
| 1594 | C-C bond in the aromatic ring | |
| 1707 | Stretching of C=O vibrations | |
| Polypropylene | 860 | C-C stretching and CH3 rocking |
| 1142 | C-C stretching and CH bending | |
| 1356 | CH stretching, CH2 wagging, and CH3 bending | |
| 1466 | CH2 bending and CH3 asymmetrical bending | |
| 1762 | C=O stretching vibration | |
| 3090–3209 | Stretching vibrations of C-H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 World Health Organization. Licensee MDPI, Basel, Switzerland. This is an open access article distributed under the terms of the Creative Commons Attribution IGO License (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any reproduction of this article there should not be any suggestion that WHO or this article endorse any specific organisation or products. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Share and Cite
Iqrar, S.A.; Bibi, A.; Chinnambedu Murugesan, R.; Hill, D.; Rozhin, A. Excitation–Emission Fluorescence Mapping Analysis of Microplastics That Are Typically Pollutants. Photochem 2024, 4, 488-500. https://doi.org/10.3390/photochem4040030
Iqrar SA, Bibi A, Chinnambedu Murugesan R, Hill D, Rozhin A. Excitation–Emission Fluorescence Mapping Analysis of Microplastics That Are Typically Pollutants. Photochem. 2024; 4(4):488-500. https://doi.org/10.3390/photochem4040030
Chicago/Turabian StyleIqrar, Syed Atif, Aisha Bibi, Raghavan Chinnambedu Murugesan, Daniel Hill, and Alex Rozhin. 2024. "Excitation–Emission Fluorescence Mapping Analysis of Microplastics That Are Typically Pollutants" Photochem 4, no. 4: 488-500. https://doi.org/10.3390/photochem4040030
APA StyleIqrar, S. A., Bibi, A., Chinnambedu Murugesan, R., Hill, D., & Rozhin, A. (2024). Excitation–Emission Fluorescence Mapping Analysis of Microplastics That Are Typically Pollutants. Photochem, 4(4), 488-500. https://doi.org/10.3390/photochem4040030







