Optimizing the Pharmacological and Optical Dosimetry for Photodynamic Therapy with Methylene Blue and Nanoliposomal Benzoporphyrin on Pancreatic Cancer Spheroids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Liposome Preparation and Characterization
2.3. Detection of HO• and 1O2 Generation
2.4. Cell and Spheroid Culture
2.5. Determination of Photosensitizer Uptake and Dark Toxicity
2.6. Determination of PDT Efficacy
2.7. Statistical Analysis
3. Results
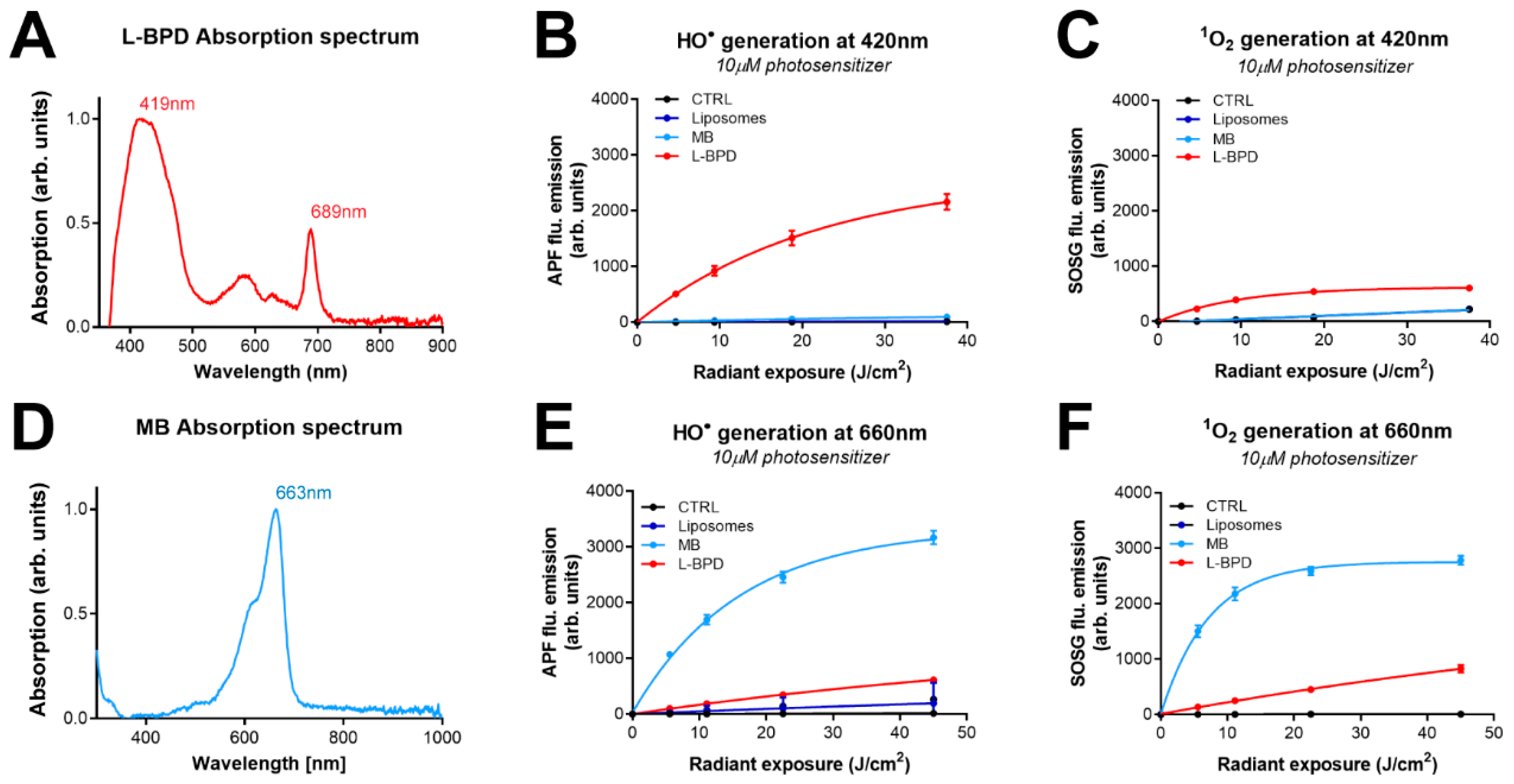
3.1. The Photodynamic Activity of L-BPD and MB
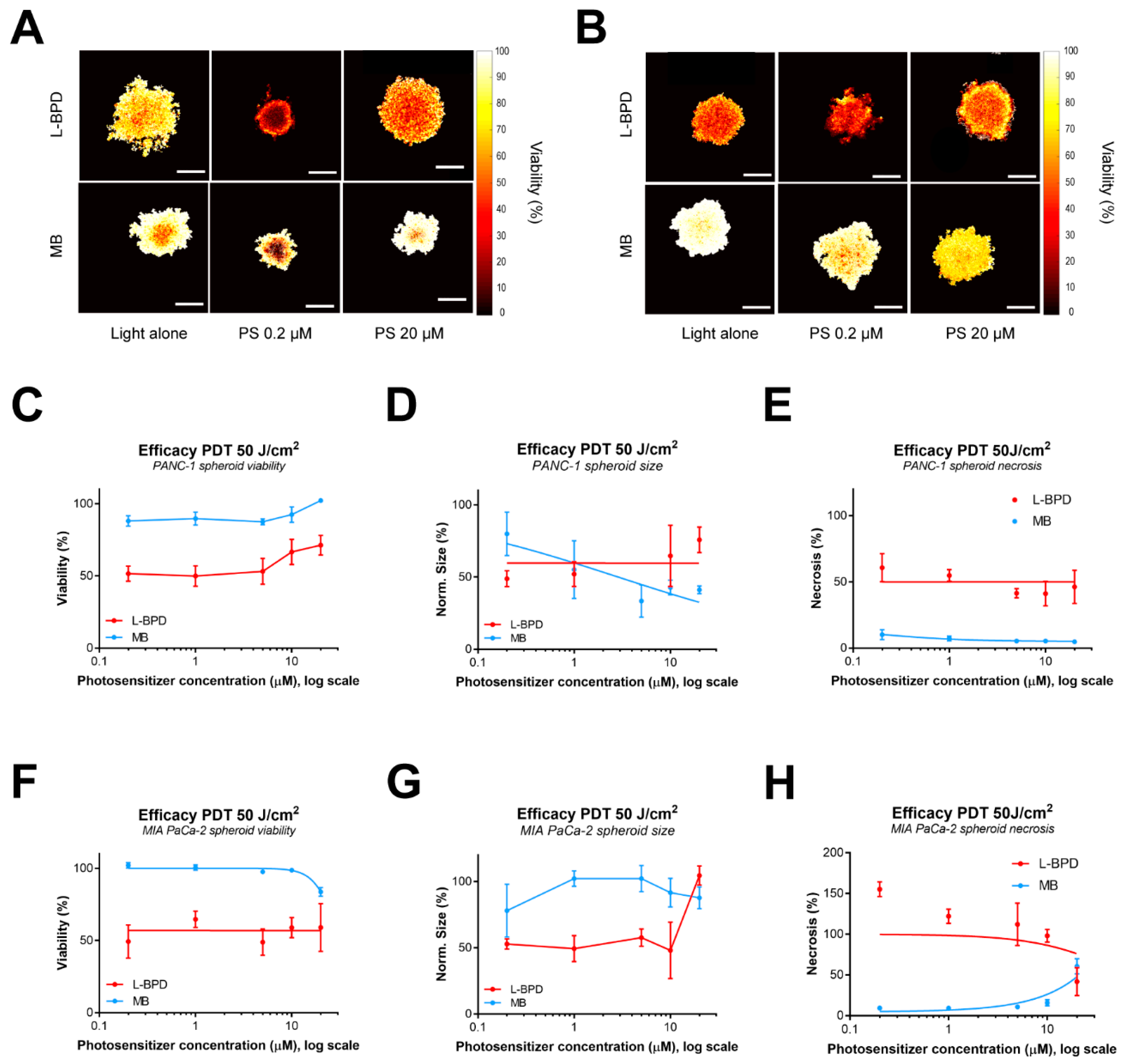
3.2. The Dark Toxicity and Uptake of L-BPD and MB in Pancreatic Cancer Spheroids
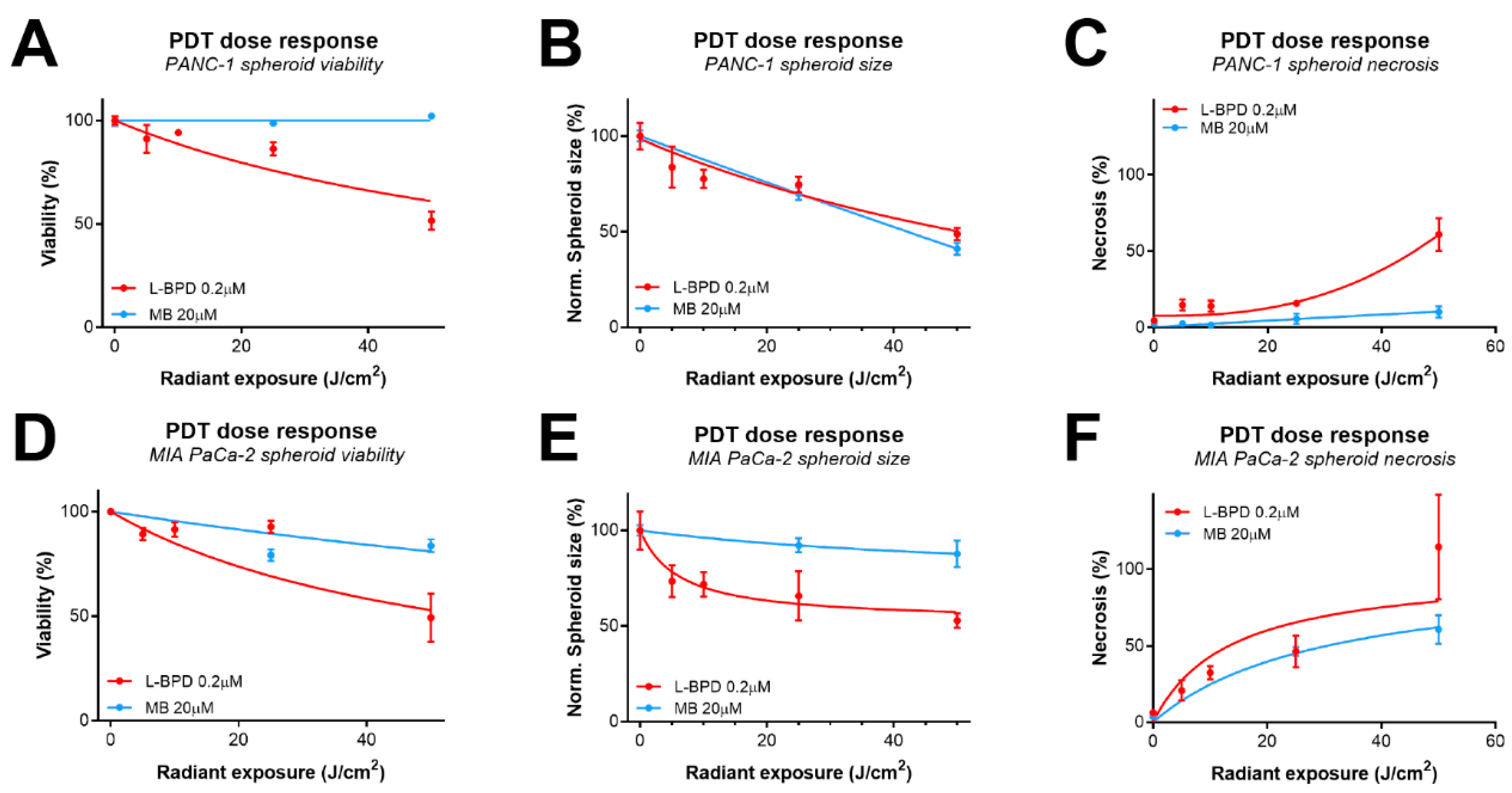
3.3. The Effect of Photosensitzer Dose on PDT Efficacy of L-BPD and MB in Pancreatic Cancer Spheroids
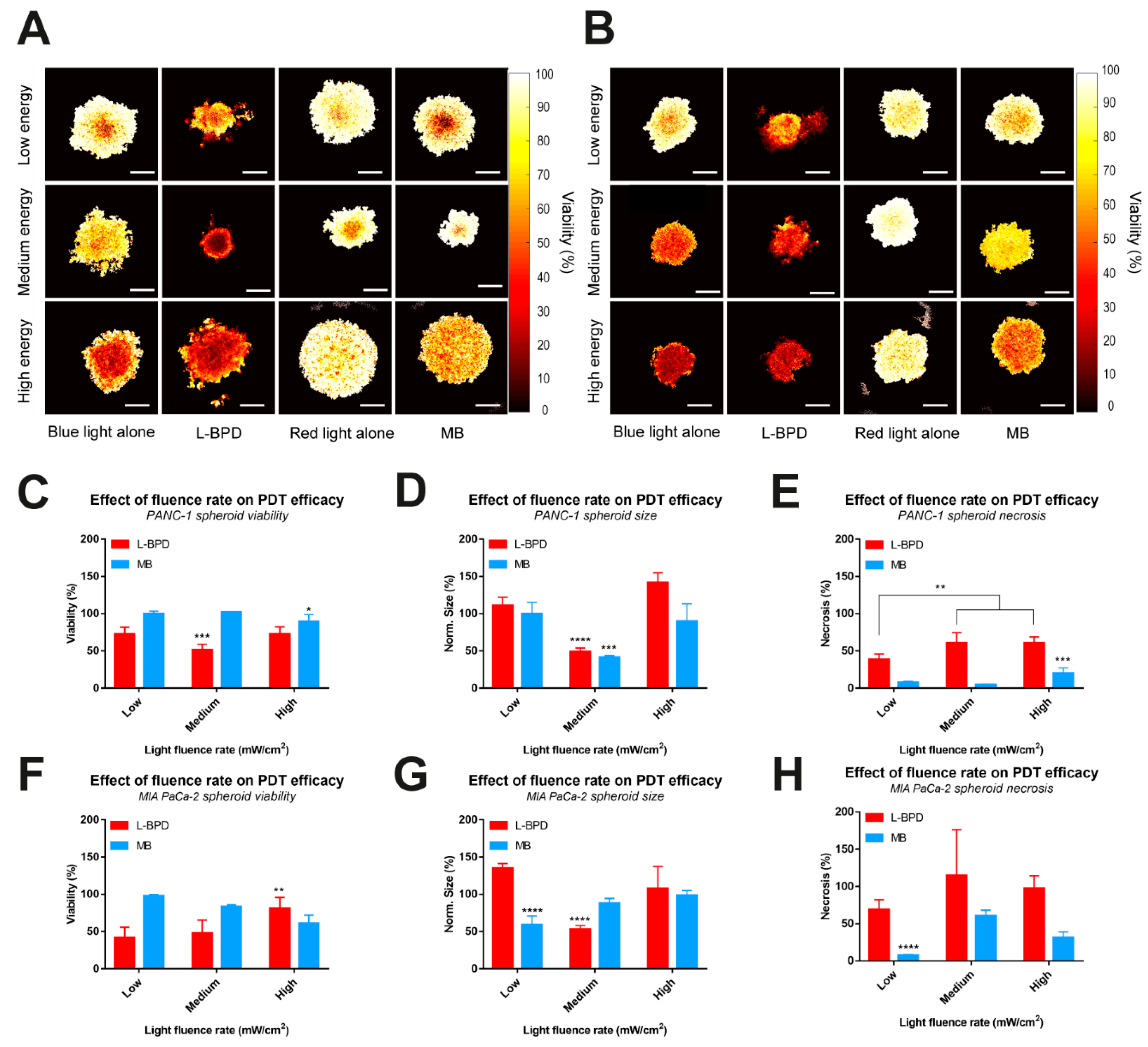
3.4. The Effect of Fluence Rate on the PDT Efficacy of L-BPD and MB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, E.; Warloe, T.; Kroon, S.; Funk, J.; Helsing, P.; Soler, A.M.; Stang, H.J.; Vatne, O.; Mørk, C. Norwegian Photodynamic Therapy (PDT) Group Guidelines for Practical Use of MAL-PDT in Non-Melanoma Skin Cancer. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 505–512. [Google Scholar] [CrossRef]
- Hopper, C.; Niziol, C.; Sidhu, M. The Cost-Effectiveness of Foscan Mediated Photodynamic Therapy (Foscan-PDT) Compared with Extensive Palliative Surgery and Palliative Chemotherapy for Patients with Advanced Head and Neck Cancer in the UK. Oral Oncol. 2004, 40, 372–382. [Google Scholar] [CrossRef]
- Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic Therapy in Head and Neck Cancer: Indications, Outcomes, and Future Prospects. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 136–141. [Google Scholar] [CrossRef]
- Ohtani, K.; Ikeda, N. Photodynamic Therapy for Lung Cancer. Kyobu Geka 2016, 69, 694–699. [Google Scholar]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic Therapy (PDT) for Lung Cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Gheewala, T.; Skwor, T.; Munirathinam, G. Photosensitizers in Prostate Cancer Therapy. Oncotarget 2017, 8, 30524–30538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windahl, T.; Andersson, S.O.; Lofgren, L. Photodynamic Therapy of Localised Prostatic Cancer. Lancet 1990, 336, 1139. [Google Scholar] [CrossRef]
- Yano, T.; Wang, K.K. Photodynamic Therapy for Gastrointestinal Cancer. Photochem. Photobiol. 2020, 96, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S. Photodynamic Therapy for Pancreatic and Biliary Tract Cancer: The United Kingdom Experience. J. Natl. Compr. Canc. Netw. 2012, 10 (Suppl. 2), S48–S51. [Google Scholar] [CrossRef] [Green Version]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II Study of Verteporfin Photodynamic Therapy in Locally Advanced Pancreatic Cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef] [Green Version]
- Celli, J.P.; Solban, N.; Liang, A.; Pereira, S.P.; Hasan, T. Verteporfin-Based Photodynamic Therapy Overcomes Gemcitabine Insensitivity in a Panel of Pancreatic Cancer Cell Lines. Lasers Surg. Med. 2011, 43, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, B.-G.; Andrén-Sandberg, A. Photodynamic Therapy for Pancreatic Cancer. Pancreas 2007, 34, 385–389. [Google Scholar] [CrossRef]
- Banerjee, S.M.; El-Sheikh, S.; Malhotra, A.; Mosse, C.A.; Parker, S.; Williams, N.R.; MacRobert, A.J.; Hamoudi, R.; Bown, S.G.; Keshtgar, M.R. Photodynamic Therapy in Primary Breast Cancer. J. Clin. Med. 2020, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One-Photosensitizers, Photochemistry and Cellular Localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Bulin, A.-L.; Hurbin, A.; Elleaume, H.; Coll, J.-L.; Broekgaarden, M. Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives. Cancers 2020, 12, 2491. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Chen, Q.; Chopp, M.; Dereski, M.O.; Wilson, B.C.; Patterson, M.S.; Schreiber, A.; Hetzel, F.W. The Effect of Light Fluence Rate in Photodynamic Therapy of Normal Rat Brain. Radiat. Res. 1992, 132, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence Rate as a Modulator of PDT Mechanisms. Lasers Surg. Med. 2006, 38, 489–493. [Google Scholar] [CrossRef]
- Iinuma, S.; Schomacker, K.T.; Wagnieres, G.; Rajadhyaksha, M.; Bamberg, M.; Momma, T.; Hasan, T. In Vivo Fluence Rate and Fractionation Effects on Tumor Response and Photobleaching: Photodynamic Therapy with Two Photosensitizers in an Orthotopic Rat Tumor Model. Cancer Res. 1999, 59, 6164–6170. [Google Scholar]
- Foster, T.H.; Hartley, D.F.; Nichols, M.G.; Hilf, R. Fluence Rate Effects in Photodynamic Therapy of Multicell Tumor Spheroids. Cancer Res. 1993, 53, 1249–1254. [Google Scholar]
- Broekgaarden, M.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Mai, Z.; Baglo, Y.; Rizvi, I.; Hasan, T. Modulation of Redox Metabolism Negates Cancer-Associated Fibroblasts-Induced Treatment Resistance in a Heterotypic 3D Culture Platform of Pancreatic Cancer. Biomaterials 2019, 222, 119421. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor Cell Survival Pathways Activated by Photodynamic Therapy: A Molecular Basis for Pharmacological Inhibition Strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef] [Green Version]
- Babilas, P.; Schacht, V.; Liebsch, G.; Wolfbeis, O.S.; Landthaler, M.; Szeimies, R.-M.; Abels, C. Effects of Light Fractionation and Different Fluence Rates on Photodynamic Therapy with 5-Aminolaevulinic Acid in Vivo. Br. J. Cancer 2003, 88, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Angell-Petersen, E.; Spetalen, S.; Madsen, S.J.; Sun, C.-H.; Peng, Q.; Carper, S.W.; Sioud, M.; Hirschberg, H. Influence of Light Fluence Rate on the Effects of Photodynamic Therapy in an Orthotopic Rat Glioma Model. J. Neurosurg. 2006, 104, 109–117. [Google Scholar] [CrossRef]
- Weijer, R.; Broekgaarden, M.; Kos, M.; van Vught, R.; Rauws, E.A.J.; Breukink, E.; van Gulik, T.M.; Storm, G.; Heger, M. Enhancing Photodynamic Therapy of Refractory Solid Cancers: Combining Second-Generation Photosensitizers with Multi-Targeted Liposomal Delivery. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 103–131. [Google Scholar] [CrossRef]
- Broekgaarden, M.; de Kroon, A.I.P.M.; Gulik, T.M.; van Heger, M. Development and in Vitro Proof-of-Concept of Interstitially Targeted Zinc- Phthalocyanine Liposomes for Photodynamic Therapy. Curr. Med. Chem. 2014, 21, 377–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obaid, G.; Broekgaarden, M.; Bulin, A.-L.; Huang, H.-C.; Kuriakose, J.; Liu, J.; Hasan, T. Photonanomedicine: A Convergence of Photodynamic Therapy and Nanotechnology. Nanoscale 2016, 8, 12471–12503. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Anbil, S.; Alagic, N.; Celli, J.; Celli, J.P.; Zheng, L.Z.; Palanisami, A.; Glidden, M.D.; Pogue, B.W.; Hasan, T. PDT Dose Parameters Impact Tumoricidal Durability and Cell Death Pathways in a 3D Ovarian Cancer Model. Photochem. Photobiol. 2013, 89, 942–952. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, W.; Wang, C.; Qin, W.; Ming, J.; Zhang, M.; Qian, H.; Jiao, T. Methylene Blue-Mediated Photodynamic Therapy Induces Macrophage Apoptosis via ROS and Reduces Bone Resorption in Periodontitis. Oxid. Med. Cell. Longev. 2019, 2019, 1529520. [Google Scholar] [CrossRef] [Green Version]
- Späth, A.; Leibl, C.; Cieplik, F.; Lehner, K.; Regensburger, J.; Hiller, K.-A.; Bäumler, W.; Schmalz, G.; Maisch, T. Improving Photodynamic Inactivation of Bacteria in Dentistry: Highly Effective and Fast Killing of Oral Key Pathogens with Novel Tooth-Colored Type-II Photosensitizers. J. Med. Chem. 2014, 57, 5157–5168. [Google Scholar] [CrossRef]
- Shen, J.J.; Jemec, G.B.E.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic Therapy Treatment of Superficial Fungal Infections: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 31, 101774. [Google Scholar] [CrossRef]
- Jesus, V.P.S.; Raniero, L.; Lemes, G.M.; Bhattacharjee, T.T.; Caetano Júnior, P.C.; Castilho, M.L. Nanoparticles of Methylene Blue Enhance Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2018, 23, 212–217. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.M.; Oliveira, T.C.; Vinícius de Morais, G.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene Blue Photodynamic Therapy Induces Selective and Massive Cell Death in Human Breast Cancer Cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [Green Version]
- Methylene Blue Mediated Photodynamic Therapy in Experimental Colorectal Tumors in Mice—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11154085/ (accessed on 29 November 2021).
- Carioli, G.; Malvezzi, M.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European Cancer Mortality Predictions for the Year 2021 with Focus on Pancreatic and Female Lung Cancer. Ann. Oncol. 2021, 32, 478–487. [Google Scholar] [CrossRef]
- Bulin, A.-L.; Broekgaarden, M.; Hasan, T. Comprehensive High-Throughput Image Analysis for Therapeutic Efficacy of Architecturally Complex Heterotypic Organoids. Sci. Rep. 2017, 7, 16645. [Google Scholar] [CrossRef] [Green Version]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring Singlet Oxygen and Hydroxyl Radical Formation with Fluorescent Probes during Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef] [Green Version]
- Bacellar, I.O.L.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Jermain, P.; Muzikansky, A.; Ross, A.; Hamblin, M.; Yaroslavsky, A.N.; Yaroslavsky, A.N. Quantifying Subcellular Localization of Methylene Blue in Cultured Brain Cells. In Proceedings of the Frontiers in Optics + Laser Science APS/DLS (2019), Washington, DC, USA, 15–19 September 2019; Optical Society of America: Washington, DC, USA, 2019; p. JTu3A.97. [Google Scholar]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell. Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.-L.; Broekgaarden, M.; Simeone, D.; Hasan, T. Low Dose Photodynamic Therapy Harmonizes with Radiation Therapy to Induce Beneficial Effects on Pancreatic Heterocellular Spheroids. Oncotarget 2019, 10, 2625–2643. [Google Scholar] [CrossRef] [Green Version]
- Broekgaarden, M.; Alkhateeb, A.; Bano, S.; Bulin, A.-L.; Obaid, G.; Rizvi, I.; Hasan, T. Cabozantinib Inhibits Photodynamic Therapy-Induced Auto- and Paracrine MET Signaling in Heterotypic Pancreatic Microtumors. Cancers 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Priwitaningrum, D.L.; Blondé, J.-B.G.; Sridhar, A.; van Baarlen, J.; Hennink, W.E.; Storm, G.; Le Gac, S.; Prakash, J. Tumor Stroma-Containing 3D Spheroid Arrays: A Tool to Study Nanoparticle Penetration. J. Control. Release 2016, 244, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Glidden, M.D.; Celli, J.P.; Massodi, I.; Rizvi, I.; Pogue, B.W.; Hasan, T. Image-Based Quantification of Benzoporphyrin Derivative Uptake, Localization, and Photobleaching in 3D Tumor Models, for Optimization of PDT Parameters. Theranostics 2012, 2, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Broekgaarden, M.; Bulin, A.-L.; Porret, E.; Musnier, B.; Chovelon, B.; Ravelet, C.; Sancey, L.; Elleaume, H.; Hainaut, P.; Coll, J.-L.; et al. Surface functionalization of gold nanoclusters with arginine: A trade-off between microtumor uptake and radiotherapy enhancement. Nanoscale 2020, 12, 6959–6963. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, M.E.; Pasquale, M.A.; Bergna, C.; Ponzinibbio, C.; Garavaglia, M. Photodynamic therapy in 2D and 3D human cervical carcinoma cell cultures employing LED light sources emitting at different wavelengths. Phys. Med. Biol. 2020, 65, 015017. [Google Scholar] [CrossRef] [PubMed]
- Alderden, R.A.; Mellor, H.R.; Modok, S.; Hall, M.D.; Sutton, S.R.; Newville, M.G.; Callaghan, R.; Hambley, T.W. Elemental tomography of cancer-cell spheroids reveals incomplete uptake of both platinum(II) and platinum(IV) complexes. J. Am. Chem. Soc. 2007, 129, 13400–13401. [Google Scholar] [CrossRef]
- Karimnia, V.; Rizvi, I.; Slack, F.J.; Celli, J.P. Photodestruction of Stromal Fibroblasts Enhances Tumor Response to PDT in 3D Pancreatic Cancer Coculture Models. Photochem. Photobiol. 2021, 97, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; Alieva, M.; Wellens, L.M.; Ariese, H.C.R.; Jamieson, P.R.; Vonk, A.M.; Amatngalim, G.D.; Hu, H.; Oost, K.C.; Snippert, H.J.G.; et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 2019, 14, 1756–1771. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Clainche, T.; Carigga Gutierrez, N.M.; Pujol-Solé, N.; Coll, J.-L.; Broekgaarden, M. Optimizing the Pharmacological and Optical Dosimetry for Photodynamic Therapy with Methylene Blue and Nanoliposomal Benzoporphyrin on Pancreatic Cancer Spheroids. Onco 2022, 2, 19-33. https://doi.org/10.3390/onco2010002
Le Clainche T, Carigga Gutierrez NM, Pujol-Solé N, Coll J-L, Broekgaarden M. Optimizing the Pharmacological and Optical Dosimetry for Photodynamic Therapy with Methylene Blue and Nanoliposomal Benzoporphyrin on Pancreatic Cancer Spheroids. Onco. 2022; 2(1):19-33. https://doi.org/10.3390/onco2010002
Chicago/Turabian StyleLe Clainche, Tristan, Nazareth Milagros Carigga Gutierrez, Núria Pujol-Solé, Jean-Luc Coll, and Mans Broekgaarden. 2022. "Optimizing the Pharmacological and Optical Dosimetry for Photodynamic Therapy with Methylene Blue and Nanoliposomal Benzoporphyrin on Pancreatic Cancer Spheroids" Onco 2, no. 1: 19-33. https://doi.org/10.3390/onco2010002
APA StyleLe Clainche, T., Carigga Gutierrez, N. M., Pujol-Solé, N., Coll, J.-L., & Broekgaarden, M. (2022). Optimizing the Pharmacological and Optical Dosimetry for Photodynamic Therapy with Methylene Blue and Nanoliposomal Benzoporphyrin on Pancreatic Cancer Spheroids. Onco, 2(1), 19-33. https://doi.org/10.3390/onco2010002







