The Relationships Among Perineural Invasion, Tumor–Nerve Interaction and Immunosuppression in Cancer
Simple Summary
Abstract
1. Introduction (Perineural Invasion, the Original Clinical Context)
2. Materials and Methods
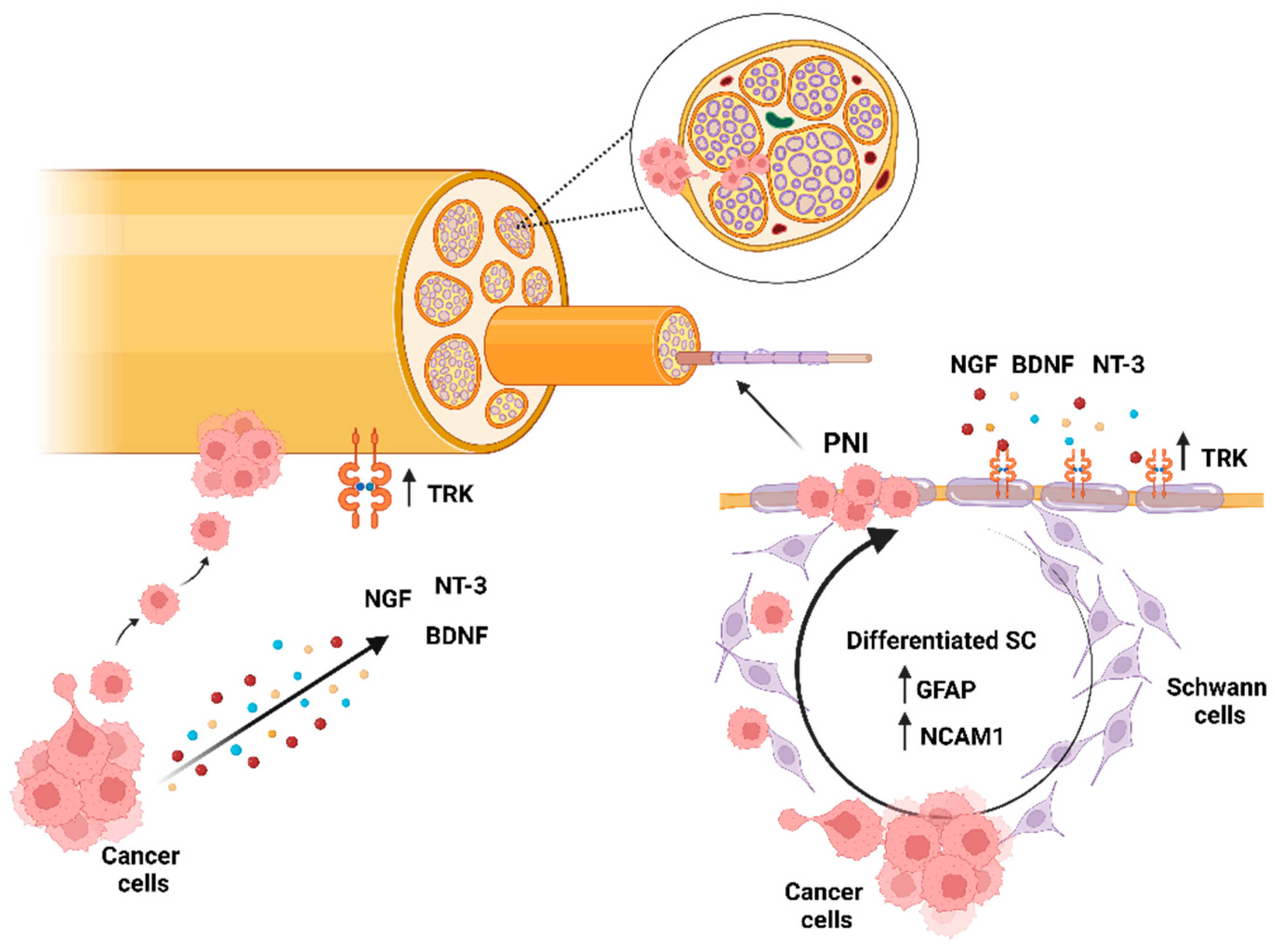
3. Nerves and Cancer
3.1. The Cancer Gets Nervous
3.2. From the Tumor Side
3.3. From the Perspective of Innervation
3.4. From the Perspective of the Tumor Microenvironment
3.5. Possible Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Binmadi, N.O.; Basile, J.R. Perineural invasion in oral squamous cell carcinoma: A discussion of significance and review of the literature. Oral Oncol. 2011, 47, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.C.; Meinel, A.; Fischer, U.; Bilek, K.; Hentschel, B. Perineural invasion in carcinoma of the cervix uteri—Prognostic impact. J. Cancer Res. Clin. Oncol. 2010, 136, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Litchman, G.H.; Fitzgerald, A.L.; Kurley, S.J.; Cook, R.W.; Rigel, D.S. Impact of a prognostic 40-gene expression profiling test on clinical management decisions for high-risk cutaneous squamous cell carcinoma. Curr. Med. Res. Opin. 2020, 36, 1295–1300. [Google Scholar] [CrossRef]
- Qin, T.; Li, J.; Xiao, Y.; Wang, X.; Gong, M.; Wang, Q.; Zhu, Z.; Zhang, S.; Zhang, W.; Cao, F.; et al. Honokiol Suppresses Perineural Invasion of Pancreatic Cancer by Inhibiting SMAD2/3 Signaling. Front. Oncol. 2021, 11, 728583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, J.; Zhang, M.; Tang, X.; Sun, G.; Zhu, S.; Liu, J.; Zhang, H.; Zhang, X.; Yin, X.; et al. The clinical significance of perineural invasion in patients with de novo metastatic prostate cancer. Andrology 2019, 7, 184–192. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef]
- Ayala, G.E.; Wheeler, T.M.; Shine, H.D.; Schmelz, M.; Frolov, A.; Chakraborty, S.; Rowley, D. In vitro dorsal root ganglia and human prostate cell line interaction: Redefining perineural invasion in prostate cancer. Prostate 2001, 49, 213–223. [Google Scholar] [CrossRef]
- Huang, Y.; He, L.; Dong, D.; Yang, C.; Liang, C.; Chen, X.; Ma, Z.; Huang, X.; Yao, S.; Liang, C.; et al. Individualized prediction of perineural invasion in colorectal cancer: Development and validation of a radiomics prediction model. Chin. J. Cancer Res. 2018, 30, 40–50. [Google Scholar] [CrossRef]
- Khorani, K.; Burkart, S.; Weusthof, C.; Han, R.; Liang, S.; Stogbauer, F.; Hess, J. Context-Dependent Regulation of Peripheral Nerve Abundance by the PI3K Pathway in the Tumor Microenvironment of Head and Neck Squamous Cell Carcinoma. Cells 2024, 13, 1033. [Google Scholar] [CrossRef]
- Huang, T.; Fan, Q.; Wang, Y.; Cui, Y.; Wang, Z.; Yang, L.; Sun, X.; Wang, Y. Schwann Cell-Derived CCL2 Promotes the Perineural Invasion of Cervical Cancer. Front. Oncol. 2020, 10, 19. [Google Scholar] [CrossRef]
- Fagan, J.J.; Collins, B.; Barnes, L.; D’Amico, F.; Myers, E.N.; Johnson, J.T. Perineural invasion in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 637–640. [Google Scholar] [CrossRef]
- Ein, L.; Bracho, O.; Mei, C.; Patel, J.; Boyle, T.; Monje, P.; Fernandez-Valle, C.; Bas, E.; Thomas, G.; Weed, D.; et al. Inhibition of tropomyosine receptor kinase B on the migration of human Schwann cell and dispersion of oral tongue squamous cell carcinoma in vitro. Head Neck 2019, 41, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Ein, L.; Mei, C.; Bracho, O.; Bas, E.; Monje, P.; Weed, D.; Sargi, Z.; Thomas, G.; Dinh, C. Modulation of BDNF-TRKB Interactions on Schwann Cell-induced Oral Squamous Cell Carcinoma Dispersion In Vitro. Anticancer Res. 2019, 39, 5933–5942. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Tanaka, Y.; Eto, K.; Ukai, N.; Sonobe, S.; Takahashi, H.; Ikegami, M.; Shimoda, M. S100-stained perineural invasion is associated with worse prognosis in stage I/II colorectal cancer: Its possible association with immunosuppression in the tumor. Pathol. Int. 2022, 72, 117–127. [Google Scholar] [CrossRef]
- Sigorski, D.; Gulczynski, J.; Sejda, A.; Rogowski, W.; Izycka-Swieszewska, E. Investigation of Neural Microenvironment in Prostate Cancer in Context of Neural Density, Perineural Invasion, and Neuroendocrine Profile of Tumors. Front. Oncol. 2021, 11, 710899. [Google Scholar] [CrossRef]
- Tao, Z.Y.; Wang, L.; Zhu, W.Y.; Zhang, G.; Su, Y.X. Lingual Denervation Improves the Efficacy of Anti-PD-1 Immunotherapy in Oral Squamous Cell Carcinomas by Downregulating TGFbeta Signaling. Cancer Res. Commun. 2024, 4, 418–430. [Google Scholar] [CrossRef]
- Ayala, G. Neuroepithelial Interactions in Cancer. Annu. Rev. Pathol. 2023, 18, 493–514. [Google Scholar] [CrossRef]
- Dewanto, A.; Dudas, J.; Glueckert, R.; Mechsner, S.; Schrott-Fischer, A.; Wildt, L.; Seeber, B. Localization of TrkB and p75 receptors in peritoneal and deep infiltrating endometriosis: An immunohistochemical study. Reprod. Biol. Endocrinol. 2016, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Q.; Liu, H.; Guo, K.; Li, F.; Li, W.; Han, L.; Wang, F.; Wu, E. Relationship between neural alteration and perineural invasion in pancreatic cancer patients with hyperglycemia. PLoS ONE 2011, 6, e17385. [Google Scholar] [CrossRef]
- Bakst, R.L.; Xiong, H.; Chen, C.H.; Deborde, S.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.; Lee, S.Y.; Olson, O.C.; et al. Inflammatory Monocytes Promote Perineural Invasion via CCL2-Mediated Recruitment and Cathepsin B Expression. Cancer Res. 2017, 77, 6400–6414. [Google Scholar] [CrossRef]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.Y.; Barajas, F.; Chen, C.H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016, 126, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Federspiel, J.; Steinbichler, T.B.; Vorbach, S.M.; Eling, M.T.; Borena, W.; Seifarth, C.; Hofauer, B.G.; Dudas, J. Patient-Derived Cancer-Associated Fibroblasts Support the Colonization of Tumor Cells in Head and Neck Squamous Cell Carcinoma. Biomedicines 2025, 13, 358. [Google Scholar] [CrossRef]
- Ding, Y.; He, D.; Florentin, D.; Frolov, A.; Hilsenbeck, S.; Ittmann, M.; Kadmon, D.; Miles, B.; Rowley, D.; Ayala, G. Semaphorin 4F as a critical regulator of neuroepithelial interactions and a biomarker of aggressive prostate cancer. Clin. Cancer Res. 2013, 19, 6101–6111. [Google Scholar] [CrossRef] [PubMed]
- Bapat, A.A.; Munoz, R.M.; Von Hoff, D.D.; Han, H. Blocking Nerve Growth Factor Signaling Reduces the Neural Invasion Potential of Pancreatic Cancer Cells. PLoS ONE 2016, 11, e0165586. [Google Scholar] [CrossRef]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J. Natl. Cancer Inst. 2014, 106, dju184. [Google Scholar] [CrossRef]
- Allen, J.K.; Armaiz-Pena, G.N.; Nagaraja, A.S.; Sadaoui, N.C.; Ortiz, T.; Dood, R.; Ozcan, M.; Herder, D.M.; Haemmerle, M.; Gharpure, K.M.; et al. Sustained Adrenergic Signaling Promotes Intratumoral Innervation through BDNF Induction. Cancer Res. 2018, 78, 3233–3242. [Google Scholar] [CrossRef]
- Cervantes-Villagrana, R.D.; Albores-Garcia, D.; Cervantes-Villagrana, A.R.; Garcia-Acevez, S.J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct. Target. Ther. 2020, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Mezey, E.; Chandross, K.J.; Harta, G.; Maki, R.A.; McKercher, S.R. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000, 290, 1779–1782. [Google Scholar] [CrossRef]
- Gregory, E.; Dugan, R.; David, G.; Song, Y.H. The biology and engineered modeling strategies of cancer-nerve crosstalk. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188406. [Google Scholar] [CrossRef]
- Dudas, J.; Riml, A.; Tuertscher, R.; Pritz, C.; Steinbichler, T.B.; Schartinger, V.H.; Sprung, S.; Glueckert, R.; Schrott-Fischer, A.; Johnson Chacko, L.; et al. Brain-Derived Neurotrophin and TrkB in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 272. [Google Scholar] [CrossRef]
- Dudas, J.; Bitsche, M.; Schartinger, V.; Falkeis, C.; Sprinzl, G.M.; Riechelmann, H. Fibroblasts produce brain-derived neurotrophic factor and induce mesenchymal transition of oral tumor cells. Oral Oncol. 2011, 47, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Jiffar, T.; Yilmaz, T.; Lee, J.; Miller, Y.; Feng, L.; El-Naggar, A.; Kupferman, M.E. Brain derived neutrophic factor (BDNF) coordinates lympho-vascular metastasis through a fibroblast-governed paracrine axis in the tumor microenvironment. Cancer Cell Microenviron. 2017, 4, e1566. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Chan, J.R.; Shooter, E.M. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 14421–14426. [Google Scholar] [CrossRef] [PubMed]
- Shurin, G.V.; Kruglov, O.; Ding, F.; Lin, Y.; Hao, X.; Keskinov, A.A.; You, Z.; Lokshin, A.E.; LaFramboise, W.A.; Falo, L.D., Jr.; et al. Melanoma-Induced Reprogramming of Schwann Cell Signaling Aids Tumor Growth. Cancer Res. 2019, 79, 2736–2747. [Google Scholar] [CrossRef]
- Pascual, G.; Dominguez, D.; Elosua-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernandez, I.; et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef]
- Mu, W.; Wang, Z.; Zoller, M. Ping-Pong-Tumor and Host in Pancreatic Cancer Progression. Front. Oncol. 2019, 9, 1359. [Google Scholar] [CrossRef]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef]
- Silva, V.M.; Gomes, J.A.; Tenorio, L.P.G.; de Omena Neta, G.C.; da Costa Paixao, K.; Duarte, A.K.F.; da Silva, G.C.B.; Ferreira, R.J.S.; Koike, B.D.V.; de Sales Marques, C.; et al. Schwann cell reprogramming and lung cancer progression: A meta-analysis of transcriptome data. Oncotarget 2019, 10, 7288–7307. [Google Scholar] [CrossRef]
- Ingruber, J.; Dudas, J.; Savic, D.; Schweigl, G.; Steinbichler, T.B.; Greier, M.D.C.; Santer, M.; Carollo, S.; Trajanoski, Z.; Riechelmann, H. EMT-related transcription factors and protein stabilization mechanisms involvement in cadherin switch of head and neck squamous cell carcinoma. Exp. Cell Res. 2022, 414, 113084. [Google Scholar] [CrossRef]
- De Wever, O.; Mareel, M. Role of tissue stroma in cancer cell invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef]
- Roger, E.; Martel, S.; Bertrand-Chapel, A.; Depollier, A.; Chuvin, N.; Pommier, R.M.; Yacoub, K.; Caligaris, C.; Cardot-Ruffino, V.; Chauvet, V.; et al. Schwann cells support oncogenic potential of pancreatic cancer cells through TGFbeta signaling. Cell Death Dis. 2019, 10, 886. [Google Scholar] [CrossRef]
- Fujii-Nishimura, Y.; Yamazaki, K.; Masugi, Y.; Douguchi, J.; Kurebayashi, Y.; Kubota, N.; Ojima, H.; Kitago, M.; Shinoda, M.; Hashiguchi, A.; et al. Mesenchymal-epithelial transition of pancreatic cancer cells at perineural invasion sites is induced by Schwann cells. Pathol. Int. 2018, 68, 214–223. [Google Scholar] [CrossRef]
- Santiago-Medina, M.; Gregus, K.A.; Nichol, R.H.; O’Toole, S.M.; Gomez, T.M. Regulation of ECM degradation and axon guidance by growth cone invadosomes. Development 2015, 142, 486–496. [Google Scholar] [CrossRef]
- Sinnberg, T.; Levesque, M.P.; Krochmann, J.; Cheng, P.F.; Ikenberg, K.; Meraz-Torres, F.; Niessner, H.; Garbe, C.; Busch, C. Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype. Mol. Cancer 2018, 17, 59. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Z.; Guo, S.; Li, J.; Liu, S.; You, Y.; Ni, B.; Wang, H.; Bie, P. Increased semaphorin 3c expression promotes tumor growth and metastasis in pancreatic ductal adenocarcinoma by activating the ERK1/2 signaling pathway. Cancer Lett. 2017, 397, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Duchalais, E.; Guilluy, C.; Nedellec, S.; Touvron, M.; Bessard, A.; Touchefeu, Y.; Bossard, C.; Boudin, H.; Louarn, G.; Neunlist, M.; et al. Colorectal Cancer Cells Adhere to and Migrate Along the Neurons of the Enteric Nervous System. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Na’ara, S.; Amit, M.; Gil, Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene 2019, 38, 596–608. [Google Scholar] [CrossRef]
- Bruschi, M.; Midjek, L.; Ajlil, Y.; Vairy, S.; Lancien, M.; Ghermaoui, S.; Kergrohen, T.; Verreault, M.; Idbaih, A.; de Biagi, C.A.O.J.; et al. Diffuse midline glioma invasion and metastasis rely on cell-autonomous signaling. Neuro Oncol. 2024, 26, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Misztal, C.I.; Green, C.; Mei, C.; Bhatia, R.; Velez Torres, J.M.; Kamrava, B.; Moon, S.; Nicolli, E.; Weed, D.; Sargi, Z.; et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers 2021, 13, 6011. [Google Scholar] [CrossRef]
- Wang, K.; Demir, I.E.; D’Haese, J.G.; Tieftrunk, E.; Kujundzic, K.; Schorn, S.; Xing, B.; Kehl, T.; Friess, H.; Ceyhan, G.O. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis 2014, 35, 103–113. [Google Scholar] [CrossRef]
- Barbonetti, A.; D’Andrea, S.; Martorella, A.; Felzani, G.; Francavilla, S.; Francavilla, F. Risk of prostate cancer in men with spinal cord injury: A systematic review and meta-analysis. Asian J. Androl. 2018, 20, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Restaino, A.C.; Walz, A.; Vermeer, S.J.; Barr, J.; Kovacs, A.; Fettig, R.R.; Vermeer, D.W.; Reavis, H.; Williamson, C.S.; Lucido, C.T.; et al. Functional neuronal circuits promote disease progression in cancer. Sci. Adv. 2023, 9, eade4443. [Google Scholar] [CrossRef] [PubMed]
- Weusthof, C.; Burkart, S.; Semmelmayer, K.; Stogbauer, F.; Feng, B.; Khorani, K.; Bode, S.; Plinkert, P.; Plath, K.; Hess, J. Establishment of a Machine Learning Model for the Risk Assessment of Perineural Invasion in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 8938. [Google Scholar] [CrossRef] [PubMed]
- Jurcak, N.; Zheng, L. Signaling in the microenvironment of pancreatic cancer: Transmitting along the nerve. Pharmacol. Ther. 2019, 200, 126–134. [Google Scholar] [CrossRef]
- Xue, M.; Zhu, Y.; Jiang, Y.; Han, L.; Shi, M.; Su, R.; Wang, L.; Xiong, C.; Wang, C.; Wang, T.; et al. Schwann cells regulate tumor cells and cancer-associated fibroblasts in the pancreatic ductal adenocarcinoma microenvironment. Nat. Commun. 2023, 14, 4600. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Qin, J.F.; Jin, F.J.; Li, N.; Guan, H.T.; Lan, L.; Ni, H.; Wang, Y. Adrenergic receptor beta2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015, 48, 295–300. [Google Scholar] [CrossRef]
- Fjaestad, K.Y.; Romer, A.M.A.; Goitea, V.; Johansen, A.Z.; Thorseth, M.L.; Carretta, M.; Engelholm, L.H.; Grontved, L.; Junker, N.; Madsen, D.H. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene 2022, 41, 1364–1375. [Google Scholar] [CrossRef]
- Yin, S.; Wang, J.; Jia, Y.; Wang, X.; Zhao, Y.; Liu, T.; Lv, W.; Duan, Y.; Zhao, S.; Wang, S.; et al. Sleep deprivation-induced sympathetic activation promotes pro-tumoral macrophage phenotype via the ADRB2/KLF4 pathway to facilitate NSCLC metastasis. iScience 2025, 28, 112321. [Google Scholar] [CrossRef]
- Martyn, G.V.; Shurin, G.V.; Keskinov, A.A.; Bunimovich, Y.L.; Shurin, M.R. Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol. Immunother. 2019, 68, 1819–1829. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Sancho, E.; Batlle, E. Overcoming TGFbeta-mediated immune evasion in cancer. Nat. Rev. Cancer 2022, 22, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, J.D.; Tseveleki, V.; Dimitrakopoulos, F.I.; Konstantinidis, K.; Kalofonos, H. Radical Tumor Denervation Activates Potent Local and Global Cancer Treatment. Cancers 2023, 15, 3758. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; McKean, M.; Tolcher, A.; Victor, A.; Hu, P.; Gao, W.; Nogueira Filho, M.A.F.; Kitzing, T.; Gleicher, S.; Holland, D.; et al. TIGIT inhibitor M6223 as monotherapy or in combination with bintrafusp alfa in patients with advanced solid tumors: A first-in-human, phase 1, dose-escalation trial. J. Immunother. Cancer 2025, 13, e010584. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudas, J.; Glueckert, R.; Greier, M.d.C.; Hofauer, B.G. The Relationships Among Perineural Invasion, Tumor–Nerve Interaction and Immunosuppression in Cancer. Onco 2025, 5, 25. https://doi.org/10.3390/onco5020025
Dudas J, Glueckert R, Greier MdC, Hofauer BG. The Relationships Among Perineural Invasion, Tumor–Nerve Interaction and Immunosuppression in Cancer. Onco. 2025; 5(2):25. https://doi.org/10.3390/onco5020025
Chicago/Turabian StyleDudas, Jozsef, Rudolf Glueckert, Maria do Carmo Greier, and Benedikt Gabriel Hofauer. 2025. "The Relationships Among Perineural Invasion, Tumor–Nerve Interaction and Immunosuppression in Cancer" Onco 5, no. 2: 25. https://doi.org/10.3390/onco5020025
APA StyleDudas, J., Glueckert, R., Greier, M. d. C., & Hofauer, B. G. (2025). The Relationships Among Perineural Invasion, Tumor–Nerve Interaction and Immunosuppression in Cancer. Onco, 5(2), 25. https://doi.org/10.3390/onco5020025






