Proteomic Variability and Nutrient-Related Proteins across Pigmented and Non-Pigmented Rice Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Rice Protein Extraction
2.3. Determination of Protein Composition via SWATH-MS
2.3.1. Sample Preparation and SWATH-Based Proteomics
2.3.2. Data Analysis and Protein Identification
2.4. Bioinformatics and Statistical Analyses
3. Results
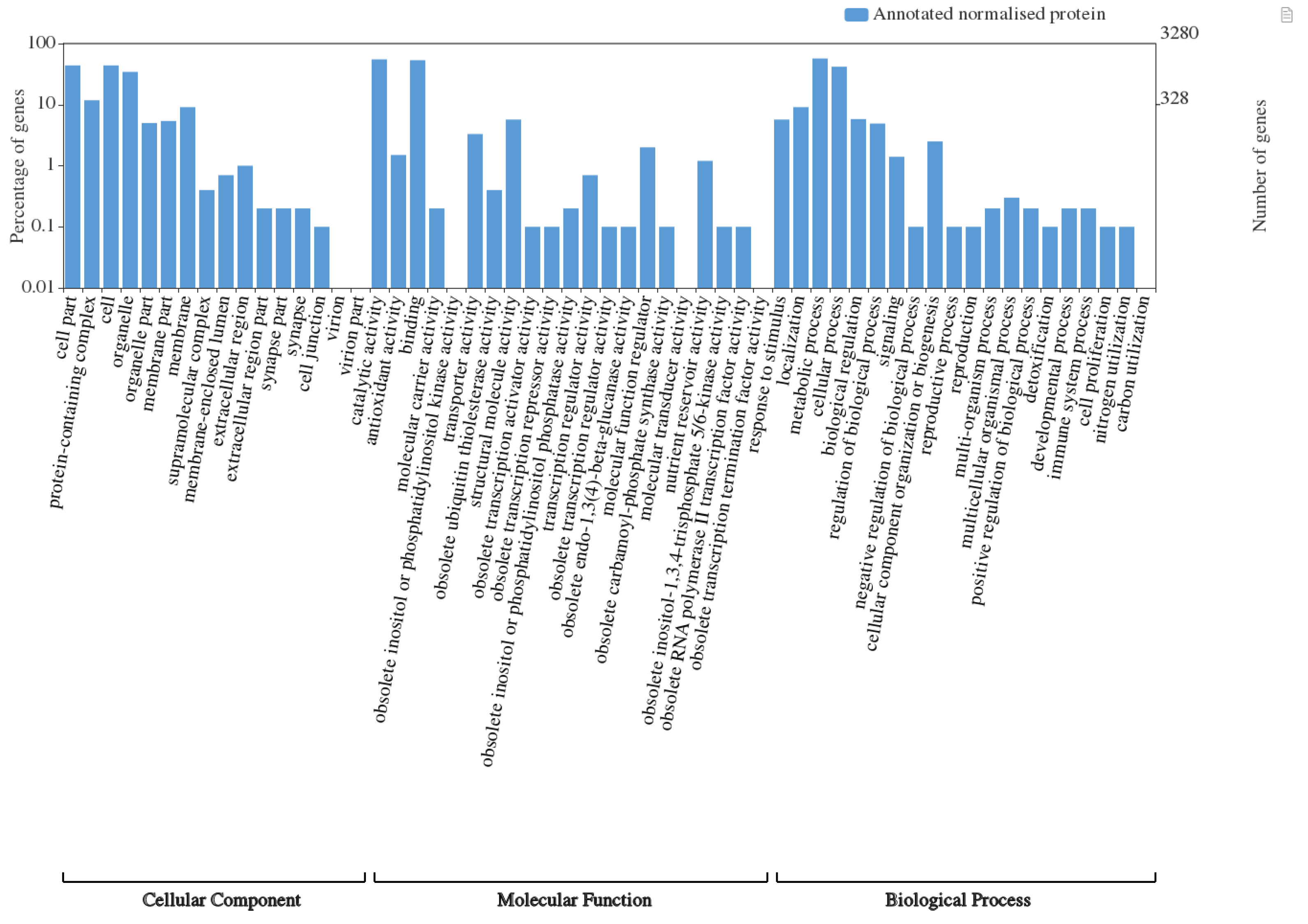
3.1. Overall Proteome Representation across Pigmented and Non-Pigmented Rice Grains
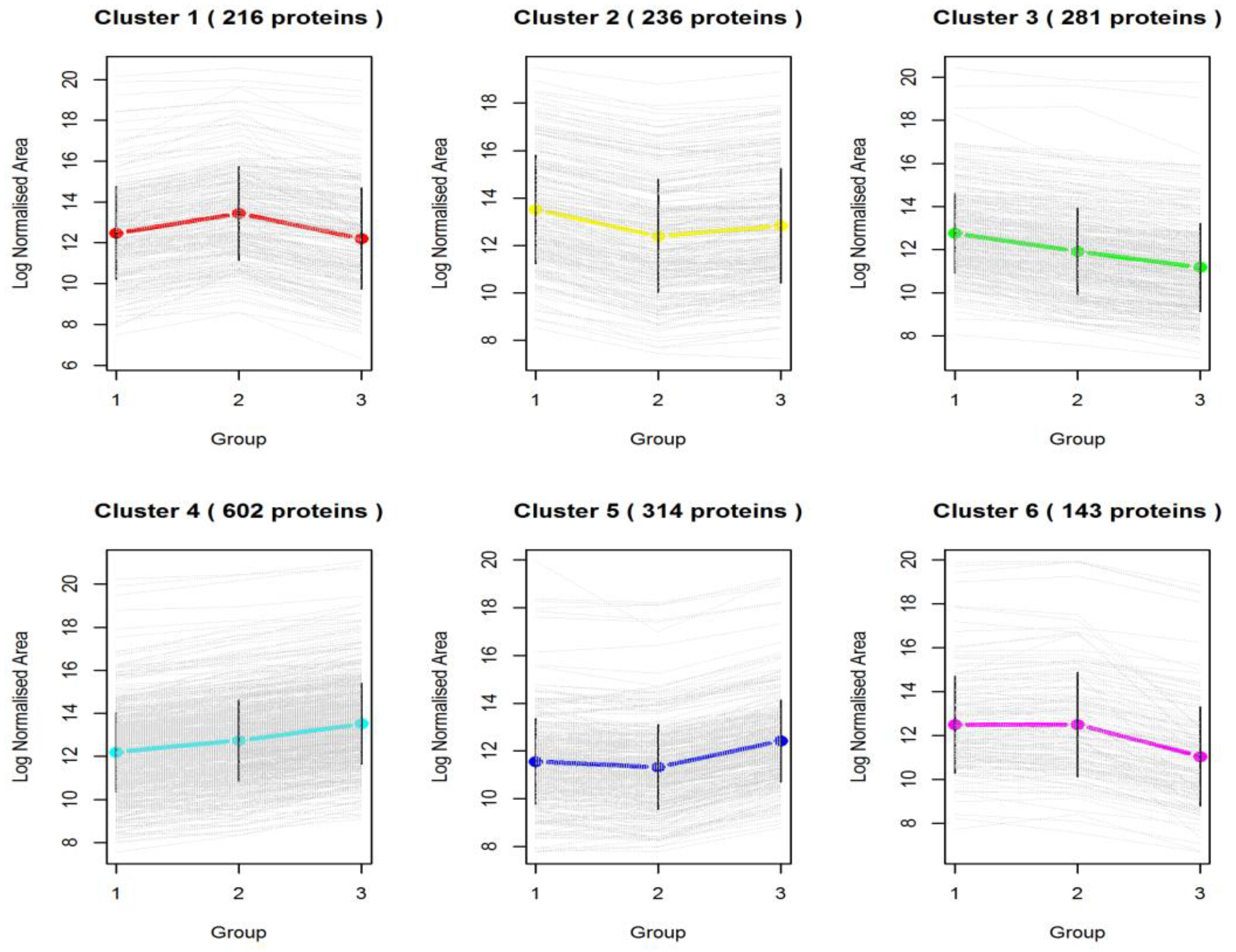
3.2. Differentially Expressed Proteins across Pigmented and Non-Pigmented Rice Varieties
3.3. Nutrient-Related Differentially Expressed Proteins between Rice Groups
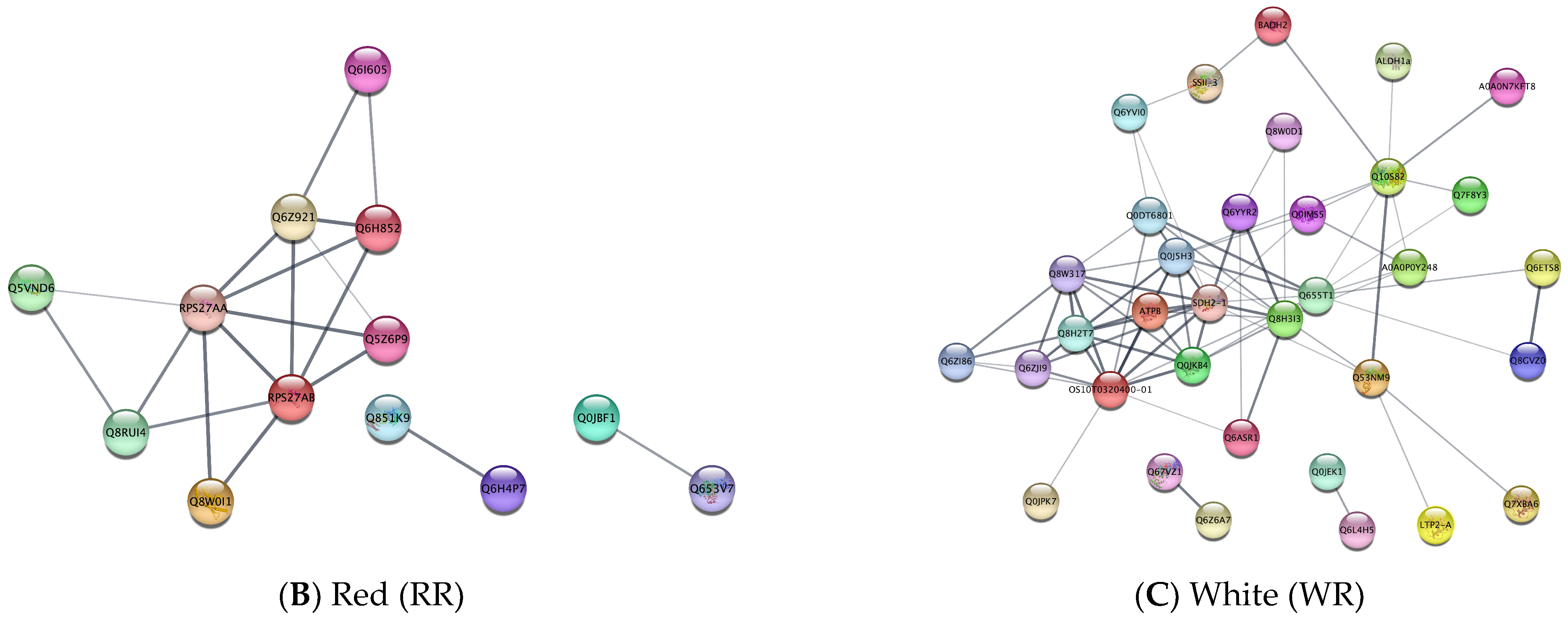
3.4. Protein–Protein Interactions among Nutrient-Related Differentially Expressed Proteins between Rice Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.; Burlingame, B.; Nguyen, V. Nutritional Contribution of Rice and Impact of Biotechnology and Biodiversity in Rice-Consuming Countries; FAO: Rome, Italy, 2003. [Google Scholar]
- Yoshida, H.; Tomiyama, Y.; Mizushina, Y. Lipid components, fatty acids and triacylglycerol molecular species of black and red rices. Food Chem. 2010, 123, 210–215. [Google Scholar] [CrossRef]
- Neoh, W.T.; Lum, M.S. Nutritional quality of rice variety in Sabah, Malaysia. Trans. Sci. Technol. 2018, 5, 88–92. [Google Scholar]
- Patrick, R.M. Protein and Amino Acid Content of Rice as Affected by Environmental Modifications; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 1971. [Google Scholar]
- Martin, M.; Fitzgerald, M. Proteins in rice grains influence cooking properties! J. Cereal Sci. 2002, 36, 285–294. [Google Scholar] [CrossRef]
- Bai, S.; Yu, H.; Wang, B.; Li, J. Retrospective and perspective of rice breeding in China. J. Genet. Genom. 2018, 45, 603–612. [Google Scholar] [CrossRef]
- Savitha, P.; Kumari, R.U. Indigenous knowledge of traditional landraces in rice (Oryza sativa L.) in situ conservation of Tamil Nadu, India. Indian J. Tradit. Knowl. 2016, 15, 321–329. [Google Scholar]
- Thompson, L.U. Antioxidants and hormone-mediated health benefits of whole grains. Crit. Rev. Food Sci. Nutr. 1994, 34, 473–497. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Osawa, T. The role of anthocyanins as an antioxidant under oxidative stress in rats. BioFactors 2000, 13, 133–139. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against oxidative stress: From molecular mechanisms to clinical applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef] [Green Version]
- Sew, Y.S.; Ahmad, M.A.; Abd Rashid, M.R.; Abu Bakar, N.; Machap, C.; Ling, A.C.K.; Zainal Abidin, R.A.; Rozano, L.; Simoh, S. Antioxidant activities and microelement composition of Malaysian local pigmented and non-pigmented rice varieties. Trans. Persat. Genet. Malays. 2016, 3, 205–212. [Google Scholar]
- Faurobert, M.; Mihr, C.; Bertin, N.; Pawlowski, T.; Negroni, L.; Sommerer, N.; Causse, M. Major proteome variations associated with cherry tomato pericarp development and ripening. Plant Physiol. 2007, 143, 1327–1346. [Google Scholar] [CrossRef] [Green Version]
- Sew, Y.S.; Aizat, W.M.; Ab Razak, M.S.F.; Zainal-Abidin, R.-A.; Simoh, S.; Abu-Bakar, N. Comprehensive proteomics data on whole rice grain of selected pigmented and non-pigmented rice varieties using SWATH-MS approach. Data Brief 2020, 31, 105927. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kermode, A.R. Regulatory mechanisms involved in the transition from seed development to germination. Crit. Rev. Plant Sci. 1990, 9, 155–195. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Wang, X.; Chen, H.; Chen, F.; Shen, S. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 2007, 7, 3358–3368. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Dai, L.; Xia, H.; Zhu, K.; Liu, H.; Chen, K. Protein profile of rice (Oryza sativa) seeds. Genet. Mol. Biol. 2013, 36, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, M.; Kumamaru, T.; Satoh, H.; Iwata, N.; Omura, T.; Kasai, Z.; Tanaka, K. Purification of protein body-I of rice seed and its polypeptide composition. Plant Cell Physiol. 1987, 28, 1517–1527. [Google Scholar]
- Zhao, W.-M.; Gatehouse, J.A.; Boulter, D. The purification and partial amino acid sequence of a polypeptide from the glutelin fraction of rice grains; homology to pea legumin. FEBS Lett. 1983, 162, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, H.; Liang, M.; Yang, L. Glutelin and prolamin, different components of rice protein, exert differently in vitro antioxidant activities. J. Cereal Sci. 2016, 72, 108–116. [Google Scholar] [CrossRef]
- Szerszunowicz, I.; Kłobukowski, J. Characteristics of potential protein nutraceuticals of plant origin with antioxidant activity. Molecules 2020, 25, 1621. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Chen, Y.-Y.; Wu, C.-T.; Yu, C.-C.; Liao, H.-F. Prolamin, a rice protein, augments anti-leukaemia immune response. J. Cereal Sci. 2010, 51, 189–197. [Google Scholar] [CrossRef]
- Liu, C.-K.; Chen, C.-A.; Lee, T.-Y.; Chang, H.-H.; Liao, H.-F.; Chen, Y.-J. Rice protein prolamin promotes anti-leukemia immunity and inhibits leukemia growth in vivo. Food Chem. Toxicol. 2018, 112, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yin, R.; Liu, Z.; Niu, Y.; Guo, E.; Cheng, R.; Diao, X.; Xue, Y.; Shen, Q. Hypoglycemic Effect of Prolamin from Cooked Foxtail Millet (Setaria italic) on Streptozotocin-Induced Diabetic Mice. Nutrients 2020, 12, 3452. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Orona-Tamayo, D.; Nieto-Rendón, B.; Paredes-López, O. Antioxidant and antihypertensive potential of protein fractions from flour and milk substitutes from canary seeds (Phalaris canariensis L.). Plant Foods Hum. Nutr. 2017, 72, 20–25. [Google Scholar] [CrossRef]
- Khuri, S.; Bakker, F.T.; Dunwell, J.M. Phylogeny, function, and evolution of the cupins, a structurally conserved, functionally diverse superfamily of proteins. Mol. Biol. Evol. 2001, 18, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Uberto, R.; Moomaw, E.W. Protein similarity networks reveal relationships among sequence, structure, and function within the cupin superfamily. PLoS ONE 2013, 8, e74477. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, G.; Bäumlein, H.; Mock, H.-P.; Himmelbach, A.; Schweizer, P. The multigene family encoding germin-like proteins of barley. Regulation and function in basal host resistance. Plant Physiol. 2006, 142, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Bernier, F.; Berna, A. Germins and germin-like proteins: Plant do-all proteins. But what do they do exactly? Plant Physiol. Biochem. 2001, 39, 545–554. [Google Scholar] [CrossRef]
- Gucciardo, S.; Wisniewski, J.-P.; Brewin, N.J.; Bornemann, S. A germin-like protein with superoxide dismutase activity in pea nodules with high protein sequence identity to a putative rhicadhesin receptor. J. Exp. Bot. 2007, 58, 1161–1171. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Maiti, M.K. Functional role of rice germin-like protein1 in regulation of plant height and disease resistance. Biochem. Biophys. Res. Commun. 2010, 394, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Manosalva, P.M.; Davidson, R.M.; Liu, B.; Zhu, X.; Hulbert, S.H.; Leung, H.; Leach, J.E. A germin-like protein gene family functions as a complex quantitative trait locus conferring broad-spectrum disease resistance in rice. Plant Physiol. 2009, 149, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.; Li, X.; Zhu, Y.; Ge, X.; Sun, Y.; Liu, N.; Jia, Y.; Li, F.; Hou, Y. GhABP19, a novel germin-like protein from Gossypium hirsutum, plays an important role in the regulation of resistance to Verticillium and Fusarium wilt pathogens. Front. Plant Sci. 2019, 10, 583. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, J.; Gantait, S.; Maiti, M.K. Physiological role of rice germin-like protein 1 (OsGLP1) at early stages of growth and development in indica rice cultivar under salt stress condition. Plant Cell Tissue Organ Cult. 2017, 131, 127–137. [Google Scholar] [CrossRef]
- He, Z.-D.; Tao, M.-L.; Leung, D.W.M.; Yan, X.-Y.; Chen, L.; Peng, X.-X.; Liu, E.-E. The rice germin-like protein OsGLP1 participates in acclimation to UV-B radiation. Plant Physiol. 2021, 186, 1254–1268. [Google Scholar] [CrossRef]
- Ke, Y.; Han, G.; He, H.; Li, J. Differential regulation of proteins and phosphoproteins in rice under drought stress. Biochem. Biophys. Res. Commun. 2009, 379, 133–138. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Wei, Z.; Yu, D.; Gan, L.; Li, F.; Wang, Z. Genome-wide characterization and expression analysis of geranylgeranyl diphosphate synthase genes in cotton (Gossypium spp.) in plant development and abiotic stresses. BMC Genom. 2020, 21, 561. [Google Scholar] [CrossRef]
- Dao, T.; Linthorst, H.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Tiwari, V.; Kumari, A.; Chaudhary, E.; Sharma, A.; Ali, U.; Garg, M. Protective and defensive role of anthocyanins under plant abiotic and biotic stresses: An emerging application in sustainable agriculture. J. Biotechnol. 2022, 361, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cheng, Z.; Zhang, X.; Guo, X.; Su, N.; Jiang, L.; Mao, L.; Wan, J. Double repression of soluble starch synthase genes SSIIa and SSIIIa in rice (Oryza sativa L.) uncovers interactive effects on the physicochemical properties of starch. Genome 2011, 54, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gilbert, R.G. Starch molecular structure: The basis for an improved understanding of cooked rice texture. Carbohydr. Polym. 2018, 195, 9–17. [Google Scholar] [CrossRef]
- Irshad, A.; Guo, H.; Rehman, S.U.; Wang, X.; Wang, C.; Raza, A.; Zhou, C.; Li, Y.; Liu, L. Soluble starch synthase enzymes in cereals: An updated review. Agronomy 2021, 11, 1983. [Google Scholar] [CrossRef]
- Babu, A.S.; Parimalavalli, R. Effect of pullulanase debranching and storage temperatures on structural characteristics and digestibility of sweet potato starch. J. Saudi Soc. Agric. Sci. 2018, 17, 208–216. [Google Scholar]
- Li, Y.; Xu, J.; Zhang, L.; Ding, Z.; Gu, Z.; Shi, G. Investigation of debranching pattern of a thermostable isoamylase and its application for the production of resistant starch. Carbohydr. Res. 2017, 446, 93–100. [Google Scholar] [CrossRef]
- Gray-Weale, A.A.; Cave, R.A.; Gilbert, R.G. Extracting physically useful information from multiple-detection size-separation data for starch. Biomacromolecules 2009, 10, 2708–2713. [Google Scholar] [CrossRef]
- Rafii, M.; Zakiah, M.; Asfaliza, R.; Haifaa, I.; Latif, M.; Malek, M. Grain quality performance and heritability estimation in selected F1 rice genotypes. Sains Malays. 2014, 43, 1–7. [Google Scholar]
- Dentin, R.; Benhamed, F.; Pégorier, J.-P.; Foufelle, F.; Viollet, B.; Vaulont, S.; Girard, J.; Postic, C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J. Clin. Investig. 2005, 115, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- Oyola-Robles, D.; Gay, D.C.; Trujillo, U.; Sánchez-Parés, J.M.; Bermúdez, M.L.; Rivera-Díaz, M.; Carballeira, N.M.; Baerga-Ortiz, A. Identification of novel protein domains required for the expression of an active dehydratase fragment from a polyunsaturated fatty acid synthase. Protein Sci. 2013, 22, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Parthibane, V.; Rajakumari, S.; Venkateshwari, V.; Iyappan, R.; Rajasekharan, R. Oleosin is bifunctional enzyme that has both monoacylglycerol acyltransferase and phospholipase activities. J. Biol. Chem. 2012, 287, 1946–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol. Mol. Biol. Plants 2018, 24, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, M.; Wang, Z.; Zhang, Y.; Wu, Q.; Yang, L. Rice protein exerts endogenous antioxidant capacity via methionine sulfoxide reductase and the nrf2 antioxidant system independent of age. J. Med. Food 2020, 23, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Xiong, L. Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses. Plant J. 2005, 44, 396–408. [Google Scholar] [CrossRef]
- Deng, B.; Jin, X.; Yang, Y.; Lin, Z.; Zhang, Y. The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production. Plant Growth Regul. 2014, 72, 269–277. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Chapman, L.M. The importance of thiamine (vitamin B1) in plant health: From crop yield to biofortification. J. Biol. Chem. 2020, 295, 12002–12013. [Google Scholar] [CrossRef]
- Esa, N.; Puteh, A.; Mat, M.; Ismail, R.; Yusop, M.R. Increasing yield of susceptible and resistant rice blast cultivars using silicon fertilization. Indones. J. Agric. Sci. 2020, 21, 49–58. [Google Scholar] [CrossRef]
- Hasan, N.; Rafii, M.Y.; Rahım, H.A.; Ahmad, F.; Ismail, N.N. Identification of bacterial leaf blight resistance genes in Malaysian local rice varieties. Gene Conserve 2020, 19, GMR18545. [Google Scholar] [CrossRef]
- Wilonita, W.; Nurliyana, R.; Asma, D.; Noorazizah, M.; Hirzun, M. Distribution of disease and pest resistance markers in Malaysian rice varieties. ASM Sci. J. 2013, 7, 105–112. [Google Scholar]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef] [PubMed]



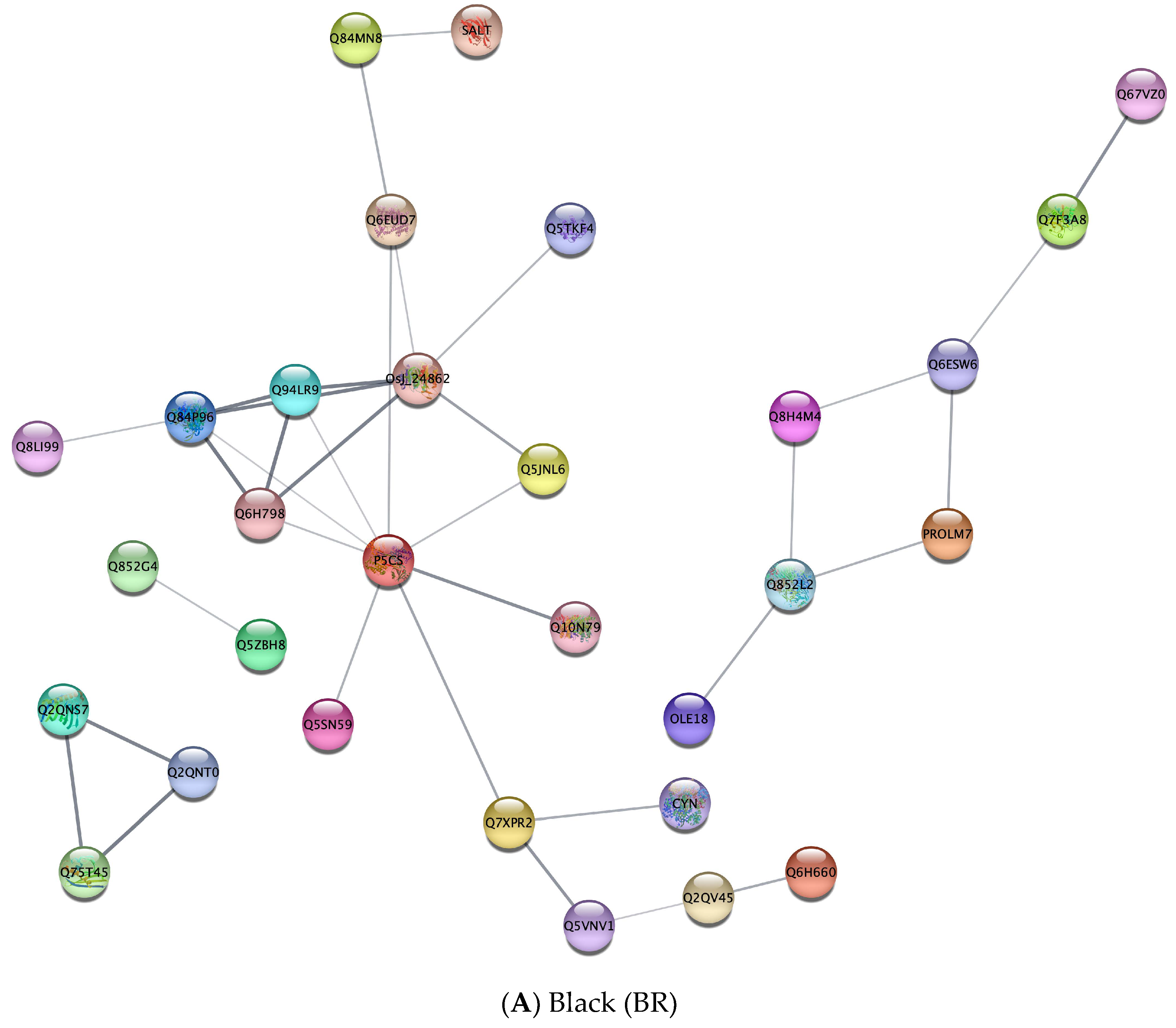
| Black (BR) | |||
| GO Type | GO ID | GO Term | p-Value |
| Biological process (BP) | GO:0006082 | Organic acid metabolic process | 0.0064 |
| GO:0009084 | Glutamine family amino acid biosynthetic process | 0.0064 | |
| GO:0008652 | Cellular amino acid biosynthetic process | 0.0135 | |
| GO:0019752 | Carboxylic acid metabolic process | 0.0161 | |
| GO:0017144 | Drug metabolic process | 0.0487 | |
| GO:1901564 | Organonitrogen compound metabolic process | 0.0487 | |
| KEGG | dosa01200 | Carbon metabolism | 5.90 × 10−4 |
| dosa00640 | Propanoate metabolism | 0.0014 | |
| dosa01040 | Biosynthesis of unsaturated fatty acids | 0.0024 | |
| dosa00071 | Fatty acid degradation | 0.0031 | |
| dosa00280 | Valine, leucine and isoleucine degradation | 0.0031 | |
| dosa00592 | Alpha-linolenic acid metabolism | 0.0031 | |
| dosa01212 | Fatty acid metabolism | 0.0052 | |
| dosa00620 | Pyruvate metabolism | 0.007 | |
| dosa04146 | Peroxisome | 0.007 | |
| dosa00230 | Purine metabolism | 0.0172 | |
| dosa01230 | Biosynthesis of amino acids | 0.0371 | |
| Red (RR) | |||
| GO Type | GO ID | GO Term | p-Value |
| Biological process (BP) | GO:0006412 | Translation | 0.0169 |
| GO:0006518 | Peptide metabolic process | 0.0169 | |
| Molecular function (MF) | GO:0003735 | Structural constituent of ribosome | 0.0012 |
| Cellular component (CC) | GO:0005840 | Ribosome | 0.0022 |
| GO:0005634 | Nucleus | 0.0309 | |
| GO:0005737 | Cytoplasm | 0.041 | |
| KEGG | dosa00052 | Galactose metabolism | 0.0018 |
| dosa00970 | Aminoacyl-tRNA biosynthesis | 0.0018 | |
| dosa03050 | Proteasome | 0.0018 | |
| dosa00500 | Starch and sucrose metabolism | 0.0027 | |
| dosa03010 | Ribosome | 0.0061 | |
| White (WR) | |||
| GO Type | GO ID | GO Term | p-Value |
| Cellular component (CC) | GO:0005743 | Mitochondrial inner membrane | 0.0164 |
| GO:0098796 | Membrane protein complex | 0.0232 | |
| KEGG | dosa00190 | Oxidative phosphorylation | 1.87 × 10−8 |
| dosa01230 | Biosynthesis of amino acids | 6.66 × 10−7 | |
| dosa01200 | Carbon metabolism | 9.64 × 10−7 | |
| dosa00260 | Glycine, serine, and threonine metabolism | 1.90 × 10−4 | |
| dosa00630 | Glyoxylate and dicarboxylate metabolism | 1.90 × 10−4 | |
| dosa00020 | Citrate cycle (TCA cycle) | 0.0047 | |
| dosa00900 | Terpenoid backbone biosynthesis | 0.0047 | |
| dosa00710 | Carbon fixation in photosynthetic organisms | 0.0077 | |
| dosa04146 | Peroxisome | 0.0087 | |
| dosa04016 | MAPK signaling pathway—plant | 0.015 | |
| dosa00010 | Glycolysis/Gluconeogenesis | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sew, Y.S.; Aizat, W.M.; Zainal-Abidin, R.-A.; Ab Razak, M.S.F.; Simoh, S.; Abu-Bakar, N. Proteomic Variability and Nutrient-Related Proteins across Pigmented and Non-Pigmented Rice Grains. Crops 2023, 3, 63-77. https://doi.org/10.3390/crops3010007
Sew YS, Aizat WM, Zainal-Abidin R-A, Ab Razak MSF, Simoh S, Abu-Bakar N. Proteomic Variability and Nutrient-Related Proteins across Pigmented and Non-Pigmented Rice Grains. Crops. 2023; 3(1):63-77. https://doi.org/10.3390/crops3010007
Chicago/Turabian StyleSew, Yun Shin, Wan Mohd Aizat, Rabiatul-Adawiah Zainal-Abidin, Mohd Shahril Firdaus Ab Razak, Sanimah Simoh, and Norliza Abu-Bakar. 2023. "Proteomic Variability and Nutrient-Related Proteins across Pigmented and Non-Pigmented Rice Grains" Crops 3, no. 1: 63-77. https://doi.org/10.3390/crops3010007
APA StyleSew, Y. S., Aizat, W. M., Zainal-Abidin, R.-A., Ab Razak, M. S. F., Simoh, S., & Abu-Bakar, N. (2023). Proteomic Variability and Nutrient-Related Proteins across Pigmented and Non-Pigmented Rice Grains. Crops, 3(1), 63-77. https://doi.org/10.3390/crops3010007





