Perfusion Bioreactor Technology for Organoid and Tissue Culture: A Mini Review
Simple Summary
Abstract
1. Introduction
2. Organoid Research and Clinical Translation
Organoid Cultures Advantages and Limitations
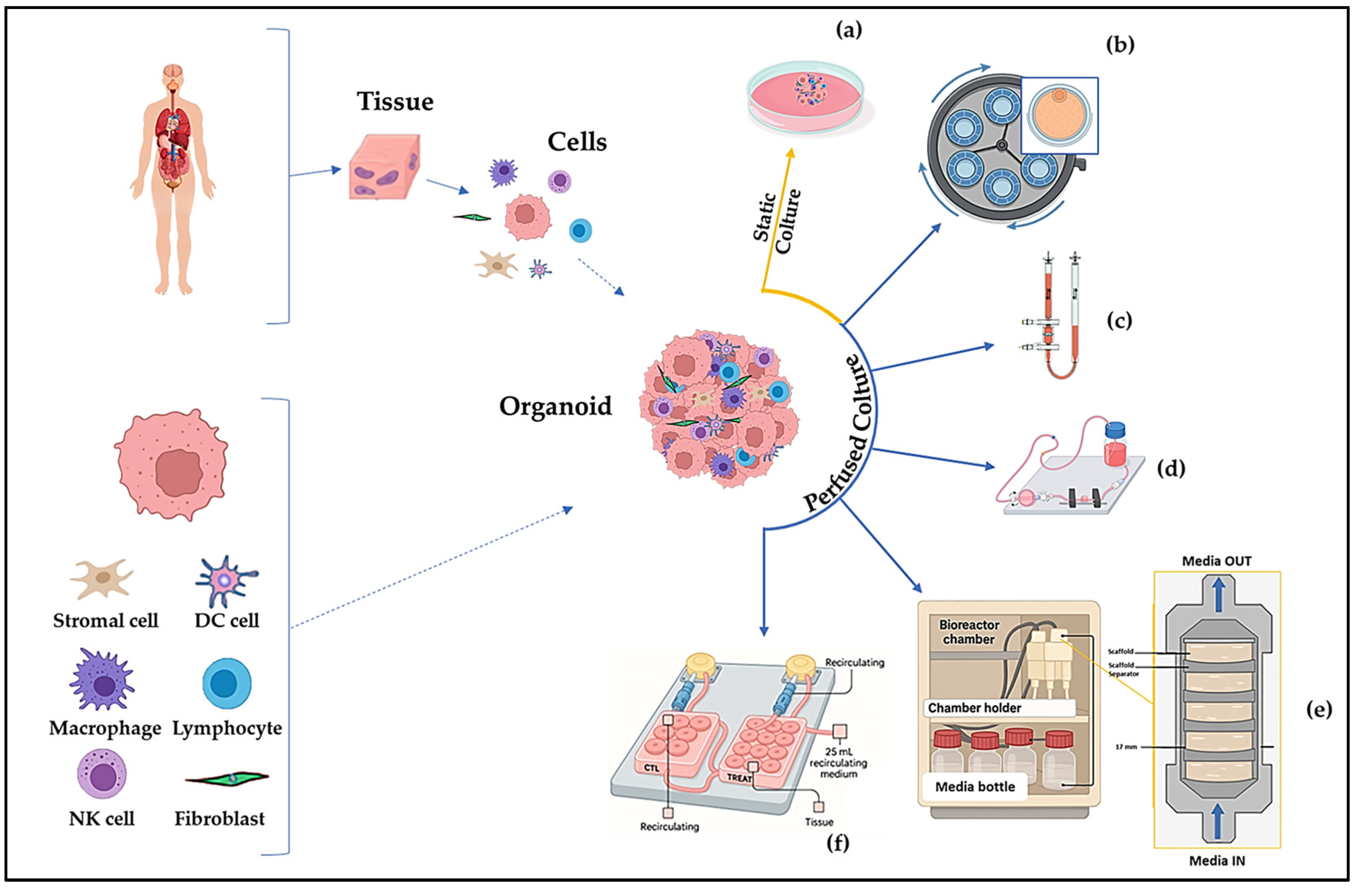
3. Bioreactors
3.1. Perfusion Bioreactor Systems a Promising Platform for Cancer Research
3.2. Perfusion Bioreactors for the Maintenance and Growth of Organoids
3.3. Perfusion Bioreactors for the Maintenance and Growth of Tumor Patient Specimens
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miserocchi, G.; Mercatali, L.; Liverani, C.; De Vita, A.; Spadazzi, C.; Pieri, F.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ibrahim, T. Management and Potentialities of Primary Cancer Cultures in Preclinical and Translational Studies. J. Transl. Med. 2017, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Sakamoto, N. Cancer Organoid Applications to Investigate Chemotherapy Resistance. Front. Mol. Biosci. 2022, 9, 1067207. [Google Scholar] [CrossRef]
- Wan, X.; Ball, S.; Willenbrock, F.; Yeh, S.; Vlahov, N.; Koennig, D.; Green, M.; Brown, G.; Jeyaretna, S.; Li, Z.; et al. Perfused Three-Dimensional Organotypic Culture of Human Cancer Cells for Therapeutic Evaluation. Sci. Rep. 2017, 7, 9408. [Google Scholar] [CrossRef]
- de Klerk, E.; Hebrok, M. Stem Cell-Based Clinical Trials for Diabetes Mellitus. Front. Endocrinol. 2021, 12, 631463. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B.; Zinöcker, S.; Holm, S.; Lewis, J.; Kavouras, P. Organoids in the Clinic: A Systematic Review of Outcomes. Cells Tissues Organs 2023, 212, 499–511. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-Derived Spheroids: Relevance to Cancer Stem Cells and Clinical Applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Aberle, M.R.; Burkhart, R.A.; Tiriac, H.; Olde Damink, S.W.M.; Dejong, C.H.C.; Tuveson, D.A.; van Dam, R.M. Patient-Derived Organoid Models Help Define Personalized Management of Gastrointestinal Cancer. Br. J. Surg. 2018, 105, e48–e60. [Google Scholar] [CrossRef]
- Nugraha, B.; Buono, M.F.; Emmert, M.Y. Modelling Human Cardiac Diseases with 3D Organoid. Eur. Heart J. 2018, 39, 4234–4237. [Google Scholar] [CrossRef]
- Schneemann, S.A.; Boers, S.N.; van Delden, J.J.M.; Nieuwenhuis, E.E.S.; Fuchs, S.A.; Bredenoord, A.L. Ethical Challenges for Pediatric Liver Organoid Transplantation. Sci. Transl. Med. 2020, 12, eaau8471. [Google Scholar] [CrossRef]
- De Miguel, M.P.; Prieto, I.; Moratilla, A.; Arias, J.; Aller, M.A. Mesenchymal Stem Cells for Liver Regeneration in Liver Failure: From Experimental Models to Clinical Trials. Stem Cells Int. 2019, 2019, 3945672. [Google Scholar] [CrossRef]
- Samimi, H.; Atlasi, R.; Parichehreh-Dizaji, S.; Khazaei, S.; Rahnama, M.A.; Seifirad, S.; Haghpanah, V. A Systematic Review on Thyroid Organoid Models: Time-Trend and Its Achievements. Am. J. Physiol. Endocrinol. Metab. 2021, 320, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Alves-Lopes, J.P.; Stukenborg, J.B. Testicular Organoids: A New Model to Study the Testicular Microenvironment in Vitro? Hum. Reprod. Update 2018, 24, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Aasen, D.M.; Vergara, M.N. New Drug Discovery Paradigms for Retinal Diseases: A Focus on Retinal Organoids. J. Ocul. Pharmacol. Ther. 2020, 36, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, J.; Razavi Bazaz, S.; Aboulkheyr Es, H.; Yaghobian Azari, D.; Thierry, B.; Ebrahimi Warkiani, M.; Ghadiri, M. Lung-on-a-Chip: The Future of Respiratory Disease Models and Pharmacological Studies. Crit. Rev. Biotechnol. 2020, 40, 213–230. [Google Scholar] [CrossRef]
- Abdollahi, S. Extracellular Vesicles from Organoids and 3D Culture Systems. Biotechnol. Bioeng. 2021, 118, 1029–1049. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-Derived Organoids Model Treatment Response of Metastatic Gastrointestinal Cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Tsai, S.; McOlash, L.; Palen, K.; Johnson, B.; Duris, C.; Yang, Q.; Dwinell, M.B.; Hunt, B.; Evans, D.B.; Gershan, J.; et al. Development of Primary Human Pancreatic Cancer Organoids, Matched Stromal and Immune Cells and 3D Tumor Microenvironment Models. BMC Cancer 2018, 18, 335. [Google Scholar] [CrossRef]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The Current Status and Biomedical Applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef]
- Hsieh, Y.K.; Chen, S.C.; Huang, W.L.; Hsu, K.P.; Gorday, K.A.V.; Wang, T.; Wang, J. Direct Micromachining of Microfluidic Channels on Biodegradable Materials Using Laser Ablation. Polymers 2017, 9, 242. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D Microfibrous Scaffolds for Engineering Endothelialized Myocardium and Heart-on-a-Chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of Human Brain Organoids with a Functional Vascular-like System. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Spier, M.R.; Vandenberghe, L.P.D.S.; Medeiros, A.B.P.; Soccol, C.R. Application of Different Types of Bioreactors in Bioprocesses; Nova Science Publishers: Hauppauge, NY, USA, 2011; ISBN 9781621001645. [Google Scholar]
- Vandermies, M.; Fickers, P. Bioreactor-Scale Strategies for the Production of Recombinant Protein in the Yeast Yarrowia Lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef]
- Grayson, W.; Stephenson, M. Recent Advances in Bioreactors for Cell-Based Therapies. F1000Research 2018, 7, 517. [Google Scholar] [CrossRef]
- Vinken, M. In Vitro Veritas. Front. Toxicol. 2020, 2, 1. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Muraro, M.G.; Muenst, S.; Mele, V.; Quagliata, L.; Iezzi, G.; Tzankov, A.; Weber, W.P.; Spagnoli, G.C.; Soysal, S.D. Ex-Vivo Assessment of Drug Response on Breast Cancer Primary Tissue with Preserved Microenvironments. Oncoimmunology 2017, 6, e1331798. [Google Scholar] [CrossRef]
- Fernandes, T.G. Organoids as Complex (Bio)Systems. Front. Cell Dev. Biol. 2023, 11, 1268540. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.; Wang, Z.; Liu, Y.; Yu, J.; Wang, W.; Chen, S.; Wu, W.; Wang, J.; Qian, G.; et al. Standardization of Organoid Culture in Cancer Research. Cancer Med. 2023, 12, 14375–14386. [Google Scholar] [CrossRef]
- Xin, M.; Li, Q.; Wang, D.; Wang, Z. Organoids for Cancer Research: Advances and Challenges. Adv. Biol. 2024, 8, 2400056. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Eng. Part C Methods 2016, 22, 221–249. [Google Scholar] [CrossRef]
- Sereti, E.; Papapostolou, I.; Dimas, K. Pancreatic Cancer Organoids: An Emerging Platform for Precision Medicine? Biomedicines 2023, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Wu, K.C.; Harnod, T.; Ding, D.C. Comparison of the Cost and Effect of Combined Conditioned Medium and Conventional Medium for Fallopian Tube Organoid Cultures. Cell Transplant. 2023, 32, 1–12. [Google Scholar] [CrossRef]
- Phelan, M.A.; Lelkes, P.I.; Swaroop, A. Mini and Customized Low-Cost Bioreactors for Optimized High-Throughput Generation of Tissue Organoids. Stem Cell Investig. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, J.E.; Santoro, M.; Williams, C.; Piard, C.M.; Smith, B.T.; Placone, J.K.; Menegaz, B.A.; Molina, E.R.; Lamhamedi-Cherradi, S.E.; Ludwig, J.A.; et al. Effects of Shear Stress Gradients on Ewing Sarcoma Cells Using 3D Printed Scaffolds and Flow Perfusion. ACS Biomater. Sci. Eng. 2018, 4, 347–356. [Google Scholar] [CrossRef]
- García-García, A.; Klein, T.; Born, G.; Hilpert, M.; Scherberich, A.; Lengerke, C.; Skoda, R.C.; Bourgine, P.E.; Martin, I. Culturing Patient-Derived Malignant Hematopoietic Stem Cells in Engineered and Fully Humanized 3D Niches. Proc. Natl. Acad. Sci. USA 2021, 118, e2114227118. [Google Scholar] [CrossRef]
- Shekarian, T.; Zinner, C.P.; Bartoszek, E.M.; Duchemin, W.; Wachnowicz, A.T.; Hogan, S.; Etter, M.M.; Flammer, J.; Paganetti, C.; Martins, T.A.; et al. Immunotherapy of Glioblastoma Explants Induces Interferon-γ Responses and Spatial Immune Cell Rearrangements in Tumor Center, but Not Periphery. Sci. Adv. 2022, 8, eabn9440. [Google Scholar] [CrossRef]
- Pasini, A.; Lovecchio, J.; Cortesi, M.; Liverani, C.; Spadazzi, C.; Mercatali, L.; Ibrahim, T.; Giordano, E. Perfusion Flow Enhances Viability and Migratory Phenotype in 3D-Cultured Breast Cancer Cells. Ann. Biomed. Eng. 2021, 49, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Goliwas, K.F.; Marshall, L.E.; Ransaw, E.L.; Berry, J.L.; Frost, A.R. A Recapitulative Three-Dimensional Model of Breast Carcinoma Requires Perfusion for Multi-Week Growth. J. Tissue Eng. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Martinez, A.; Buckley, M.S.; Scalise, C.B.; Wang, D.; Katre, A.A.; Birrer, M.J.; Berry, J.L.; Arend, R.C. Utilization of a 3-D Tissue Engineered Model to Investigate the Effects of Perfusion on Gynecologic Cancer Biology. J. Tissue Eng. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Jasuja, H.; Kar, S.; Katti, D.R.; Katti, K.S. Perfusion Bioreactor Enabled Fluid-Derived Shear Stress Conditions for Novel Bone Metastatic Prostate Cancer Testbed. Biofabrication 2021, 13, 035004. [Google Scholar] [CrossRef]
- Manfredonia, C.; Muraro, M.G.; Hirt, C.; Mele, V.; Governa, V.; Papadimitropoulos, A.; Däster, S.; Soysal, S.D.; Droeser, R.A.; Mechera, R.; et al. Maintenance of Primary Human Colorectal Cancer Microenvironment Using a Perfusion Bioreactor-Based 3D Culture System. Adv. Biosyst. 2019, 3, 1800300. [Google Scholar] [CrossRef] [PubMed]
- Calamaio, S.; Serzanti, M.; Boniotti, J.; Fra, A.; Garrafa, E.; Cominelli, M.; Verardi, R.; Poliani, P.L.; Dotti, S.; Villa, R.; et al. Human IPSC-Derived 3D Hepatic Organoids in a Miniaturized Dynamic Culture System. Biomedicines 2023, 11, 2114. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, M.; D’Agostino, S.; Veltri, G.; Bacchiega, M.; Tombolan, L.; Zanon, C.; Gamba, P.; Serafin, V.; Muraro, M.G.; Martin, I.; et al. A Perfusion-Based Three-Dimensional Cell Culture System to Model Alveolar Rhabdomyosarcoma Pathological Features. Sci. Rep. 2023, 13, 9444. [Google Scholar] [CrossRef]
- Dupard, S.J.; Garcia, A.G.; Bourgine, P.E. Customizable 3D Printed Perfusion Bioreactor for the Engineering of Stem Cell Microenvironments. Front. Bioeng. Biotechnol. 2023, 10, 1081145. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Bilang, R.; Supuran, C.T.; von der Weid, N.; Bruder, E.; Holland-Cunz, S.; Martin, I.; Muraro, M.G.; Gros, S.J. Perfusion-Based Bioreactor Culture and Isothermal Microcalorimetry for Preclinical Drug Testing with the Carbonic Anhydrase Inhibitor SLC-0111 in Patient-Derived Neuroblastoma. Int. J. Mol. Sci. 2022, 23, 3128. [Google Scholar] [CrossRef]
- Avena, P.; De Luca, A.; Chimento, A.; Nocito, M.C.; Sculco, S.; La Padula, D.; Zavaglia, L.; Giulietti, M.; Hantel, C.; Sirianni, R.; et al. Estrogen Related Receptor Alpha (ERRα) a Bridge between Metabolism and Adrenocortical Cancer Progression. Cancers 2022, 14, 3885. [Google Scholar] [CrossRef]
- Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R.; et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers 2023, 15, 1050. [Google Scholar] [CrossRef]
| Advantages | Limitations |
|---|---|
| Mimic tumor environment | Increased analytical costs |
| Constant and continuous nutrient exchange and waste constituents’ removal | Long validation time |
| Constant and continuous control and monitoring of chemical–physical parameters | Variability in organoid growth |
| High cell density | Challenges in large-scale reproduction |
| Improve proliferation, differentiation, and the formation of new ECM progression | Incomplete tumor microenvironment reconstruction |
| Very convenient for long term study | Poor compatibility and integration with advanced imaging |
| Challenges in integrating immune cells | |
| Limited adaptability to specific histological tumor type |
| Main Cancer Type | Maximun Culture Time | Bioreactor Effects | Reference |
|---|---|---|---|
| Prostate cancer | 23 + 20 days | Increased proliferation and differentiation | [41] |
| Hepatic cancer | 7 days | Increase and support hepatocytes maturation | [43] |
| Rhabdomyosarcoma | 7 days | Increased tumor progression and aggressiveness | [44] |
| Ewing sarcoma | 10 days | Enhanced cell proliferation | [35] |
| Myeloproliferative tumor and acute myeloid leukemia | 3 weeks | Extended maintenance and expansion of patient-derived malignant HSPCs. | [36] |
| Breast cancer | 7 days | More aggressive phenotype | [38] |
| Human mesenchymal stromal cells | 2 weeks | Tumor cells and stromal structures retained their characteristics | [45] |
| Main Cancer Type | Maximun Culture Time | Bioreactor Effects | Reference |
|---|---|---|---|
| Glioblastoma | 7 days | Antitumor immune response activation | [37] |
| Epithelial ovarian cancer | 7 days | Increased ovarian cancer cell density | [40] |
| Colorectal cancer | 3 days | Conservation and maintenance of cellular architecture and density | [42] |
| Breast cancer | 14 days | Preservation of cellar composition and interaction of cancer cells and TME | [27] |
| Neuroblastoma | 7 days | The system enables the preservation of high-quality tissue, maintaining both intact tumor cells and stromal structure | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avena, P.; Zavaglia, L.; Casaburi, I.; Pezzi, V. Perfusion Bioreactor Technology for Organoid and Tissue Culture: A Mini Review. Onco 2025, 5, 17. https://doi.org/10.3390/onco5020017
Avena P, Zavaglia L, Casaburi I, Pezzi V. Perfusion Bioreactor Technology for Organoid and Tissue Culture: A Mini Review. Onco. 2025; 5(2):17. https://doi.org/10.3390/onco5020017
Chicago/Turabian StyleAvena, Paola, Lucia Zavaglia, Ivan Casaburi, and Vincenzo Pezzi. 2025. "Perfusion Bioreactor Technology for Organoid and Tissue Culture: A Mini Review" Onco 5, no. 2: 17. https://doi.org/10.3390/onco5020017
APA StyleAvena, P., Zavaglia, L., Casaburi, I., & Pezzi, V. (2025). Perfusion Bioreactor Technology for Organoid and Tissue Culture: A Mini Review. Onco, 5(2), 17. https://doi.org/10.3390/onco5020017







