Unravelling Insights into the Evolution and Management of SARS-CoV-2
Abstract
:1. Introduction
2. Clinical Description of SARS-CoV-2
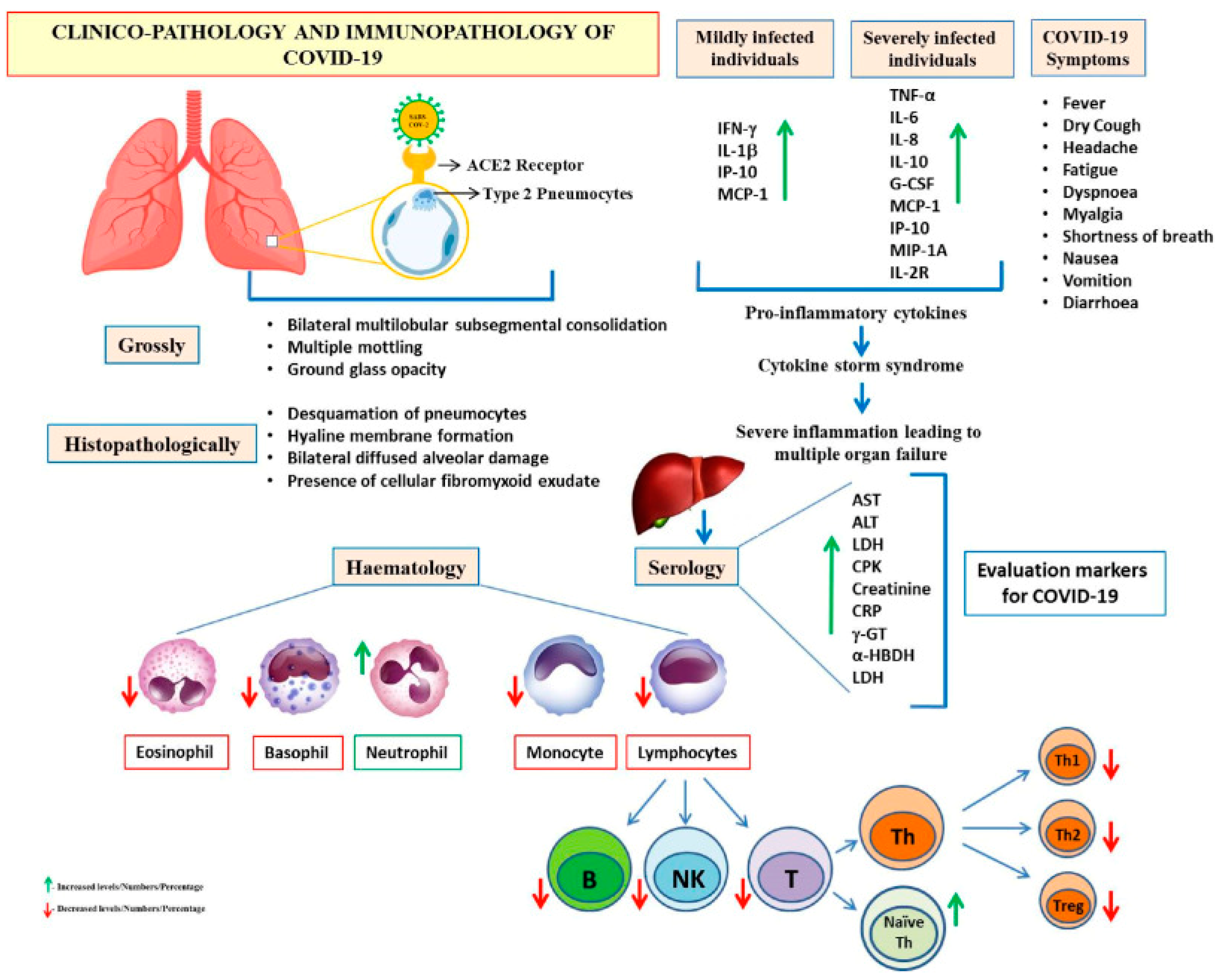
3. SARS-CoV-2 Prevalence and Pathology
3.1. SARS-CoV-2 Transmission, Clinical Presentation, and Risk Factors for Severe Disease and Fatality
3.2. Profile Characteristics and Prognostic Markers of COVID-19/SARS-CoV-2
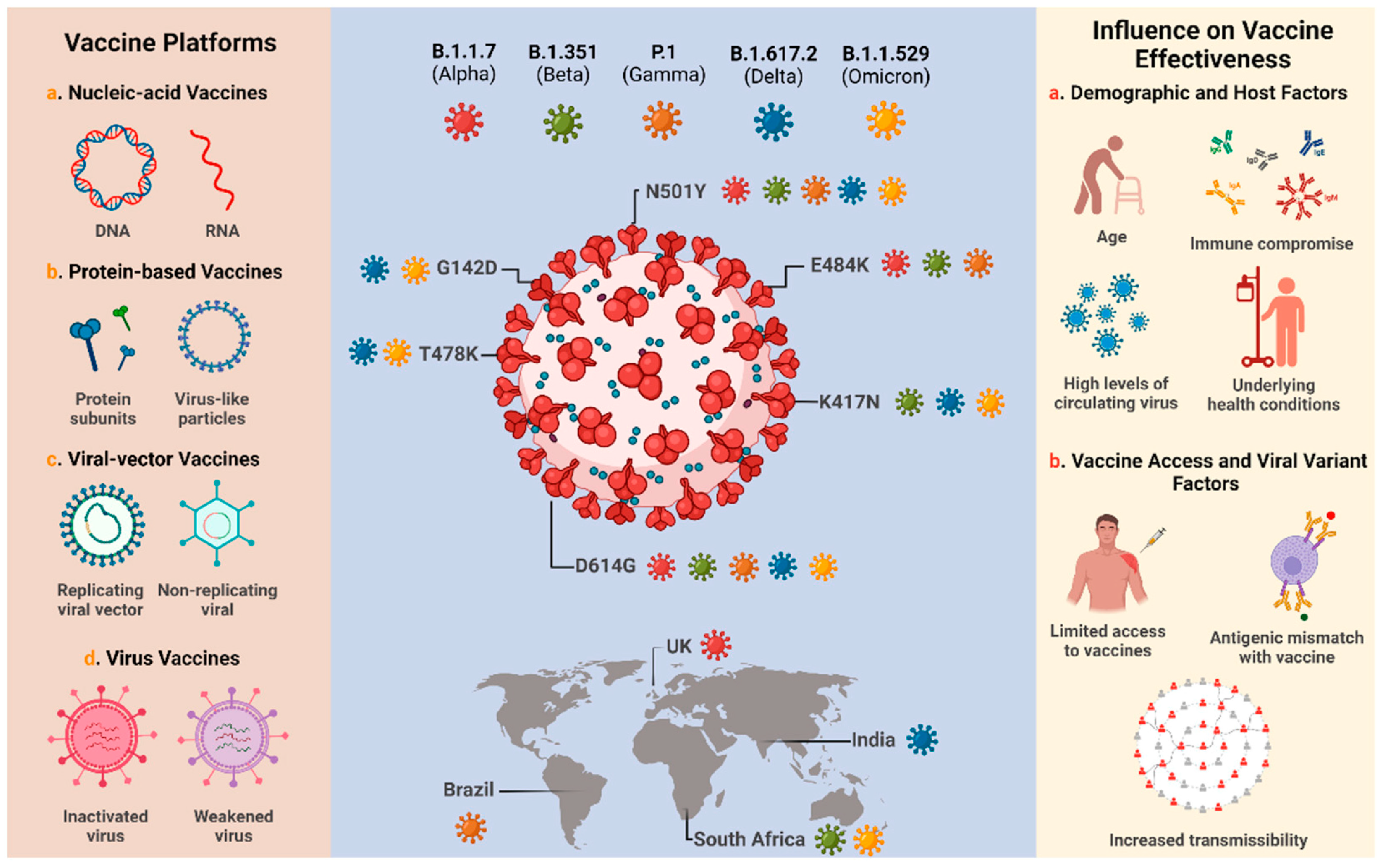
4. Discussion on the Postulated Hypothesis on COVID-19 Mutations
5. Advancements in SARS-CoV-2/COVID-19 Management
5.1. COVID-19 Therapies, Vaccines, and Other Ongoing Clinical Trial Therapies
| Approved SARS-CoV-2 Vaccines | ||||||
|---|---|---|---|---|---|---|
| Vaccine Name | Manufacturer | Type | Dosage/Post-dosage | Efficacy | Target | Ref. |
| BNT162b2 | Pfizer, BioNTech | mRNA | Two doses, 4–8 weeks apart. A booster (4–6 months after). | 95% | Spike protein | [115,116] |
| mRNA-1273 | Moderna | mRNA | Two doses, 4–8 weeks apart. Dose 3 at least 4 weeks after Dose 2. A single booster dose (0.25 mL) may be administered at least 5 months after completing a primary series. | 94.1% | Spike protein | [115,117] |
| Ad26.COV2.S | Johnson and Johnson | Viral vector | One dose (preferred). Administration of the second dose to increase level of protection against symptomatic infection. W.H.O recommends interval of 2 to 6 months. Booster dose after 90 days. | 72% (in the U.S.) | Spike protein | [115,118] |
| ChAdOx1-S [recombinant] | AstraZeneca, University of Oxford | Viral vector | Two doses, with an interval of 8 to 12 weeks. A booster dose may be considered 4–6 months after completion of the primary vaccination series. | 70.4% (average) | Spike protein | [115,119] |
| SARS-CoV-2 therapeutics | ||||||
| Drug name | Manufacturer | Type | Target | Antiviral Agent | Status | Ref. |
| Remdesivir | Gilead Sciences | Antiviral | RNA polymerase | Nucleotide analogue | FDA-approved for emergency use in hospitalised patients | [120] |
| Baricitinib | Eli Lilly and Company | Anti-inflammatory | AP-1 | Janus kinase inhibitor | FDA-approved for emergency use along with remdesivir | [121,122] |
| Tocilizumab | Roche | Anti-inflammatory | IL-6 | The monoclonal antibody (mAb) | FDA-approved for emergency use in hospitalised patients | [123,124] |
| Sotrovimab | GlaxoSmithKline and Vir Biotechnology | The monoclonal antibody (mAb) | Spike protein | The monoclonal antibody (mAb) | FDA-approved for emergency use in high-risk individuals | [125,126] |
| Molnupiravir | Merck & Co. | Antiviral | RNA polymerase | Nucleotide analogue | Currently under review for emergency use authorisation | [127,128] |
| Ongoing Clinical Trials for SARS-CoV-2 | ||||||
| Study name | Sponsor | Type | Phase | Target | Antiviral Agent/Status | Ref. |
| ACTIV-6 | NIH | Therapeutics | 3 | Various | Various/Ongoing | [129,130] |
| COMET-ICE | NIAID, Lilly | Therapeutics | 3 | Various | Various/Ongoing | [126,131] |
| REGN-COV2 | Regeneron | Therapeutics | 3 | Spike protein | The monoclonal antibody (mAb)/Ongoing | [132,133] |
| COV-BOOST | University of Oxford | Vaccine | 2 | Spike protein | N/A/Ongoing | [134] |
| COV-FLU | Novavax | Vaccine | 3 | Influenza virus | N/A/Ongoing | [135] |
5.2. Drug Repurposing for COVID-19
5.2.1. Vaccine Development and Challenges
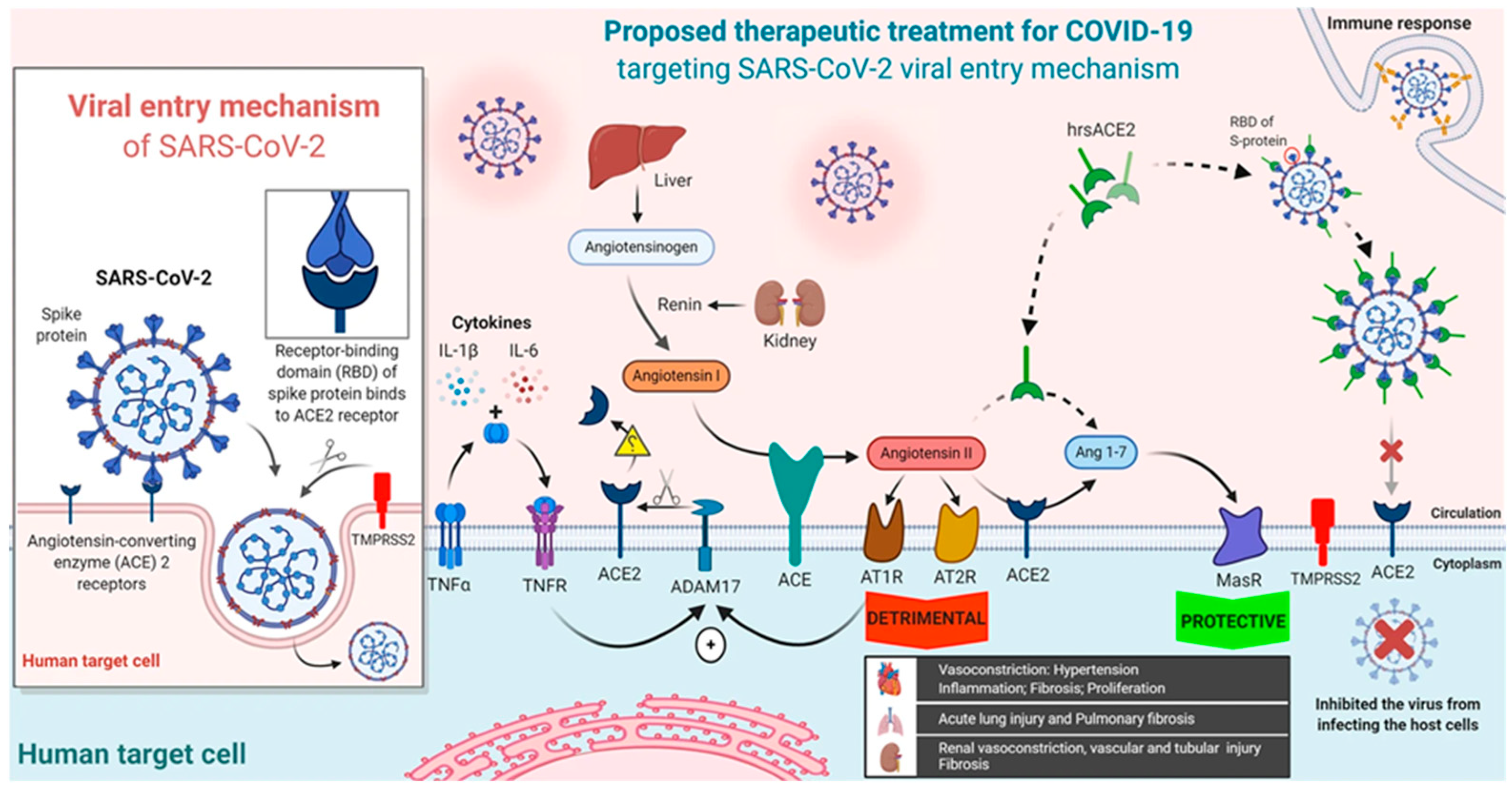
5.2.2. Experimental Therapeutic Interventions
Convalescent Plasma (CP) Therapy
Soluble Human Angiotensin-Converting Enzyme 2
6. Non-Pharmacological Interventions
7. Conclusions and Future Perspectives for COVID-19 Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acter, T.; Uddin, N.; Das, J.; Akhter, A.; Choudhury, T.R.; Kim, S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020, 730, 138996. [Google Scholar] [CrossRef]
- Kamel Boulos, M.N.; Geraghty, E.M. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: How 21st century GIS technologies are supporting the global fight against outbreaks and epidemics. Int. J. Health Geogr. 2020, 19, 8. [Google Scholar] [PubMed]
- Nassar, A.; Ibrahim, I.M.; Amin, F.G.; Magdy, M.; Elgharib, A.M.; Azzam, E.B.; Nasser, F.; Yousry, K.; Shamkh, I.M.; Mahdy, S.M.; et al. A review of human coronaviruses’ receptors: The host-cell targets for the crown bearing viruses. Molecules 2021, 26, 6455. [Google Scholar] [CrossRef]
- Ye, Z.-W.; Yuan, S.; Yuen, K.-S.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686. [Google Scholar] [CrossRef]
- Chikowe, I.; Mtewa, A.G.; Tembo, D.; Smith, D.; Ibrahim, E.; Mwamatope, B.; Nkhungulu, J.; Kumpalume, P.; Maroyi, A. Potential of Malawi’s medicinal plants in COVID-19 disease management: A review. Malawi Med. J. 2021, 33, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Prasad, R.; Gupta, A.; Das, K.; Gupta, N. Severe acute respiratory syndrome coronavirus-2 and pulmonary tuberculosis: Convergence can be fatal. Monaldi Arch. Chest Dis. 2020, 90. [Google Scholar] [CrossRef]
- Bleibtreu, A.; Bertine, M.; Bertin, C.; Houhou-Fidouh, N.; Visseaux, B. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV). Med. Mal. Infect. 2020, 50, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Chamola, V.; Hassija, V.; Gupta, V.; Guizani, M. A comprehensive review of the COVID-19 pandemic and the role of IoT, drones, AI, blockchain, and 5G in managing its impact. IEEE Access 2020, 8, 90225–90265. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, J.-Z.; Wan, X.-F.; Hua, Y.; Li, L.; Zhou, J.; Wang, X.; Hou, F.; Chen, J.; Zou, J. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020, 16, e1008421. [Google Scholar] [CrossRef]
- Machado, D.J.; Scott, R.; Guirales, S.; Janies, D.A. Fundamental evolution of all Orthocoronavirinae including three deadly lineages descendent from Chiroptera-hosted coronaviruses: SARS-CoV, MERS-CoV and SARS-CoV-2. Cladistics 2021, 37, 461. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Pathak, M.; Yatoo, M.I.; Tiwari, R.; Malik, Y.S.; Singh, R.; Sah, R.; Rabaan, A.A.; Bonilla-Aldana, D.K. An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med. Infect. Dis. 2020, 37, 101755. [Google Scholar] [CrossRef]
- Seitz, B.M.; Aktipis, A.; Buss, D.M.; Alcock, J.; Bloom, P.; Gelfand, M.; Harris, S.; Lieberman, D.; Horowitz, B.N.; Pinker, S.; et al. The pandemic exposes human nature: 10 evolutionary insights. Proc. Natl. Acad. Sci. USA 2020, 117, 27767–27776. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Brunialti, E.; Dellavedova, J.; Meda, C.; Rebecchi, M.; Conti, M.; Donnici, L.; De Francesco, R.; Reggiani, A.; Lionetti, V.; et al. DNA aptamers masking angiotensin converting enzyme 2 as an innovative way to treat SARS-CoV-2 pandemic. Pharmacol. Res. 2022, 175, 105982. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Effective drugs used to combat SARS-CoV-2 infection and the current status of vaccines. Biomed. Pharmacother. 2021, 137, 111330. [Google Scholar] [CrossRef]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its impact on patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Abu Haleeqa, M.; Alshamsi, I.; Al Habib, A.; Noshi, M.; Abdullah, S.; Kamour, A.; Ibrahim, H. Optimizing supportive care in COVID-19 patients: A multidisciplinary approach. J. Multidiscip. Healthc. 2020, 13, 877–880. [Google Scholar] [CrossRef]
- Formenti, B.; Gregori, N.; Crosato, V.; Marchese, V.; Tomasoni, L.R.; Castelli, F. The impact of COVID-19 on communicable and non-communicable diseases in Africa: A narrative review. Le Infez. Med. 2022, 30, 30. [Google Scholar]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.-S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef]
- Jeong, G.U.; Song, H.; Yoon, G.Y.; Kim, D.; Kwon, Y.-C. Therapeutic strategies against COVID-19 and structural characterization of SARS-CoV-2: A review. Front. Microbiol. 2020, 11, 1723. [Google Scholar] [CrossRef]
- Chauhan, S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed. J. 2020, 43, 334–340. [Google Scholar] [CrossRef]
- Liu, N.-N.; Tan, J.-C.; Li, J.; Li, S.; Cai, Y.; Wang, H. COVID-19 pandemic: Experiences in China and implications for its prevention and treatment worldwide. Curr. Cancer Drug Targets 2020, 20, 410–416. [Google Scholar]
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): An update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed]
- Loeffler-Wirth, H.; Schmidt, M.; Binder, H. COVID-19 transmission trajectories–monitoring the pandemic in the worldwide context. Viruses 2020, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 27 October 2023).
- Xiang, M.; Zhang, Z.; Kuwahara, K. Impact of COVID-19 pandemic on children and adolescents’ lifestyle behavior larger than expected. Prog. Cardiovasc. Dis. 2020, 63, 531. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Pustake, M.; Tambolkar, I.; Giri, P.; Gandhi, C. SARS, MERS and COVID-19: An overview and comparison of clinical, laboratory and radiological features. J. Fam. Med. Prim. Care 2022, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Pan, C.; Yang, X.; Zhong, M.; Shang, X.; Wu, Z.; Yu, Z.; Zhang, W.; Zhong, Q.; Zheng, X. Management of critically ill patients with COVID-19 in ICU: Statement from front-line intensive care experts in Wuhan, China. Ann. Intensive Care 2020, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobial Resistance Threats in the emerging COVID-19 pandemic: Where do we stand? J. Infect. Public Health 2021, 14, 555–560. [Google Scholar] [CrossRef]
- Rabi, F.A.; Al Zoubi, M.S.; Kasasbeh, G.A.; Salameh, D.M.; Al-Nasser, A.D. SARS-CoV-2 and coronavirus disease 2019: What we know so far. Pathogens 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Meekins, D.A.; Gaudreault, N.N.; Richt, J.A. Natural and experimental SARS-CoV-2 infection in domestic and wild animals. Viruses 2021, 13, 1993. [Google Scholar] [CrossRef] [PubMed]
- Damialis, A.; Gilles, S.; Sofiev, M.; Sofieva, V.; Kolek, F.; Bayr, D.; Plaza, M.P.; Leier-Wirtz, V.; Kaschuba, S.; Ziska, L.H.; et al. Higher airborne pollen concentrations correlated with increased SARS-CoV-2 infection rates, as evidenced from 31 countries across the globe. Proc. Natl. Acad. Sci. USA 2021, 118, e2019034118. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Mustafa, F.; Rizvi, T.A.; Loney, T.; Al Suwaidi, H.; Al-Marzouqi, A.H.H.; Kamal Eldin, A.; Alsabeeha, N.; Adrian, T.E.; Stefanini, C.; et al. SARS-CoV-2/COVID-19: Viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses 2020, 12, 526. [Google Scholar] [CrossRef]
- Johnson, K.D.; Harris, C.; Cain, J.K.; Hummer, C.; Goyal, H.; Perisetti, A. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front. Med. 2020, 7, 526. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. COVID-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Callow, M.A.; Callow, D.D.; Smith, C. Older adults’ intention to socially isolate once COVID-19 stay-at-home orders are replaced with “safer-at-home” public health advisories: A survey of respondents in Maryland. J. Appl. Gerontol. 2020, 39, 1175–1183. [Google Scholar] [CrossRef]
- Azuma, K.; Yanagi, U.; Kagi, N.; Kim, H.; Ogata, M.; Hayashi, M. Environmental factors involved in SARS-CoV-2 transmission: Effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. Med. 2020, 25, 1–16. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Ge, J. Scientific research progress of COVID-19/SARS-CoV-2 in the first five months. J. Cell. Mol. Med. 2020, 24, 6558–6570. [Google Scholar] [CrossRef] [PubMed]
- Jit, M.; Cook, A.R. Informing Public Health Policies with Models for Disease Burden, Impact Evaluation, and Economic Evaluation. Annu. Rev. Public Health 2023, 45. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Al-Ahmed, S.H.; Albayat, H.; Alwarthan, S.; Alhajri, M.; Najim, M.A.; AlShehail, B.M.; Al-Adsani, W.; Alghadeer, A.; Abduljabbar, W.A.; et al. Variants of SARS-CoV-2: Influences on the Vaccines’ Effectiveness and Possible Strategies to Overcome Their Consequences. Medicina 2023, 59, 507. [Google Scholar] [CrossRef] [PubMed]
- Killingley, B.; Mann, A.J.; Kalinova, M.; Boyers, A.; Goonawardane, N.; Zhou, J.; Lindsell, K.; Hare, S.S.; Brown, J.; Frise, R.; et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat. Med. 2022, 28, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.P. SARS-CoV-2-infection (COVID-19): Clinical course, viral acute respiratory distress syndrome (ARDS) and cause(s) of death. Med. Sci. 2022, 10, 58. [Google Scholar] [CrossRef]
- Walsh, K.A.; Spillane, S.; Comber, L.; Cardwell, K.; Harrington, P.; Connell, J.; Teljeur, C.; Broderick, N.; De Gascun, C.F.; Smith, S.M.; et al. The duration of infectiousness of individuals infected with SARS-CoV-2. J. Infect. 2020, 81, 847–856. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, W.R.; Lukas, H.; Torres, M.D.; Gao, W.; de la Fuente-Nunez, C. Low-Cost Biosensor Technologies for Rapid Detection of COVID-19 and Future Pandemics. ACS Nano 2024, 18, 1757–1777. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cao, Q.; Qin, L.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and non-specific clinical manifestations and symptoms: The current state of knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef]
- Banerjee, D. The impact of COVID-19 pandemic on elderly mental health. Int. J. Geriatr. Psychiatry 2020, 35, 1466. [Google Scholar] [CrossRef]
- Suzuki, H.; Miyamoto, T.; Hamada, A.; Nakano, A.; Okoshi, H.; Yamasawa, F. A guide for businesses and employers responding to novel coronavirus disease (COVID-19). J. Occup. Health 2021, 63, e12225. [Google Scholar]
- Yuen, K.-S.; Ye, Z.-W.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef]
- Mueller, M.R.; Ganesh, R.; Hurt, R.T.; Beckman, T.J. Post-COVID conditions. Mayo Clin. Proc. 2023, 98, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.; Mudara, C.; Vika, C.; Blumberg, L.; Mayet, N.; Cohen, C.; Tempia, S.; Parker, A.; Nel, J.; Perumal, R. Post-COVID-19 condition 3 months after hospitalisation with SARS-CoV-2 in South Africa: A prospective cohort study. Lancet Glob. Health 2022, 10, e1247–e1256. [Google Scholar] [CrossRef] [PubMed]
- Haidar, M.A.; Shakkour, Z.; Reslan, M.A.; Al-Haj, N.; Chamoun, P.; Habashy, K.; Kaafarani, H.; Shahjouei, S.; Farran, S.H.; Shaito, A. SARS-CoV-2 involvement in central nervous system tissue damage. Neural Regen. Res. 2022, 17, 1228. [Google Scholar] [PubMed]
- Marjenberg, Z.; Leng, S.; Tascini, C.; Garg, M.; Misso, K.; El Guerche Seblain, C.; Shaikh, N. Risk of long COVID main symptoms after SARS-CoV-2 infection: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 15332. [Google Scholar] [CrossRef]
- Kenny, G.; Townsend, L.; Savinelli, S.; Mallon, P.W. Long COVID: Clinical characteristics, proposed pathogenesis and potential therapeutic targets. Front. Mol. Biosci. 2023, 10, 1157651. [Google Scholar] [CrossRef]
- Derksen, C.; Rinn, R.; Gao, L.; Dahmen, A.; Cordes, C.; Kolb, C.; Becker, P.; Lippke, S. Longitudinal Evaluation of an Integrated Post–COVID-19/Long COVID Management Program Consisting of Digital Interventions and Personal Support: Randomized Controlled Trial. J. Med. Internet Res. 2023, 25, e49342. [Google Scholar] [CrossRef] [PubMed]
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 2023, 12, e86002. [Google Scholar] [CrossRef]
- Singh, S.J.; Baldwin, M.M.; Daynes, E.; Evans, R.A.; Greening, N.J.; Jenkins, R.G.; Lone, N.I.; McAuley, H.; Mehta, P.; Newman, J.; et al. Respiratory sequelae of COVID-19: Pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir. Med. 2023, 11, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Leveringhaus, E.S. Investigations of Cellular Determinants Involved in the Entry Process of Bovine and Porcine Pestiviruses. Ph.D. Thesis, Stiftung Tierärztliche Hochschule Hannover, Hannover, Germany, 2022. [Google Scholar]
- Samprathi, M.; Jayashree, M. Biomarkers in COVID-19: An up-to-date review. Front. Pediatr. 2021, 8, 607647. [Google Scholar] [CrossRef]
- Siavoshi, F.; Safavi-Naini, S.A.A.; Shirzadeh Barough, S.; Azizmohammad Looha, M.; Hatamabadi, H.; Ommi, D.; Jalili Khoshnoud, R.; Fatemi, A.; Pourhoseingholi, M.A. On-admission and dynamic trend of laboratory profiles as prognostic biomarkers in COVID-19 inpatients. Sci. Rep. 2023, 13, 6993. [Google Scholar] [CrossRef]
- Hachim, M.Y.; Hachim, I.Y.; Naeem, K.B.; Hannawi, H.; Salmi, I.A.; Hannawi, S. D-dimer, troponin, and urea level at presentation with COVID-19 can predict ICU admission: A single centered study. Front. Med. 2020, 7, 585003. [Google Scholar] [CrossRef]
- Ryabkova, V.A.; Churilov, L.P.; Shoenfeld, Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm–the common denominator and the lessons to be learned. Clin. Immunol. 2021, 223, 108652. [Google Scholar] [CrossRef]
- Campbell, G.R.; To, R.K.; Hanna, J.; Spector, S.A. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. Iscience 2021, 24, 102295. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Belizna, C.; Selva-O’Callaghan, A.; Pardos-Gea, J.; Quintana, A.; Mekinian, A.; Anunciacion-Llunell, A.; Miró-Mur, F. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun. Rev. 2020, 19, 102569. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; García-Crespo, C.; Lobo-Vega, R.; Perales, C. Mutation rates, mutation frequencies, and proofreading-repair activities in RNA virus genetics. Viruses 2021, 13, 1882. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Lin, X.-Y.; Peng, C.-W.; Zheng, W.-J.; Liu, X.-H.; Wen, W.-J.; Jiang, Y.; Zhan, S.-F.; Huang, X.-F. Network-based analysis between SARS-CoV-2 receptor ACE2 and common host factors in COVID-19 and asthma: Potential mechanistic insights. Biomed. Signal Process. Control 2024, 87, 105502. [Google Scholar] [CrossRef]
- Chen, P.; Wu, M.; He, Y.; Jiang, B.; He, M.-L. Metabolic alterations upon SARS-CoV-2 infection and potential therapeutic targets against coronavirus infection. Signal Transduct. Target. Ther. 2023, 8, 237. [Google Scholar] [CrossRef]
- Lipman, D.; Safo, S.E.; Chekouo, T. Multi-omic analysis reveals enriched pathways associated with COVID-19 and COVID-19 severity. PLoS ONE 2022, 17, e0267047. [Google Scholar] [CrossRef]
- Sawalha, A.H.; Zhao, M.; Coit, P.; Lu, Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin. Immunol. 2020, 215, 108410. [Google Scholar] [CrossRef]
- Yildirim, Z.; Sahin, O.S.; Yazar, S.; Bozok Cetintas, V. Genetic and epigenetic factors associated with increased severity of COVID-19. Cell Biol. Int. 2021, 45, 1158–1174. [Google Scholar] [CrossRef]
- Gemmati, D.; Longo, G.; Gallo, I.; Silva, J.A.; Secchiero, P.; Zauli, G.; Hanau, S.; Passaro, A.; Pellegatti, P.; Pizzicotti, S. Host genetics impact on SARS-CoV-2 vaccine-induced immunoglobulin levels and dynamics: The role of TP53, ABO, APOE, ACE2, HLA-A, and CRP genes. Front. Genet. 2022, 13, 1028081. [Google Scholar] [CrossRef]
- Ovsyannikova, I.G.; Haralambieva, I.H.; Crooke, S.N.; Poland, G.A.; Kennedy, R.B. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol. Rev. 2020, 296, 205–219. [Google Scholar] [CrossRef]
- Van Damme, W.; Dahake, R.; Delamou, A.; Ingelbeen, B.; Wouters, E.; Vanham, G.; Van De Pas, R.; Dossou, J.-P.; Ir, P.; Abimbola, S.; et al. The COVID-19 pandemic: Diverse contexts; different epidemics—How and why? BMJ Glob. Health 2020, 5, e003098. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020, 85, 104422. [Google Scholar] [CrossRef] [PubMed]
- Poduri, R.; Joshi, G.; Jagadeesh, G. Drugs targeting various stages of the SARS-CoV-2 life cycle: Exploring promising drugs for the treatment of COVID-19. Cell. Signal. 2020, 74, 109721. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Gautam, A.; Elsworth, J.; Kumar, M.; Chawla, R.; Kumar, P. An updated insight into the molecular pathogenesis, secondary complications and potential therapeutics of COVID-19 pandemic. Life Sci. 2020, 257, 118105. [Google Scholar] [CrossRef]
- Qu, P.; Faraone, J.N.; Evans, J.P.; Zheng, Y.-M.; Carlin, C.; Anghelina, M.; Stevens, P.; Fernandez, S.; Jones, D.; Panchal, A.R.; et al. Enhanced evasion of neutralizing antibody response by Omicron XBB.1.5, CH.1.1, and CA.3.1 variants. Cell Rep. 2023, 42, 112443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Møhlenberg, M.; Thakor, J.C.; Tuli, H.S.; Wang, P.; Assaraf, Y.G.; Dhama, K.; Jiang, S. Sensitivity to vaccines, therapeutic antibodies, and viral entry inhibitors and advances to counter the SARS-CoV-2 Omicron variant. Clin. Microbiol. Rev. 2022, 35, e0001422. [Google Scholar] [CrossRef] [PubMed]
- Lima Neto, J.X.; Vieira, D.S.; de Andrade, J.; Fulco, U.L. Exploring the Spike-hACE 2 Residue–Residue Interaction in Human Coronaviruses SARS-CoV-2, SARS-CoV, and HCoV-NL63. J. Chem. Inf. Model. 2022, 62, 2857–2868. [Google Scholar] [PubMed]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Tuttle, K.S.; Marquez, A.C.; Sekirov, I.; Subramaniam, S. SARS-CoV-2 Omicron variant: Antibody evasion and cryo-EM structure of spike protein–ACE2 complex. Science 2022, 375, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.J.R.D.; Kohl, A.; Pena, L.; Pardee, K. Recent insights into SARS-CoV-2 omicron variant. Rev. Med. Virol. 2023, 33, e2373. [Google Scholar] [PubMed]
- Rees-Spear, C.; Muir, L.; Griffith, S.A.; Heaney, J.; Aldon, Y.; Snitselaar, J.L.; Thomas, P.; Graham, C.; Seow, J.; Lee, N.; et al. The effect of spike mutations on SARS-CoV-2 neutralization. Cell Rep. 2021, 34, 108890. [Google Scholar] [CrossRef] [PubMed]
- Thomson, E.C.; Rosen, L.E.; Shepherd, J.G.; Spreafico, R.; da Silva Filipe, A.; Wojcechowskyj, J.A.; Davis, C.; Piccoli, L.; Pascall, D.J.; Dillen, J.; et al. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell 2021, 184, 1171–1187.e20. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Q.; Ge, J.; Ren, W.; Zhang, R.; Lan, J.; Ju, B.; Su, B.; Yu, F.; Chen, P. Analysis of SARS-CoV-2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species. Immunity 2021, 54, 1611–1621.e15. [Google Scholar] [CrossRef]
- Zhang, L.; Jackson, C.B.; Mou, H.; Ojha, A.; Peng, H.; Quinlan, B.D.; Rangarajan, E.S.; Pan, A.; Vanderheiden, A.; Suthar, M.S.; et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 2020, 11, 6013. [Google Scholar] [CrossRef]
- Mushebenge, A.G.-A.; Ugbaja, S.C.; Mbatha, N.A.; Khan, R.B.; Kumalo, H.M. A Comprehensive Analysis of Structural and Functional Changes Induced by SARS-CoV-2 Spike Protein Mutations. COVID 2023, 3, 1454–1472. [Google Scholar] [CrossRef]
- Zhou, B.; Thao, T.T.N.; Hoffmann, D.; Taddeo, A.; Ebert, N.; Labroussaa, F.; Pohlmann, A.; King, J.; Steiner, S.; Kelly, J.N.; et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature 2021, 592, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Escalera, A.; Gonzalez-Reiche, A.S.; Aslam, S.; Mena, I.; Laporte, M.; Pearl, R.L.; Fossati, A.; Rathnasinghe, R.; Alshammary, H.; van de Guchte, A.; et al. Mutations in SARS-CoV-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe 2022, 30, 373–387.e377. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, X.; Tan, S.; Dan, Y.; Lu, Y.; Zhang, J.; Xu, J.; Tan, Z.; Xiang, X.; Zhou, Y. SARS-CoV-2 quasispecies provides an advantage mutation pool for the epidemic variants. Microbiol. Spectr. 2021, 9, e0026121. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Choudhary, S.; Kumar, P.; Tomar, S. Emerging SARS-CoV-2 variants: Genetic variability and clinical implications. Curr. Microbiol. 2022, 79, 1–18. [Google Scholar] [CrossRef]
- Volz, E.; Hill, V.; McCrone, J.T.; Price, A.; Jorgensen, D.; O’Toole, Á.; Southgate, J.; Johnson, R.; Jackson, B.; Nascimento, F.F.; et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell 2021, 184, 64–75.e11. [Google Scholar] [CrossRef]
- Mengist, H.M.; Kombe Kombe, A.J.; Mekonnen, D.; Abebaw, A.; Getachew, M.; Jin, T. Mutations of SARS-CoV-2 spike protein: Implications on immune evasion and vaccine-induced immunity. Semin. Immunol. 2021, 55, 101533. [Google Scholar] [CrossRef]
- De Maio, N.; Walker, C.R.; Turakhia, Y.; Lanfear, R.; Corbett-Detig, R.; Goldman, N. Mutation rates and selection on synonymous mutations in SARS-CoV-2. Genome Biol. Evol. 2021, 13, evab087. [Google Scholar] [CrossRef]
- Karakose, T.; Polat, H.; Papadakis, S. Examining teachers’ perspectives on school principals’ digital leadership roles and technology capabilities during the COVID-19 pandemic. Sustainability 2021, 13, 13448. [Google Scholar] [CrossRef]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020, 182, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Fakhar, Z.; Hussain, A.; Ahmad, A.; Jairajpuri, D.S.; Alajmi, M.F.; Hassan, M.I. Structure-based identification of potential SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 3595–3608. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A. SARS-CoV-2 (COVID-19): A short update on molecular biochemistry, pathology, diagnosis and therapeutic strategies. Ann. Clin. Biochem. 2022, 59, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Razzaque, M.S. COVID-19 pandemic: Can zinc supplementation provide an additional shield against the infection? Comput. Struct. Biotechnol. J. 2021, 19, 1371–1378. [Google Scholar] [CrossRef]
- Robinson, P.C.; Liew, D.F.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.-P.; Hussell, T. COVID-19 therapeutics: Challenges and directions for the future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef]
- Gerardi, V.; Rohaim, M.A.; Naggar, R.F.E.; Atasoy, M.O.; Munir, M. Deep Structural Analysis of Myriads of Omicron Sub-Variants Revealed Hotspot for Vaccine Escape Immunity. Vaccines 2023, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Parkkila, S.; Wu, X.; Aspatwar, A. SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies. Int. Rev. Immunol. 2023, 42, 393–414. [Google Scholar] [CrossRef]
- Lawrence, H.Y. Vaccine Rhetorics; The Ohio State University Press: Columbus, OH, USA, 2020. [Google Scholar]
- Jaeger, B.R.; Arron, H.E.; Kalka-Moll, W.M.; Seidel, D. The potential of heparin-induced extracorporeal LDL/fibrinogen precipitation (HELP)-apheresis for patients with severe acute or chronic COVID-19. Front. Cardiovasc. Med. 2022, 9, 1007636. [Google Scholar] [CrossRef]
- Dioh, W.; Chabane, M.; Tourette, C.; Azbekyan, A.; Morelot-Panzini, C.; Hajjar, L.; Lins, M.; Nair, G.; Whitehouse, T.; Mariani, J.; et al. Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 42. [Google Scholar] [CrossRef]
- Lobo, S.M.; Plantefève, G.; Nair, G.; Cavalcante, A.J.; de Moraes, N.F.; Nunes, E.; Barnum, O.; Stadnik, C.M.B.; Lima, M.P.; Lins, M.; et al. Efficacy of oral 20-hydroxyecdysone (BIO101), a MAS receptor activator, in adults with severe COVID-19 (COVA): A randomized, placebo-controlled, phase 2/3 trial. eClinicalMedicine 2024, 102383. [Google Scholar] [CrossRef]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the main anti-SARS-CoV-2 vaccines: Mechanism of action, efficacy and safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Noor, R. Developmental Status of the Potential Vaccines for the Mitigation of the COVID-19 Pandemic and a Focus on the Effectiveness of the Pfizer-BioNTech and Moderna mRNA Vaccines. Curr. Clin. Microbiol. Rep. 2021, 8, 178–185. [Google Scholar] [CrossRef]
- Costanzo, M.; De Giglio, M.A.; Roviello, G.N. Anti-coronavirus vaccines: Past investigations on SARS-CoV-1 and MERS-CoV, the approved vaccines from BioNTech/Pfizer, Moderna, Oxford/AstraZeneca and others under Development Against SARSCoV-2 Infection. Curr. Med. Chem. 2022, 29, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.P.-Y.; Connolly, C.M.; Ruddy, J.A.; Boyarsky, B.J.; Alejo, J.L.; Werbel, W.A.; Massie, A.; Christopher-Stine, L.; Garonzik-Wang, J.; Segev, D.L.; et al. Antibody response to the Janssen/Johnson & Johnson SARS-CoV-2 vaccine in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021, 80, 1365–1366. [Google Scholar]
- Gillion, V.; Jadoul, M.; Demoulin, N.; Aydin, S.; Devresse, A. Granulomatous vasculitis after the AstraZeneca anti–SARS-CoV-2 vaccine. Kidney Int. 2021, 100, 706. [Google Scholar] [CrossRef]
- Lo, H.S.; Hui, K.P.Y.; Lai, H.-M.; He, X.; Khan, K.S.; Kaur, S.; Huang, J.; Li, Z.; Chan, A.K.; Cheung, H.H.-Y.; et al. Simeprevir potently suppresses SARS-CoV-2 replication and synergizes with remdesivir. ACS Cent. Sci. 2021, 7, 792–802. [Google Scholar] [CrossRef]
- Drożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybyciński, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Łos, M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updates 2021, 59, 100794. [Google Scholar] [CrossRef]
- Naik, R.R.; Shakya, A.K.; Aladwan, S.M.; El-Tanani, M. Kinase inhibitors as potential therapeutic agents in the treatment of COVID-19. Front. Pharmacol. 2022, 13, 806568. [Google Scholar] [CrossRef] [PubMed]
- Masiá, M.; Fernández-González, M.; Padilla, S.; Ortega, P.; García, J.A.; Agulló, V.; García-Abellán, J.; Telenti, G.; Guillén, L.; Gutiérrez, F. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: A prospective cohort study. eBioMedicine 2020, 60, 102999. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Calabrese, C.; Garofalo, E.; Bruni, A.; Vatrella, A.; Pelaia, G. Therapeutic role of tocilizumab in SARS-CoV-2-induced cytokine storm: Rationale and current evidence. Int. J. Mol. Sci. 2021, 22, 3059. [Google Scholar]
- Ashoor, D.; Marzouq, M.; Fathallah, M.-D. In silico evaluation of anti SARS-CoV-2 antibodies neutralization power: A blueprint with monoclonal antibody Sotrovimab. Res. Sq. 2023, PREPRINT. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Casal, M.C.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 2022, 327, 1236–1246. [Google Scholar] [CrossRef]
- Ashour, N.A.; Abo Elmaaty, A.; Sarhan, A.A.; Elkaeed, E.B.; Moussa, A.M.; Erfan, I.A.; Al-Karmalawy, A.A. A systematic review of the global intervention for SARS-CoV-2 combating: From drugs repurposing to molnupiravir approval. Drug Des. Dev. Ther. 2023, 16, 685–715. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Hsieh, C.-C.; Ko, W.-C. Molnupiravir—A novel oral anti-SARS-CoV-2 agent. Antibiotics 2021, 10, 1294. [Google Scholar] [CrossRef] [PubMed]
- Bhimraj, A.; Gallagher, J.C. Lack of Benefit of Fluvoxamine for COVID-19. JAMA 2023, 329, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.K.; Dehgani-Mobaraki, P. The mechanisms of action of ivermectin against SARS-CoV-2—An extensive review. J. Antibiot. 2022, 75, 60–71. [Google Scholar] [CrossRef]
- Tuccori, M.; Ferraro, S.; Convertino, I.; Cappello, E.; Valdiserra, G.; Blandizzi, C.; Maggi, F.; Focosi, D. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: Clinical pipeline. mAbs 2020, 12, 1854149. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.K.; Yin, J.; James, M.N. Treatment and prevention strategies for the COVID 19 pandemic: A review of immunotherapeutic approaches for neutralizing SARS-CoV-2. Int. J. Biol. Macromol. 2021, 186, 490–500. [Google Scholar] [CrossRef]
- Taha, Y.; Wardle, H.; Evans, A.B.; Hunter, E.R.; Marr, H.; Osborne, W.; Bashton, M.; Smith, D.; Burton-Fanning, S.; Schmid, M.L.; et al. Persistent SARS-CoV-2 infection in patients with secondary antibody deficiency: Successful clearance following combination casirivimab and imdevimab (REGN-COV2) monoclonal antibody therapy. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 85. [Google Scholar] [CrossRef]
- Liu, X.; Munro, A.P.; Feng, S.; Janani, L.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: Three month analyses of the COV-BOOST trial. J. Infect. 2022, 84, 795–813. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, P.; Hao, T.; Liu, S.; Wang, X.; Xie, Y.; Xu, K.; Lei, W.; Zhang, C.; Han, P. Rational design of an influenza-COVID-19 chimeric protective vaccine with HA-stalk and S-RBD. Emerg. Microbes Infect. 2023, 12, 2231573. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.A. Efficacy of repurposed antiviral drugs: Lessons from COVID-19. Drug Discov. Today 2022, 27, 1954–1960. [Google Scholar] [CrossRef]
- Mushebenge, A.G.-A.; Ugbaja, S.C.; Mbatha, N.A.; Khan, R.B.; Kumalo, H.M. Assessing the Potential Contribution of In Silico Studies in Discovering Drug Candidates That Interact with Various SARS-CoV-2 Receptors. Int. J. Mol. Sci. 2023, 24, 15518. [Google Scholar] [CrossRef] [PubMed]
- Consortium, W.S.T. Repurposed antiviral drugs for COVID-19—Interim WHO solidarity trial results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.; Roviello, G.N. SARS-CoV-2: Recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr. Med. Chem. 2020, 27, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, R.K.; Petruschke, R.A. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J. Antimicrob. Chemother. 2004, 53, 4–9. [Google Scholar] [CrossRef]
- Jain, M.S.; Barhate, S.D. Favipiravir has been investigated for the treatment of life-threatening pathogens such as Ebola virus, Lassa virus, and now COVID-19: A review. Asian J. Pharm. Res. 2021, 11, 39–42. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Pastick, K.A.; Okafor, E.C.; Wang, F.; Lofgren, S.M.; Skipper, C.P.; Nicol, M.R.; Pullen, M.F.; Rajasingham, R.; McDonald, E.G.; Lee, T.C.; et al. Review: Hydroxychloroquine and Chloroquine for Treatment of SARS-CoV-2 (COVID-19). Open Forum Infect. Dis. 2020, 7, ofaa130. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, A.; Shaikh, A.; Singh, R.; Misra, A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Gbinigie, K.; Frie, K. Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review. BJGP Open 2020, 4, bjgpopen20X101069. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.D.; Gray, A.L.; Kredo, T.; De Waal, R.; Cohen, K.; Parrish, A.G.; Dawood, H. Managing therapeutic uncertainty in the COVID-19 pandemic: Rapid evidence syntheses and transparent decision-making. S. Afr. Health Rev. 2021, 2021, 41–49. [Google Scholar] [CrossRef]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G.; Wang, D.D.; Huitsing, K.; Brar, I.; Alangaden, G.J.; Ramesh, M.S.; McKinnon, J.E.; et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020, 97, 396–403. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Alejandro, R.; Caprio, M.; Fabbri, A. Hydroxychloroquine in the COVID-19 pandemic era: In pursuit of a rational use for prophylaxis of SARS-CoV-2 infection. Expert Rev. Anti-Infect. Ther. 2021, 19, 5–16. [Google Scholar] [CrossRef]
- Kalra, R.S.; Tomar, D.; Meena, A.S.; Kandimalla, R. SARS-CoV-2, ACE2, and hydroxychloroquine: Cardiovascular complications, therapeutics, and clinical readouts in the current settings. Pathogens 2020, 9, 546. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Memorandum Explaining Basis for Revocation of Emergency Use Authorization for Chloroquine Phosphate and Hydroxychloroquine Sulfate. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-revokes-emergency-use-authorization-chloroquine-and (accessed on 27 October 2023).
- U.S. Food and Drug Administration (FDA). FDA Cautions against Use of Hydroxychloroquine or Chloroquine for COVID-19 Outside of the Hospital Setting or a Clinical Trial Due to Risk of Heart Rhythm Problems. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-COVID-19-outside-hospital-setting-or (accessed on 27 October 2023).
- Figueredo, J.; Lopez, L.F.; Leguizamon, B.F.; Samudio, M.; Pederzani, M.; Apelt, F.F.; Añazco, P.; Caballero, R.; Bianco, H. Clinical evolution and mortality of critically ill patients with SARS-CoV-2 pneumonia treated with remdesivir in an adult intensive care unit of Paraguay. BMC Infect. Dis. 2024, 24, 37. [Google Scholar] [CrossRef]
- Murmu, N.; Sarkar, M.; Dey, S.; Manna, R.; Roy, S.; Mondal, T.; Halder, S.; Bhattacharjee, N.; Dash, S.K.; Giri, B. Efficacy and limitations of repurposed drugs and vaccines for COVID-19. J. Med. Surg. Public Health 2023, 2, 100041. [Google Scholar] [CrossRef]
- Waseem, W.; Zafar, R.; Jan, M.S.; Alomar, T.S.; Almasoud, N.; Rauf, A.; Khattak, H. Drug repurposing of FDA-approved anti-viral drugs via computational screening against novel 6M03 SARS-COVID-19. Ir. J. Med. Sci. 2023, 193, 73–83. [Google Scholar] [CrossRef]
- Rabie, A.M.; Abdel-Dayem, M.A.; Abdalla, M. Promising experimental anti-SARS-CoV-2 agent “SLL-0197800”: The prospective universal inhibitory properties against the coming versions of the coronavirus. ACS Omega 2023, 8, 35538–35554. [Google Scholar] [CrossRef]
- Akter, R.; Rahman, M.R.; Ahmed, Z.S.; Afrose, A. Plausibility of natural immunomodulators in the treatment of COVID-19—A comprehensive analysis and future recommendations. Heliyon 2023, 9, e17478. [Google Scholar] [CrossRef]
- Velásquez, P.A.; Hernandez, J.C.; Galeano, E.; Hincapié-García, J.; Rugeles, M.T.; Zapata-Builes, W. Effectiveness of Drug Repurposing and Natural Products Against SARS-CoV-2: A Comprehensive Review. Clin. Pharmacol. Adv. Appl. 2024, 16, 1–25. [Google Scholar] [CrossRef]
- Mushebenge, A.G.; Ugbaja, S.C.; Mtambo, S.E.; Ntombela, T.; Metu, J.I.; Babayemi, O.; Chima, J.I.; Appiah-Kubi, P.; Odugbemi, A.I.; Ntuli, M.L.; et al. Unveiling the Inhibitory Potentials of Peptidomimetic Azanitriles and Pyridyl Esters towards SARS-CoV-2 Main Protease: A Molecular Modelling Investigation. Molecules 2023, 28, 2641. [Google Scholar] [CrossRef]
- Das, A.; Khan, S.; Roy, S.; Das, S. Phytochemicals for mitigating the COVID-19 crisis: Evidence from pre-clinical and clinical studies. Explor. Drug Sci. 2023, 1, 336–376. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Patel, K.J.; Ranjan, K. COVID-19: Unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules 2021, 11, 993. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Hannan, M.A.; Rahman, S.; Hossain, M.; Hasan, M.; Khan, M.K.; Khatun, A.; Dash, R.; Uddin, M.J. Revisiting potential druggable targets against SARS-CoV-2 and repurposing therapeutics under preclinical study and clinical trials: A comprehensive review. Drug Dev. Res. 2020, 81, 919–941. [Google Scholar] [CrossRef] [PubMed]
- Velikova, T.; Georgiev, T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol. Int. 2021, 41, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, U.; Barouch, D.H.; Rizzi, R.; Lagkadinou, E.; Türeci, Ö.; Pather, S.; Neels, P. Clinical development and approval of COVID-19 vaccines. Expert Rev. Vaccines 2022, 21, 609–619. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Liu, Z. Nanomedicine approaches against SARS-CoV-2 and variants. J. Control. Release 2024, 365, 101–111. [Google Scholar] [CrossRef]
- D’Acunto, E.; Muzi, A.; Marchese, S.; Donnici, L.; Chiarini, V.; Bucci, F.; Pavoni, E.; Ferrara, F.F.; Cappelletti, M.; Arriga, R.; et al. Isolation and characterization of neutralizing monoclonal antibodies from a large panel of murine antibodies against RBD of the SARS-CoV-2 Spike protein. Antibodies 2024, 13, 5. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Takatori, M.; Mochizuki, H.; Omata, M. Effectiveness of the severe acute respiratory syndrome coronavirus 2 Omicron BA. 5 bivalent vaccine on symptoms in healthcare workers with BA.5 infection. Vaccine X 2024, 17, 100433. [Google Scholar] [CrossRef]
- Aydillo, T.; Rombauts, A.; Stadlbauer, D.; Aslam, S.; Abelenda-Alonso, G.; Escalera, A.; Amanat, F.; Jiang, K.; Krammer, F.; Carratala, J.; et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat. Commun. 2021, 12, 3781. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Hodel, K.V.S.; Fonseca, L.M.D.S.; Pires, V.C.; Mascarenhas, L.A.B.; da Silva Andrade, L.P.C.; Moret, M.A.; Badaró, R. The importance of vaccination in the context of the COVID-19 pandemic: A brief update regarding the use of vaccines. Vaccines 2022, 10, 591. [Google Scholar] [CrossRef]
- Malik, J.A.; Mulla, A.H.; Farooqi, T.; Pottoo, F.H.; Anwar, S.; Rengasamy, K.R. Targets and strategies for vaccine development against SARS-CoV-2. Biomed. Pharmacother. 2021, 137, 111254. [Google Scholar] [CrossRef] [PubMed]
- Piperno, A.; Sciortino, M.T.; Giusto, E.; Montesi, M.; Panseri, S.; Scala, A. Recent advances and challenges in gene delivery mediated by polyester-based nanoparticles. Int. J. Nanomed. 2021, 16, 5981–6002. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, S.; Sharma, P.C. Recent advances in the vaccine development for the prophylaxis of SARS COVID-19. Int. Immunopharmacol. 2022, 111, 109175. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, S.; Arshadi, M.; Akrami, S.; Koupaei, M.; Ghahramanpour, H.; Shariati, A.; Sadeghifard, N.; Heidary, M. An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. J. Clin. Lab. Anal. 2022, 36, e24418. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Yang, S.; Boyer, J.D.; Reuschel, E.L.; Patel, A.; Christensen-Quick, A.; Andrade, V.M.; Morrow, M.P.; Kraynyak, K.; Agnes, J.; et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. eClinicalMedicine 2021, 31, 100689. [Google Scholar] [CrossRef]
- Dokoupilová, E.; Vetchý, D.; Pavloková, S.; Hanuštiaková, M. Effect of treatment with original or biosimilar adalimumab on SARS-CoV2 vaccination antibody titers. Int. J. Pharm. X 2024, 7, 100229. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Yuan, D.; Kellman, B.; Gonzalez, I.G.d.S.; Clemens, R.; Milan, E.P.; Sprinz, E.; Cerbino Neto, J.; Smolenov, I.; Alter, G.; et al. Boosting with adjuvanted SCB-2019 elicits superior Fcγ-receptor engagement driven by IgG3 to SARS-CoV-2 spike. NPJ Vaccines 2024, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Maldeney, A.R.; Yuan, X.; Richer, M.J.; Renshaw, S.E.; Luo, W. Ipsilateral immunization after a prior SARS-CoV-2 mRNA vaccination elicits superior B cell responses compared to contralateral immunization. Cell Rep. 2024, 43, 113665. [Google Scholar] [CrossRef]
- Song, N.-J.; Chakravarthy, K.B.; Jeon, H.; Bolyard, C.; Reynolds, K.; Weller, K.P.; Reisinger, S.; Wang, Y.; Li, A.; Jiang, S. mRNA vaccines against SARS-CoV-2 induce divergent antigen-specific T-cell responses in patients with lung cancer. J. Immunother. Cancer 2024, 12, e007922. [Google Scholar] [CrossRef]
- Shrotri, M.; Swinnen, T.; Kampmann, B.; Parker, E.P. An interactive website tracking COVID-19 vaccine development. Lancet Glob. Health 2021, 9, e590–e592. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, P.; Dai, R.; Lv, X.; Yuan, S.; Zhang, Y.; Guo, Y.; Li, R.; Yu, Q.; Zhu, K. Convalescent plasma may be a possible treatment for COVID-19: A systematic review. Int. Immunopharmacol. 2021, 91, 107262. [Google Scholar] [CrossRef]
- Baldeón, M.E.; Maldonado, A.; Ochoa-Andrade, M.; Largo, C.; Pesantez, M.; Herdoiza, M.; Granja, G.; Bonifaz, M.; Espejo, H.; Mora, F.; et al. Effect of convalescent plasma as complementary treatment in patients with moderate COVID-19 infection. Transfus. Med. 2022, 32, 153–161. [Google Scholar] [CrossRef]
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Hussham Arshad, M.; et al. COVID-19 pandemic: From origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez. Med. 2021, 29, 20–36. [Google Scholar]
- Chadha, R.; Raghav, A.; Banerjee, B.; Sengar, A.; Sengar, M.; Raghav, P.K. Targets of SARS-CoV-2: Therapeutic implications for COVID-19. In Stem Cells; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–14. [Google Scholar]
- Wang, Y.; Zhang, Z.; Yang, M.; Xiong, X.; Yan, Q.; Cao, L.; Wei, P.; Zhang, Y.; Zhang, L.; Lv, K.; et al. Identification of a broad sarbecovirus neutralizing antibody targeting a conserved epitope on the receptor-binding domain. Cell Rep. 2024, 43, 113653. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Turelli, P.; Perez, L.; Pellaton, C.; Esteves-Leuenberger, L.; Farina, A.; Campos, J.; Lana, E.; Fiscalini, F.; Raclot, C.; et al. A highly potent antibody effective against SARS-CoV-2 variants of concern. Cell Rep. 2021, 37, 109814. [Google Scholar] [CrossRef]
- Jones, B.E.; Brown-Augsburger, P.L.; Corbett, K.S.; Westendorf, K.; Davies, J.; Cujec, T.P.; Wiethoff, C.M.; Blackbourne, J.L.; Heinz, B.A.; Foster, D.; et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci. Transl. Med. 2021, 13, eabf1906. [Google Scholar] [CrossRef]
- Sahoo, P.; Dey, J.; Mahapatra, S.R.; Ghosh, A.; Jaiswal, A.; Padhi, S.; Prabhuswamimath, S.C.; Misra, N.; Suar, M. Nanotechnology and COVID-19 convergence: Toward new planetary health interventions against the pandemic. OMICS J. Integr. Biol. 2022, 26, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Michler, T.; Merkel, O.M. siRNA therapeutics against respiratory viral infections—What have we learned for potential COVID-19 therapies? Adv. Healthc. Mater. 2021, 10, 2001650. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, F.; Wang, R.; Guan, K.; Jiang, T.; Xu, G.; Sun, J.; Chang, C. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020, 109, 102434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Seyedpour, S.; Khodaei, B.; Loghman, A.H.; Seyedpour, N.; Kisomi, M.F.; Balibegloo, M.; Nezamabadi, S.S.; Gholami, B.; Saghazadeh, A.; Rezaei, N. Targeted therapy strategies against SARS-CoV-2 cell entry mechanisms: A systematic review of in vitro and in vivo studies. J. Cell. Physiol. 2021, 236, 2364–2392. [Google Scholar] [CrossRef]
- Ita, K. Coronavirus disease (COVID-19): Current status and prospects for drug and vaccine development. Arch. Med. Res. 2021, 52, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Miners, S.; Kehoe, P.G.; Love, S. Cognitive impact of COVID-19: Looking beyond the short term. Alzheimer’s Res. Ther. 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.M.; Al-Sabi, A.; Stockand, J.D. Human recombinant soluble ACE2 (hrsACE2) shows promise for treating severe COVID19. Signal Transduct. Target. Ther. 2020, 5, 258. [Google Scholar] [CrossRef]
- Zanganeh, S.; Goodarzi, N.; Doroudian, M.; Movahed, E. Potential COVID-19 therapeutic approaches targeting angiotensin-converting enzyme 2; an updated review. Rev. Med. Virol. 2022, 32, e2321. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, S.; Liu, R.; Krall, A.; Wang, Y.; Ventura, M.; Deflitch, C. Epidemic informatics and control: A holistic approach from system informatics to epidemic response and risk management in public health. In AI and Analytics for Public Health-Proceedings of the 2020 INFORMS International Conference on Service Science; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–46. [Google Scholar]
- Di Domenico, L.; Pullano, G.; Sabbatini, C.E.; Boëlle, P.-Y.; Colizza, V. Impact of lockdown on COVID-19 epidemic in Île-de-France and possible exit strategies. BMC Med. 2020, 18, 240. [Google Scholar] [CrossRef]
- Simandan, D.; Rinner, C.; Capurri, V. The academic left, human geography, and the rise of authoritarianism during the COVID-19 pandemic. Geogr. Ann. Ser. B Hum. Geogr. 2023, 1–21. [Google Scholar] [CrossRef]
- Behera, R.K.; Bala, P.K.; Rana, N.P.; Kayal, G. Self-promotion and online shaming during COVID-19: A toxic combination. Int. J. Inf. Manag. Data Insights 2022, 2, 100117. [Google Scholar] [CrossRef]
- Mendez-Brito, A.; El Bcheraoui, C.; Pozo-Martin, F. Systematic review of empirical studies comparing the effectiveness of non-pharmaceutical interventions against COVID-19. J. Infect. 2021, 83, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.M.; Choocharukul, K. Passengers’ Intentions to Use Public Transport during the COVID-19 Pandemic: A Case Study of Bangkok and Jakarta. Sustainability 2023, 15, 5273. [Google Scholar] [CrossRef]
- Hanson, K.E.; Caliendo, A.M.; Arias, C.A.; Englund, J.A.; Lee, M.J.; Loeb, M.; Patel, R.; El Alayli, A.; Kalot, M.A.; Falck-Ytter, Y. Infectious Diseases Society of America guidelines on the diagnosis of coronavirus disease 2019. Clin. Infect. Dis. 2020, ciaa760. [Google Scholar] [CrossRef] [PubMed]
- Sala, G.; Chakraborti, R.; Ota, A.; Miyakawa, T. Association of BCG vaccination policy and tuberculosis burden with incidence and mortality of COVID-19. medRxiv 2020, 3. [Google Scholar] [CrossRef]
- Aminu, J. The implications of misconceptions about coronavirus disease (COVID-19) pandemic in relation to its daily increases from Nigerian perspective. J. Infect. Dis. Epidemiol. 2020, 6, 156. [Google Scholar]
- Yang, J.; Li, X.; He, T.; Ju, F.; Qiu, Y.; Tian, Z. Impact of physical activity on COVID-19. Int. J. Environ. Res. Public Health 2022, 19, 14108. [Google Scholar] [CrossRef] [PubMed]
- Ayouni, I.; Maatoug, J.; Dhouib, W.; Zammit, N.; Fredj, S.B.; Ghammam, R.; Ghannem, H. Effective public health measures to mitigate the spread of COVID-19: A systematic review. BMC Public Health 2021, 21, 1015. [Google Scholar] [CrossRef]
- Cheng, V.C.-C.; Wong, S.-C.; Chuang, V.W.-M.; So, S.Y.-C.; Chen, J.H.-K.; Sridhar, S.; To, K.K.-W.; Chan, J.F.-W.; Hung, I.F.-N.; Ho, P.-L.; et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020, 81, 107–114. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.-W.; Sridhar, S.; Chiu, K.H.-Y.; Hung, D.L.-L.; Li, X.; Hung, I.F.-N.; Tam, A.R.; Chung, T.W.-H.; Chan, J.F.-W.; Zhang, A.J.-X.; et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes Infect. 2021, 10, 507–535. [Google Scholar] [CrossRef] [PubMed]
- Dumache, R.; Enache, A.; Macasoi, I.; Dehelean, C.A.; Dumitrascu, V.; Mihailescu, A.; Popescu, R.; Vlad, D.; Vlad, C.S.; Muresan, C. SARS-CoV-2: An overview of the genetic profile and vaccine effectiveness of the five variants of concern. Pathogens 2022, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Genomic Sequencing of SARS-CoV-2: A Guide to Implementation for Maximum Impact on Public Health; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Adly, A.S.; Adly, A.S.; Adly, M.S. Approaches based on artificial intelligence and the internet of intelligent things to prevent the spread of COVID-19: Scoping review. J. Med. Internet Res. 2020, 22, e19104. [Google Scholar] [CrossRef] [PubMed]
- McCall, B. COVID-19 and artificial intelligence: Protecting health-care workers and curbing the spread. Lancet Digit. Health 2020, 2, e166–e167. [Google Scholar] [CrossRef]
- Yu, K.-H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef]
- Salvatore, M.; Purkayastha, S.; Ganapathi, L.; Bhattacharyya, R.; Kundu, R.; Zimmermann, L.; Ray, D.; Hazra, A.; Kleinsasser, M.; Solomon, S. Lessons from SARS-CoV-2 in India: A data-driven framework for pandemic resilience. Sci. Adv. 2022, 8, eabp8621. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Mir, A.; Shinde, M.; Bender, O.; Alshammari, F.; Ansari, M.; Anwar, S. The SARS-CoV-2 mutations versus vaccine effectiveness: New opportunities to new challenges. J. Infect. Public Health 2022, 15, 228–240. [Google Scholar] [CrossRef]
- Gulseven, O.; Al Harmoodi, F.; Al Falasi, M.; ALshomali, I. How the COVID-19 Pandemic Will Affect the UN Sustainable Development Goals? 2020. Available online: https://ssrn.com/abstract=3592933 (accessed on 27 October 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushebenge, A.G.-A.; Ugbaja, S.C.; Mbatha, N.A.; Khan, R.B.; Kumalo, H.M. Unravelling Insights into the Evolution and Management of SARS-CoV-2. BioMedInformatics 2024, 4, 385-409. https://doi.org/10.3390/biomedinformatics4010022
Mushebenge AG-A, Ugbaja SC, Mbatha NA, Khan RB, Kumalo HM. Unravelling Insights into the Evolution and Management of SARS-CoV-2. BioMedInformatics. 2024; 4(1):385-409. https://doi.org/10.3390/biomedinformatics4010022
Chicago/Turabian StyleMushebenge, Aganze Gloire-Aimé, Samuel Chima Ugbaja, Nonkululeko Avril Mbatha, Rene B. Khan, and Hezekiel M. Kumalo. 2024. "Unravelling Insights into the Evolution and Management of SARS-CoV-2" BioMedInformatics 4, no. 1: 385-409. https://doi.org/10.3390/biomedinformatics4010022
APA StyleMushebenge, A. G.-A., Ugbaja, S. C., Mbatha, N. A., Khan, R. B., & Kumalo, H. M. (2024). Unravelling Insights into the Evolution and Management of SARS-CoV-2. BioMedInformatics, 4(1), 385-409. https://doi.org/10.3390/biomedinformatics4010022







