Abstract
Amaranthus caudatus is a nutrient-rich Andean pseudocereal with wide genetic variability. Six productive varieties (Oscar Blanco, Pucara, Tomina, Cotahuasi, Barbechos, and Guindo Criollo) were compared by proximate, mineral, and fatty acid composition. The proximal content showed certain singularities in the varieties. Barbechos and Guindo Criollo stood out for their fat content (9.50% and 9.01%, respectively), while Tomina stood out for their carbohydrate content (72.6%), and Pucara and Oscar Blanco for their fiber content (4.59% and 4.48%, respectively). The mineral content presented differences, highlighting the Ca content for Pucara (108 mg/100 g), and Tomina with micro-minerals (Zn, Mn, Fe, and Cu, 4.67, 5.90, 9.13 and 1.03 mg/100 g, respectively). All varieties showed high tricosanic acid (C23:0) content, and Cotahuasi was highlighted for its high linoleic acid (C18:2) content. Multivariate analysis showed negative correlations between proteins and carbohydrates, and between fat and fiber in their proximal content, as well as between Fe and Na for their mineral content, and C18:1 and C18:2 for the fatty acids. Although certain differences were found, the total nutritional composition tended to have minor differences between the investigated varieties.
1. Introduction
Amaranth is an ancient crop cultivated in pre-Columbian North, middle and South America, but it also includes several species, some of them, Amaranthus hypochondriacus, Amaranthus cruentus and Amaranthus caudatus [1]. A. caudatus, Foxtail amaranth, is a species cultivated in the Andean highlands, mainly in Bolivia, Peru, and Ecuador [2]. This crop presents high genetic variability with the ability to adapt to diverse agro-climatic habitats and edaphic conditions, i.e., cold, drought, different altitudes, and salinity [3,4].
Amaranthus species have a higher nutritional potential than common cereals due to the high amounts of macro- and micronutrients. The protein content is higher (12.5–16.0%) than wheat [1,5], which can be affected by the growing conditions between species or the varieties cultivated [6,7]. The fat content is also relevant in amaranth grains [8,9], where previous studies of the fatty acid profile oil have shown slight differences between species and varieties [7,9]. However, other authors have established that location was more important than the effect of variety on fatty acid composition for A. cruentus [9]. Moreover, ash content was similar between varieties of the same species cultivated in the same locality or nearby [8]. In addition, several amaranth varieties had higher contents of P, Ca, K and Mg than common cereals [5,8]. Amaranth is also considered a good source of dietary fiber compared to wheat or corn, but it might be due to differences between varieties [10].
The agro-industrial promotion and support foundation PROINPA (‘Fundación Promoción e Investigación de Productos Andinos’, Foundation for Promotion and Research of Andean Products) has collected six varieties of amaranth in Bolivia (Oscar Blanco, Pucara, Tomina, Cotahuasi, Barbechos and Guindo Criollo) that they are breeding and promoting [11]. As a part of these activities there is a need for understanding their compositional properties.
The aim of this study was to compare the proximate, mineral, and fatty acid composition of six productive Amaranth caudatus varieties cultivated in their natural and optimal location in sub-Andean valleys. This could optimize their use and the products developed from them.
2. Material and Methods
2.1. Amaranth Samples
The selected grains for this study included six varieties of the Amaranthus caudatus, Foxtail amaranth, species from Bolivia. Whole grains were obtained from ‘Fundación Promoción e Investigación de Productos Andinos’, PROINPA, Cochabamba, Bolivia (Foundation for Promotion and Research of Andean Products). Some cultivation data about these varieties are shown in Table 1. The grains were cultivated in their natural locations: Mojocoya, Padilla, and Tomina, at 2339, 2102, and 2300 m above sea level, respectively.

Table 1.
Amaranthus caudatus varieties: origin, altitude, growth cycle and yield.
All grains were thoroughly screened to separate impurities, cleaned with running water, and dried at 30 °C for 12 h, before being packaged and stored at 4 °C until further use.
For the chemical and mineral analyses, 150 g of seeds were milled (Retsch, ZM 200, Hann, Germany) using sieves at 0.5 mm and 1 mm. The obtained flours were packaged and stored at 4 °C until further use.
2.2. Proximate Analysis
Flours were evaluated for proximate analysis according the AOAC official methods [12]. Moisture content in flours was determined by drying approximately 2 g of samples at 105 °C until a constant weight (925.10). The protein content was determined by the micro-Kjeldahl method and a factor of 6.25 for pseudocereals was used to calculate the protein content from the nitrogen content (984.13). The fat content was determined using the Soxhlet apparatus with hexane extraction (963.15). Ash content was determined by ignition of flours at 550 °C until light gray ash resulted (923.03). Crude fiber was determined by acid–alkali hydrolysis (962.09). The carbohydrate content was estimated by differences using the formula CHO = 1 − (protein + fat + ash + crude fiber).
2.3. Starch Content
The starch content in the flours was determined enzymatically (Megazyme International Ltd. Co., Wicklow, Ireland), as described by other authors [13,14], with some modifications. 100 mg of sample, contained in a tube (glass, 10 mL capacity), was mixed with 200 µL of aqueous ethanol (80% v/v). Thereafter, 3 mL sodium acetate buffer (prepared with 2.9 mL of glacial acetic acid, 15 mL NaOH 1 M and 0.3784 g CaCl2 in 400 mL of deionized water, made up to 500 mL) and 100 µL of thermostable α-amylase was added, boiled for 10 min while stirred every second minute. The sample was cooled down to 50 °C and 100 µL amyloglucosidase was added, and thereafter stirred and incubated over a heating plate (IKA, RET control-visc, Bundesländer, Germany) at 50 °C for 30 min, while stirred every fifth minute. Subsequently, it was transferred to a 100 mL volumetric flask. Approx. 50 mL was centrifuged at 4000 rpm for 10 min. 100 μL of the supernatant was incubated at 50 °C for 20 minutes with 2 mL glucose oxidase/peroxidase reagent [15]. 100 μL of water (blank) and 100 μL of standard glucose solution (1 mg/mL) were obtained in the same way. The absorbance (Model UV/VIS EZ 301, Perkin Elmer Corporation, Shelton, CT, USA) was read at 510 nm.
2.4. Mineral Composition
The content of the macro-minerals sodium (Na), potassium (K), calcium (Ca) and magnesium (Mg), and the micro-minerals zinc (Zn), manganese (Mn), iron (Fe), and copper (Cu) in the flours were determined according to the 999.10 AOAC official Methods and Lazarte et al. [12,16]. 5 mL of nitric acid (65% v/v, Merck, Darmstadt, Germany) and 3 mL of hydrogen peroxide (30% w/v, Merck, Darmstadt, Germany) were added to 0.5 ± 0.0001 g of sample in a Teflon container, which thereafter was acid digested in a microwave system (Model Multiwave PRO, Anton Paar Co., Graz, Austria) at 160 °C for 1.25 h. The obtained solution was diluted to 50 mL with deionized water (0.055 μS/cm). Minerals were quantified by atomic absorption spectroscopy with an air–acetylene flame (Model AAnalyst 200, Perkin Elmer Corporation, Shelton, CT, USA). Measurements were performed at the following emission lines (nm): Na 589.0, K 766.5, Ca 422.7, Mg 285.2, Zn 213.9, Mn 279.5, Fe 248.3, and Cu 324.7. For the determination of macro-minerals, 1:5 dilutions were made, but for magnesium, it was 1:10, for calcium, lanthanum chloride was added in approximately 1% (w/v) both to the standards and to the samples before reading them in the spectrophotometer to avoid interferences due to the presence of phosphates. A certified reference quinoa flour (LP-CMQ-0247-2019, IBMETRO, Bolivia) was used to validate the mineral analysis.
A calibration curve of six points (0.05–6.00 mg/L) was prepared for each mineral from certified atomic absorption standard solutions (Merck, Darmstadt, Germany).
Phosphorus (P) was evaluated by gravimetric quinolinium molybdophosphate method, Method 3098–3100 AOAC Official Methods [17] in a spectrophotometer (Model UV/VIS EZ 301, Perkin Elmer Corporation, Shelton, CT, USA) at 700 nm.
2.5. Fatty Acid Composition
Fatty acid (FA) analysis was conducted in the following steps: extraction, derivatization, and analysis by gas chromatography. The procedure by Folch [18] was used since it has been reported to be the most reliable quantitative method for a variety of food products [19]. Oil was extracted from 1 g of seeds using 20 mL of chloroform/methanol solution (2:1, v/v) by stirring (T-25 Ultraturrax®, IKA®, Staufen, Germany) for 5 min at room temperature. Two phases were formed, and the lower phase (organic phase) was collected and washed with first 19 mL of KCl solution (0.88%), and then with 76 mL of methanol/water solution (1:1, v/v). The lower phase was collected, and the solvent was removed through a rotary vacuum evaporator.
The fatty acid composition was analyzed as fatty acids methyl esters (FAME), this conversion process of fatty acids into FAMEs is called derivatization. The derivatization was performed according to other studies [20,21] with some modifications: 10 mL alkaline–methanol solution (0.1 M KOH) at 70 °C was added, after 1 h, 4 mL of methanol/HCL (1.2 M) was added, and it was kept at 70 °C for 20 min. Then, it was cooled at room temperature and stirred with 20 mL of hexane and 10 mL of deionized water, using a vortex for 2 min. The extracted oil was stored under nitrogen atmosphere at −20 °C until further analysis. The supernatant was collected for GC analysis.
FAMEs were analyzed by gas chromatography (GC) [21]. Analyses were carried out using a system GC-FID 7890B (Agilent Technologies, Wilmington, DE, USA). The chromatographic conditions established were as follows: capillary column Rt®-2560 column (100 m × 0.25 mm × 0.20 µm) impregnated with a 100% cyano propyl polysiloxane stationary phase (Restek, Part N° 13199, Bellefonte, PA, USA). The analysis was performed with helium at 1.58 mL/min, air at 350 mL/min and hydrogen gas at 40 mL/min, as a carrier gas at the following temperature program: 140 °C held for 10 min, after which the temperature was increased to 240 °C at a rate of 3 °C/min. The temperature was kept at 240 °C for 14 min. The injector and detector temperatures were set at 260 °C. For the determination and quantification of FAME, Tritridecanoin (Sigma Aldrich, St. Louis, MO, USA) was used as an internal standard and a certified FAME reference standard mixture (from C4 to C24, 37 FAs) were used from Supelco (Bellefonte, PA, USA). All analyses were expressed as g/100 g of oil.
2.6. Statistical Analyses
All analyses were performed in triplicate. Results were expressed as means with standard deviations. The data were tested for significance using the one-way ANOVA followed by Tukey with the SPSS Statistics 24 package (SPSS Inc., IBM Corporation, Armok, NY, USA). The Unscrambler® X 10.2 software (CAMO Software AS, Oslo, Norway) was used to perform principal component analysis (PCA). PCA compared the amaranth varieties according to the proximate, mineral, and fatty acid composition. All the data were centered and normalized.
3. Results
3.1. Proximate Analysis
Table 2 shows that Pucara (14.8%), Guindo Criollo (14.9%) and Oscar Blanco (14.5%) did not differ significantly from Cotahuasi (15.4%), while Barbechos (13.4%) and Tomina (13.1%) had a significantly lower protein content. In our results, the fat content varied between 8.08 and 9.50%, four A. caudatus varieties: Oscar Blanco, Pucara, Tomina, and Cotahuasi, had similar content, while Guindo Criollo and Barbechos contained significantly higher amounts of fat. The ash content varied between 2.39 and 2.83%, with the highest ash content in Barbechos and the lowest in Guindo Criollo. Crude fiber also differed between the varieties, especially Guindo Criollo (3.60%) from Pucara (4.59%). Tomina had significantly higher amounts of carbohydrates, compared with the others, with 72.6% versus 69.8–70.1%. Starch content varied between 60.0 and 64.7% and Guindo Criollo had the highest starch content concerning the carbohydrate content (91.3%).

Table 2.
Proximate and mineral composition of six Amaranthus caudatus varieties. Concentrations are presented in dry weight (dw), as mean ± standard deviation (n = 3). For each compound, different superscript letters in rows indicate significant differences between the amaranth varieties (Tukey’s test, p < 0.05).
3.2. Mineral Composition
Nine minerals were analyzed in total, five macro-minerals (P, Na, K, Ca, and Mg) and four micro-minerals (Zn, Mn, Fe, and Cu), shown in Table 2. P, Ca, and Mg contents were higher in Barbechos and Pucara varieties, and both were significantly different from each other as well. Oscar Blanco had the highest content of Na and K. Pucara had the highest Ca content (108 mg/100) and Oscar Blanco the lowest.
In terms of micro-minerals content, Tomina had the highest Zn (4.67 mg/100 g) and Mn (5.90 mg/100 g) content, while the Fe (9.13 mg/100 g) was similar to Cotahuasi (9.85 mg/100 g). The highest Fe (14.8 mg/100) content was found in Guindo Criollo which also had the highest Cu content (1.23 mg/100 g), while Cotahuasi had the lowest (0.74 mg/100 g).
3.3. Fatty Acid Composition
Data on fatty acid methyl esters (FAME) in the different varieties are presented in Table 3. Fatty acids (FAs) were dominated by C16:0 palmitic acid (16.2–17.5%), C18:1 oleic acid (26.8 and 34.6%), and C18:2 linoleic acid (41.3–49.1%). C18:3 linolenic acid was similar between the varieties (0.40–0.57%). C23:0 tricosanic acid was the most dominant long-chain FA (C20 to C24) (3.21–4.24%). Three-quarters of the total FAs were unsaturated (UFA), 41.9 to 49.5% were polyunsaturated (PUFAs), and 19.8 to 22.7% were saturated FAs (SFA). Finally, the ω6/ω3 ratio was different in all the varieties, with the lowest ratio for Cotahuasi (86/1) and the highest for Guindo Criollo (128/1).

Table 3.
Fatty acid composition of six Amaranthus caudatus varieties. The results are presented in relative percentage as mean ± standard deviation (n = 3). For each compound, different superscript letters in rows indicate significant differences between the amaranth varieties (Tukey’s test, p < 0.05).
3.4. PCA Analysis
A PCA analysis explained more relationships and characteristics of the six amaranth varieties by proximate, mineral, and fatty acid composition. Figure 1 shows PCAs for PC−1 and PC−2 with 67, 71, and 62% explained variance from the dataset of proximate, mineral, and fatty acid analyses, respectively. The PC−1 for proximate composition explained 40% of dataset variation and was positively correlated with protein and fiber, and negatively correlated with carbohydrates and starch. Guindo Criollo stood out for its fat content, Tomina for carbohydrates, Oscar Blanco and Pucara for fiber content, Cotahuasi for protein, and Barbechos with the fat and ash content (in the middle), presenting the highest fat and ash content.
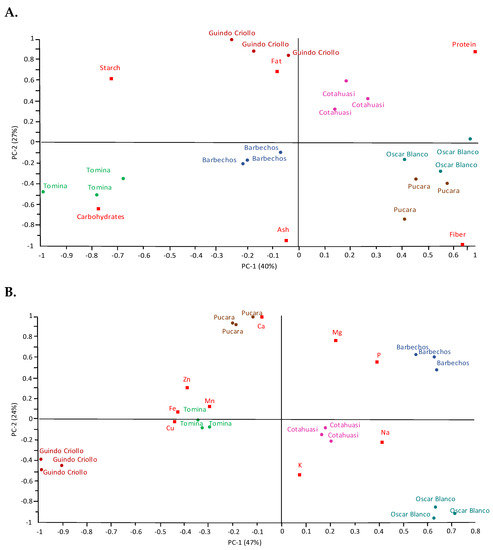
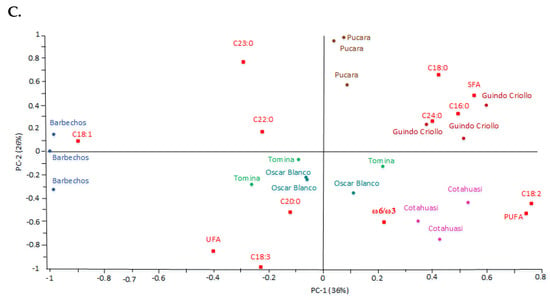
Figure 1.
Principal component analysis (PCA) biplot for PC−1 and PC−2. The dataset includes six Amaranth caudatus varieties (n = 3). (A) Proximate composition. (B) Mineral composition. (C) Fatty acid composition. All data were normalized and centered.
The mineral content (Figure 1B) had 47% explained variation of the dataset for PC−1, and it was negatively correlated with micro-minerals (Zn, Mn, Fe and Cu). The Fe content had a negative correlation to the Na content. Pucara stood out for its Ca content, Tomina had a higher micro-mineral content (Zn, Mn, Fe and Cu) and Barbechos had a higher Mg and P content.
The fatty acids (FAs) were scattered (Figure 1C). Both C18:1 and C18:2 had a high contribution to the correlation of FA correlation and showed a strong negative relationship. This multiple correlation analysis also showed a negative relationship between C18:3 and saturated FAs (SFAs). Barbechos was related to C18:1, Cotahuasi to C18:2 and Guindo Criollo to SFAs.
4. Discussion
Amaranthus caudatus is a valuable pseudocereal with a good source of nutrients. There is little information on the typical varieties of a region cultivated with good agronomic practices that guarantee pure, clean, and optimized varieties. A. caudatus varieties showed some differences in their proximal and mineral composition, mainly due to a variety effect, as found by other authors [22]. Amaranth has a capacity for adaptability, termed ‘phenotypic plasticity’. In the plant kingdom, phenotypic plasticity is every measured variable, which depends both on the biological characteristic of the plant, called the genotype (G), on the environment (E), and the interaction of both (GxE) [23]. The agricultural management developed by foundation PROINPA (‘Fundación Promoción e Investigación de Productos Andinos’, Foundation for Promotion and Research of Andean Products) was quite technical, so that it made it possible to obtain these unique varieties between native and optimized varieties. Currently these six varieties of amaranth are the most cultivated in Bolivia and the most commercialized is the Oscar Blanco variety.
The protein content was similar between all varieties (Table 2), which has also been reported by other authors [22], this might be due to the fact that in Bolivia the cultivation locations are close to each other (20 to 60 km), and the differences in altitude range are only between 198 and 237 m. Remarkably, the protein content obtained is higher than that of common cereals, i.e., corn and rice [24].
These varieties belong to this region, and the altitude and origin were not systematic factors of variation among them. Although, other authors indicate that the environmental conditions could favor some contents, such as high altitudes could increase fat content [25], which confer better stability to avoid the oxidation [26]. Our findings showed some characteristics for each of the varieties. The proximal content indicated that the more protein, the less carbohydrate it had, and if it had a higher fiber, the less starch it had. It could be profitable when a process requires a higher starch content, i.e., the production of snacks by extrusion [27], or if the starch content is not relevant and the protein content is preferred.
In the present study, five were native varieties and one introduced from another region, Oscar Blanco, a commercial variety from Peru. Previous studies of this Peruvian variety have shown a higher fiber content (7.27%) [1] compared to our results (4.48%), which could be due to adaptations to another habitat. However, even so, this variety stood out from the others for its higher fiber content (Figure 1A).
The low content of carbohydrates and starch was found previously by other authors, who compared amaranth with common cereals, i.e., corn and rice [24]. The mean starch content (62.5%) was similar to other studies for Amaranthus hypochondriacus (62%) and higher than Amaranthus cruentus (48%) [24,28], both of which have been studied more than A. caudatus.
In this study, the dominant macro-minerals were K, P, Mg, Ca, and Na, in decreasing order (Table 2), which agrees with previous findings [3]. Amaranth species, such as quinoa, are tolerant to saline stress in the soil, called a halophyte crop, which results in an accumulation of Na in the plant [29]. Other studies indicate that this saline stress changes the composition of minerals and fatty acids of quinoa [30]. In our findings, the Fe content was negatively correlated with the Na content. These varieties showed that the higher the Na content, the lower Fe content (Figure 1B).
The Fe and Ca are crucial minerals in the nutritional diet [31]. Guindo Criollo and Pucara had the highest content of Fe (14.8 mg/100 g) and Ca (108 mg/100 g), respectively (Table 2 and Figure 1B). While the variety with the highest micro-mineral (Zn, Fe, Mn, and Cu) content was Tomina (Figure 1B). Although Pucara and Tomina belong to the same locality, they presented different micro-mineral compositions. This phenotypic response may be because they have different growth cycles, 90–110 and 120–130 days, respectively, as found by other authors in amaranth and wheat [32,33].
In fatty acid (FA) composition, the higher C18:2 content than C16:0 content (Table 3) confirmed that it is a cold-tolerant plant [34]. The C18:2 content was like corn oil, but the high C23:0 content was unusual for a plant-based oil [35]. The high content of PUFAs is necessary for these (vascular) plants to survive the cold [34]. Many factors can affect the composition of FAs in amaranth, such as the location in A. cruentus [6] or the growth cycle of the plant [36]. The FAs synthesis in plants is complex. However, Figure 1C showed a negative correlation between saturated fats (SFAs) and unsaturated fats (UFAs), other authors have explained that C16:0 and C18:0 (UFAs) are monounsaturated fat precursors (C18:1) (MUFAs), and polyunsaturated (C18:2 and C18:3) (PUFAs) [37]. These correlations also indicated that not all varieties are rich in all FAs, but, for example, Guindo Criollo was rich in SFAs, Barbechos in MUFAs, and Cotahuasi in C18:2 (PUFAs).
This study found that in the commonly cultivated A. caudatus crops, there are small differences between varieties according to their proximate, mineral, and fatty acid composition. The causes of these characteristics open many new studies related to the genotypes, growth conditions, and the interaction of both factors.
5. Conclusions
Although the varieties presented certain differences in the proximate, mineral, and fatty acid content, these differences tend to be minor, indicating that these varieties present similar characteristics in terms of these contents but maintain the qualities for which amaranth is known.
Author Contributions
Conceptualization, J.M.-L. and C.C.R.; methodology, J.M.-L. and C.C.R.; formal analysis, J.M.-L., C.C.R., B.B., J.P. and S.J.P.; data curation, J.M.-L., C.C.R., B.B., J.P. and S.J.P.; writing—original draft preparation, J.M.-L.; writing—review and editing, J.M.-L., C.C.R., B.B., J.P. and S.J.P.; visualization, J.M.-L., J.P. and C.C.R.; supervision, C.C.R., J.P. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been founded by the Swedish International Development Agency (SIDA, contribution 2021:13486).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Sincere thanks to the Food and Natural Products Center (CAPN) of the San Simon University, Cochabamba, Bolivia and Fundación PROINPA. We thank Rocio Morales Vargas of CAPN for measurements of fatty acids. This paper is dedicated to the memory of brothers Antonio and Edson Gandarillas, PROINPA Foundation, Bolivia, who passed away in July 2020 and July 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Repo-Carrasco-Valencia, R.; Peña, J.; Kallio, H.; Salminen, S. Dietary fiber and other functional components in two varieties of crude and extruded kiwicha (Amaranthus caudatus). J. Cereal Sci. 2009, 49, 219–224. [Google Scholar] [CrossRef]
- Rojas, W.; Pinto, M.; Soto, J.L. Distribución geográfica y variabilidad genética. In Granos Andinos: Avances, Logros y Experiencias Desarrolladas en Quinoa, Cañahua y Amaranto en Bolivia; Rojas, W., Soto, J.L., Pinto, M., Jager, M., Padulosi, S., Eds.; Bioversity Internacional: Roma, Italia, 2010; pp. 11–23. [Google Scholar]
- Nascimento, A.C.; Mota, C.; Coelho, I.; Gueifão, S.; Santos, M.; Matos, A.S.; Gimenez, A.; Lobo, M.; Samman, N.; Castanheira, I. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: Proximates, minerals and trace elements. Food Chem. 2014, 148, 420–426. [Google Scholar] [CrossRef]
- Di Fabio, A.; Parraga, G. Origin, production and utilization of pseudocereals. In Pseudocereals: Chemistry and Technology; Haros, C.M.S.R., Ed.; Jhon Wiley: Hoboken, NJ, USA, 2017; pp. 1–27. [Google Scholar]
- Ikeda, S.; Yamashita, Y.; Tomura, K.; Kreft, I. Nutritional comparison in mineral characteristics between buckwheat and cereals. Fagopyrum 2006, 23, 61–65. [Google Scholar]
- Mustafa, A.F.; Seguin, P.; Gélinas, B. Chemical composition, dietary fibre, tannins and minerals of grain amaranth genotypes. Int. J. Food Sci. Nutr. 2011, 62, 750–754. [Google Scholar] [CrossRef]
- Bressani, R.; Gonzáles, J.M.; Zúñiga, J.; Breuner, M.; Elías, L.G. Yield, selected chemical composition and nutritive value of 14 selections of amaranth grain representing four species. J. Sci. Food Agric. 1987, 38, 347–356. [Google Scholar] [CrossRef]
- Bressani, R. Composition and nutritional properties of amaranth. In Amaranth-Biology, Chemistry and Technology, 1st ed.; Taylor & Francis Group: Oxford, UK, 1994; pp. 185–205. [Google Scholar]
- Berganza, B.E.; Moran, A.W.; Rodríguez, G.M.; Coto, N.M.; Santamaría, M.; Bressani, R. Effect of variety and location on the total fat, fatty acids and squalene content of amaranth. Plant Foods Hum. Nutr. 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.A.M. Dietary Fibre and Bioactive Compounds of Kernels. In Psedocereals: Chemistry and Techonology; Scgoenlechner, C.M.H.a.R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 71–93. [Google Scholar]
- Fuentes, W.; Calle, C. Manejo del Cultivo del Amaranto; Latincrop—PROINPA: La Paz, Bolivia, 2017; p. 28. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Rockville, MD, USA, 2019. [Google Scholar]
- Fuentes, C.; Perez-Rea, D.; Bergenståhl, B.; Carballo, S.; Sjöö, M.; Nilsson, L. Physicochemical and structural properties of starch from five Andean crops grown in Bolivia. Int. J. Biol. Macromol. 2019, 125, 829–838. [Google Scholar] [CrossRef]
- Perez-Rea, D.; Bergenståhl, B.; Nilsson, L. Development and evaluation of methods for starch dissolution using asymmetrical flow field-flow fractionation. Part I: Dissolution of amylopectin. Anal. Bioanal. Chem. 2015, 407, 4315–4326. [Google Scholar] [CrossRef]
- Holm, J.; Björck, I.; Drews, A.; Asp, N.G. A Rapid Method for the Analysis of Starch. Starch Stärke 1986, 38, 224–226. [Google Scholar] [CrossRef]
- Lazarte, C.E.; Carlsson, N.-G.; Almgren, A.; Sandberg, A.-S.; Granfeldt, Y. Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. J. Food Compos. Anal. 2015, 39, 111–119. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 14th ed.; AOAC: Washington, DC, USA, 1984. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.; Wickramasinghe, I. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Ce 2—66: Preparation of Methyl Esters of Fatty Acids, 5th ed.; Oil Chemists’ Society: Champaign, IL, USA, 1998. [Google Scholar]
- Golay, P.-A.; Moulin, J. Determination of labeled fatty acids content in milk products, infant formula, and adult/pediatric nutritional formula by capillary gas chromatography: Collaborative study, final action 2012.13. J. AOAC Int. 2016, 99, 210–222. [Google Scholar] [CrossRef]
- Derkanosova, N.; Stakhurlova, A.; Pshenichnaya, I.; Ponomareva, I.; Peregonchaya, O.; Sokolova, S. Amaranth as a bread enriching ingredient. Foods Raw Mater. 2020, 8, 223–231. [Google Scholar] [CrossRef]
- El-Soda, M.; Malosetti, M.; Zwaan, B.J.; Koornneef, M.; Aarts, M.G. Genotype× environment interaction QTL mapping in plants: Lessons from Arabidopsis. Trends Plant Sci. 2014, 19, 390–398. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef]
- Villacrés, E.; Pástor, G.; Quelal, M.B.; Zambrano, I.; Morales, S. Effect of processing on the content of fatty acids, tocopherols and sterols in the oils of quinoa (Chenopodium quinoa Willd), lupine (Lupinus mutabilis Sweet), amaranth (Amaranthus caudatus L.) and sangorache (Amaranthus quitensis L.). Glob. Adv. Res. J. Food Sci. Technol. 2013, 2, 44–53. [Google Scholar]
- Gamel, T.H.; Mesallam, A.S.; Damir, A.A.; Shekib, L.A.; Linssen, J.P. Characterization of amaranth seed oils. J. Food Lipids 2007, 14, 323–334. [Google Scholar] [CrossRef]
- Bhattacharya, S. Raw materials for extrusion of foods. In Advances in Food Extrusion Technology; Medeni Maskan, A.A., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Becker, R.; Wheleer, E.L.; Lorenz, K.; Stafford, A.E.; Grosjean, O.K.; Betschart, A.A.; Saunders, R.M. A Compositional Study of Amaranth Grain. J. Food Sci. 1981, 46, 1175–1180. [Google Scholar] [CrossRef]
- Kiani-Pouya, A.; Roessner, U.; Jayasinghe, N.S.; Lutz, A.; Rupasinghe, T.; Bazihizina, N.; Bohm, J.; Alharbi, S.; Hedrich, R.; Shabala, S. Epidermal bladder cells confer salinity stress tolerance in the halophyte quinoa and Atriplex species. Plant Cell Environ. 2017, 40, 1900–1915. [Google Scholar] [CrossRef]
- Toderich, K.; Mamadrahimov, A.; Khaitov, B.; Karimov, A.; Soliev, A.; Nanduri, K.; Shuyskaya, E. Differential Impact of Salinity Stress on Seeds Minerals, Storage Proteins, Fatty Acids, and Squalene Composition of New Quinoa Genotype, Grown in Hyper-Arid Desert Environments. Front. Plant Sci. 2020, 11, 607102. [Google Scholar] [CrossRef]
- FAO. Nutrición humana en el mundo en desarrollo. In De las Naciones Unidas para la Agricultura y la Alimentación; Latham, M.C., Ed.; FAO: Rome, Italy, 2002. [Google Scholar]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Minerals, phytic acid, tannin and rutin in buckwheat seed milling fractions. J. Sci. Food Agric. 2001, 81, 1094–1100. [Google Scholar] [CrossRef]
- Iba, K. Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef]
- Coultate, T. Food: The Chemistry of Its Components, 6th ed.; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Peiretti, P.; Meineri, G.; Longato, E.; Tassone, S. Chemical composition, in vitro digestibility and fatty acid profile of Amaranthus caudatus herbage during its growth cycle. Anim. Nutr. Feed. Technol. 2018, 18, 107–116. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).