Abstract
Amid a rapidly growing global population and increasing threats to crop yields, this review focuses on Speed Breeding (SB) in crop genetics. It traces SB’s development from carbon arc lamp experiments 150 years ago to its modern use with LED technology which significantly accelerates breeding cycles. SB has applications in genetic mapping, genetic modification, and trait stacking, enhancing crop resilience by leveraging allelic diversity. It aligns well with breeding methods like single plant selection and single seed descent. The integration of SB with gene editing, genotyping, and genomic selection holds great promise. However, SB faces challenges related to infrastructure, genotypic variations, and potential stress responses. In summary, SB is a powerful and promising approach to address food security concerns and advancing crop genetics.
1. Introduction
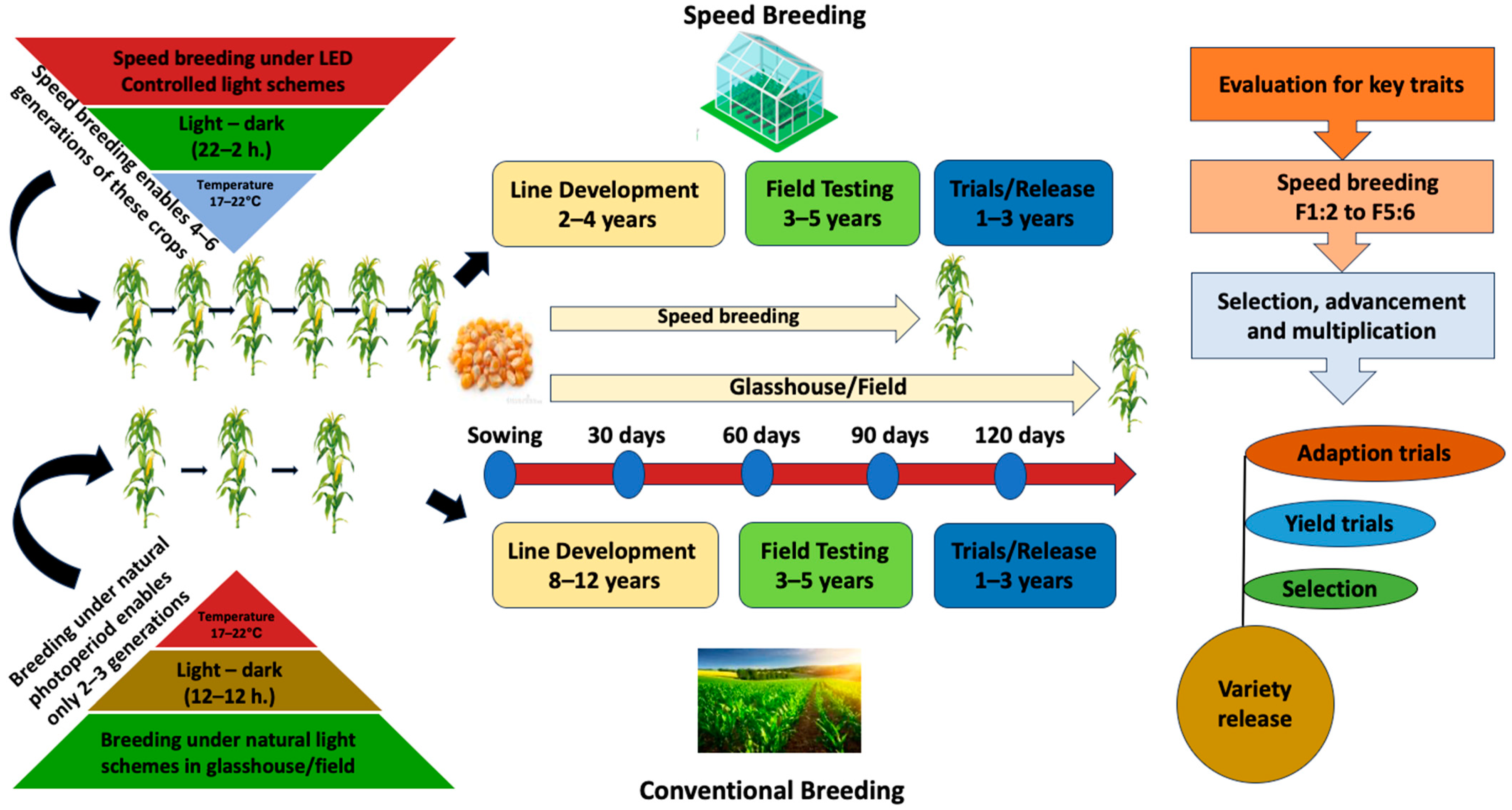
Urgent investments in crop improvements, enhancing their resilience to both biotic and abiotic stresses while ensuring high-quality and optimal yields, are needed to attain food security [1]. It is projected that by 2050, the present pace of agricultural advancement will not be adequate to provide sustenance for the expanding global population, which is anticipated to experience a 25% growth and reach 10 billion [2]. The traditional breeding techniques used for developing new crop varieties are often time-consuming and cannot keep up with the exponentially rising demand for food production [3]. However, with the advancement of technologies and breakthroughs, researchers and breeders are able to accelerate the advancement of novel varieties [4,5,6]. Speed Breeding (SB) is one such technique that involves utilizing controlled environments, which promotes rapid and accelerated growth and development from the vegetative to the reproductive stage in high-density planting (HDP) (Figure 1) [7,8,9].
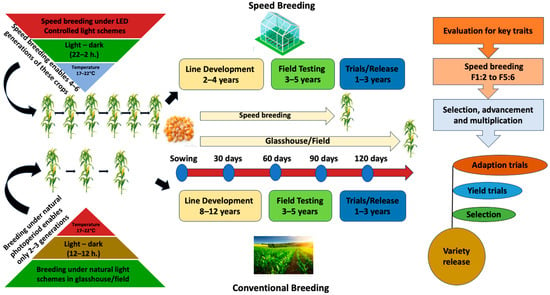
Figure 1.
Overview of conventional and speed breeding.
By doing so, SB reduces generation time through the optimal utilization of light and temperature, and immature seed propagation, thereby enhancing biomass and seed production in long-day crops like lettuce [10] and wheat [11], as well as a few short-day crops like rice, cotton, and sorghum [9,12], and day-neutral crops such as amaranth [13]. SB protocols are now available for multiple crops, and they can enhance rapid advancement in crop improvement research, ranging from crossing, developing transgenic pipelines, and creating mapping populations [5,14,15].
SB can significantly increase the progress toward combating challenges associated with food security through the development of genetic gain, especially in areas with harsh environmental conditions. By developing crops with higher yields that can withstand climate changes and utilize water more efficiently, SB can help provide people with healthier and more nutritious foods while also reducing the environmental impact of agriculture [6,15,16]. This review provides an in-depth exploration of SB, covering its historical development, contemporary applications in genetic mapping, genetic modification, and trait stacking, and addressing the limitations, including the need for controlled environments and potential genotypic variations with a focus on attaining genetic gain and food security.
2. The History and Development of SB in Enhancing Crop Genetics
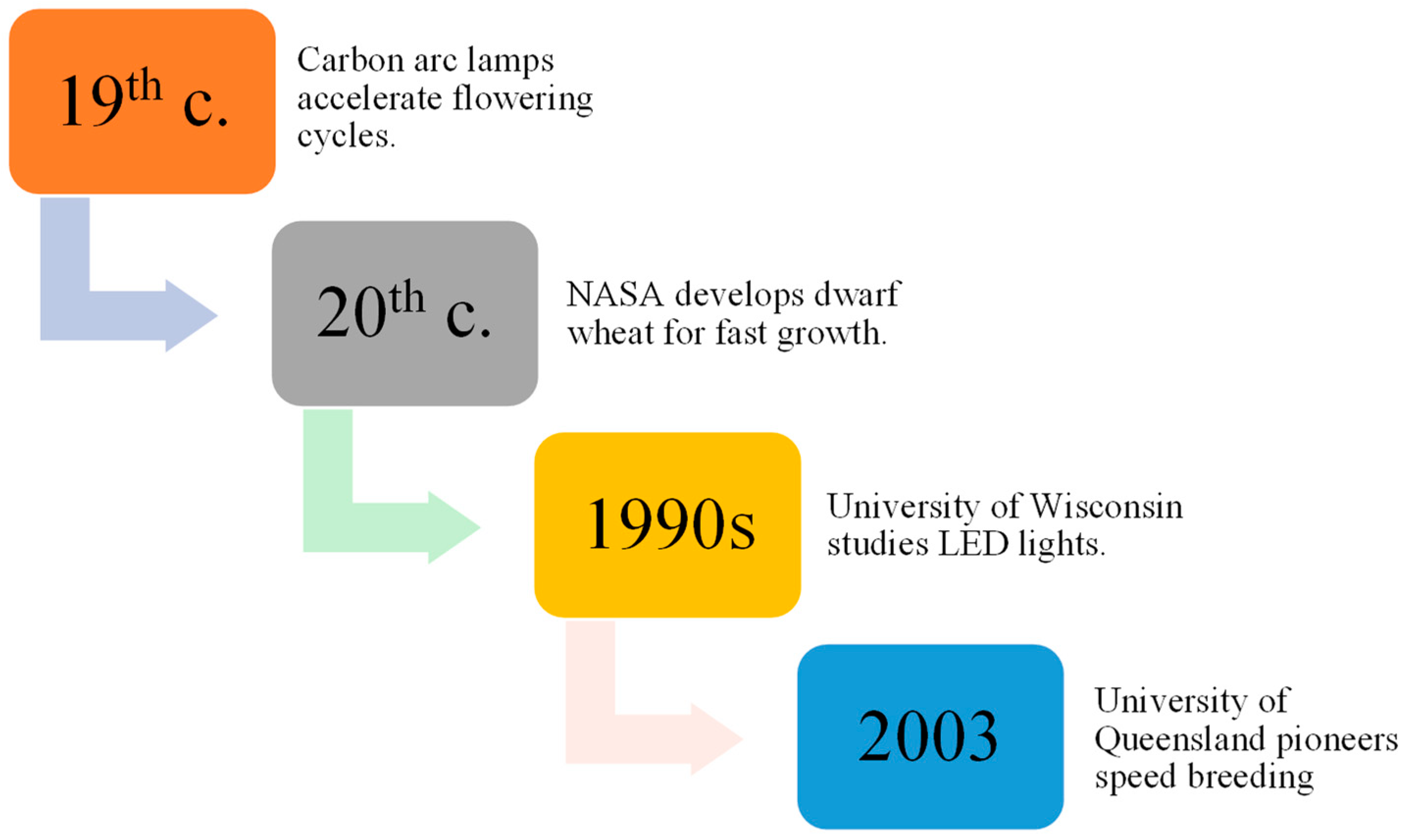
Research dating back to 150 years ago, using carbon arc lamps, resulted in the discovery of plants being able to grow and procreate under conditions with artificial lights, speeding up the flowering cycle when subjected to continuous light [17,18]. Later, Utah State University collaborated with the National Aeronautics and Space Administration (NASA) to develop a dwarf wheat line selected for fast growth and development in a perpetual light environment. Additionally, space mirror utilization was proposed by Russian scientists to improve agricultural productivity [19,20].
Thereafter, the effects of light-emitting diode (LED) lights on plant development were evaluated in the 1990s by the University of Wisconsin, and improvements in LED technology have made indoor plant propagation systems more affordable, leading to increased crop productivity [21]. As a result of NASA’s research efforts, researchers at the University of Queensland named the technology SB (Figure 2), which they utilized in 2003 to hasten wheat breeding without specialized labs [22]. Specific protocols for inducing flowering in crops with environmental cues can be used to reduce the space and cost associated with inbred line development. Seed chipping and barcoding can assist marker-assisted selection (MAS), crossing, mapping population development, adult plant phenotyping, trait stacking, and development of Genetic modification (GM) pipelines [11,23]. This approach holds the potential to substantially accelerate the exploration and utilization of allelic diversity inherent in traditional landraces and the wild progenitors of cultivated crops. Through the application of this method, researchers can uncover and harness previously undiscovered reservoirs of resistance, ultimately contributing to the diversification and enhancement of crop resilience [24].
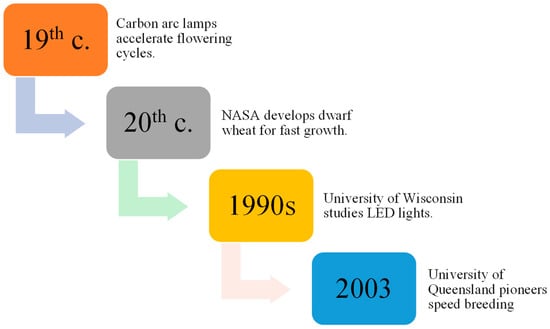
Figure 2.
Evolution of SB techniques over time.
Early studies indicated that growing lettuce under red LEDs and blue fluorescent lamps resulted in growth rates comparable to cool-white fluorescent and incandescent lamps [25]. Further research showed that red LEDs promoted the elongation of hypocotyls and cotyledons, while blue LEDs prevented this elongation [26]. These findings spurred the development and use of LED illumination systems in plant growth chambers for various plant species, including wheat, Brassica rapa, potato leaf cuttings, Arabidopsis thaliana, and soybeans [27,28,29,30]. Comparison with conventional lighting sources revealed similar photosynthetic responses, showcasing the potential of LEDs and combinations of red, green, and blue light at different ratios for studying terrestrial plants [26]. Moreover, recent studies have demonstrated that the narrow blue + narrow amber LED light mixture outperforms white LEDs, HPS lamps, and narrow amber light treatments, resulting in the highest tomato mass (479 g). Dry mass and plant height showed only minor variations. Additionally, supplementing narrow amber light with 430 nm blue light increased chlorophyll content by 20%, emphasizing the importance of precise wavelength selection for tomato growth [31]. The study also suggests that winter wheat can be successfully grown indoors using various LED lighting configurations. Among these configurations, the treatment employing a 4:1 ratio of red and green LEDs yielded the highest crop production, assimilation rates, and flour quality. Elevated levels of blue light negatively impacted yield [32].
3. SB Applications and Selection Methods in Plant Breeding
Due to the lengthy and extended cycles of selection and inbreeding inherent in traditional selection techniques, including pure line, recurrent, bulk, mass, and pedigree selection, they are considered unsuitable for application in SB. These conventional methods do not align with the accelerated breeding demands and precision required by SB in modern agricultural contexts [33]. In contrast, it is worth noting that certain breeding methods, such as single plant selection (SPS), and single seed descent (SSD), align exceptionally well with the principles of SB [34]. These techniques offer distinct benefits, as they require less cultivation space and labor during initial generations. This streamlined approach enables researchers to efficiently advance offspring under the conditions of HDP within controlled growth chambers and relatively compact nurseries, excluding greenhouse environments. This expedites breeding cycles while optimizing resource allocation for enhanced crop development. Furthermore, this method can be extended to field environments [9].
SB using SSD programs is highly efficient, especially for cereal crops like wheat and barley. Higher sowing densities in SB allowed rapid cycling of multiple generations annually. Under specific LED-supplemented glasshouse setups, up to six generations of wheat and barley per year were achieved at a density of 1000 plants/m2. Extending the photoperiod from 16 to 22 h significantly accelerated development [16]. Studies revealed that manipulating light quality, specifically the red to far-red ratio, significantly influences the flowering time of cool-season grain legumes such as peas, chickpeas, faba beans, lentils, and lupins. These findings are crucial for expediting SSD in legume breeding, offering practical applications in biotechnological tools for enhancing legume crops [35]. In another study, SSD was applied to create recombinant inbred lines in chickpeas, focusing on salinity tolerance. Researchers successfully identified multiple genetic loci (QTLs) associated with salinity tolerance, providing valuable insights for developing salt-tolerant chickpea varieties [10]. Similar approaches have been used in peanut breeding research, where controlled conditions, continuous light, optimal temperature, and SSD were combined in a greenhouse setting. These efforts consistently reduced the generation time for full-season maturity peanut cultivars from 145 to 89 days. This innovative speed breeding technique has the potential to significantly expedite peanut variety development, shortening the traditional breeding timeline from the first cross to commercial release to approximately six to seven years [36].
By utilization of various methods such as biparental population mapping, phenotyping, and genetic transformation experiments [16,37], SB stands out as a superior alternative to the doubled haploid (DH) technique, primarily due to its remarkable ability to overcome the hurdles of poor vigor and germination rates [38]. The speed breeding technique was applied in the cultivation of durum wheat. The study conducted a phenotypic analysis on a biparental population derived from Outrob 4 and Caoaroi, focusing on various traits such as leaf rust resistance, crown rot susceptibility, plant stature, root angle, and root number. Astonishingly, they managed to complete an entire generation of durum wheat in a mere 77 days [39]. In contrast to the DH method, which necessitates specific genotypes and well-equipped facilities, SB is more versatile and does not have these limitations. Nevertheless, the technology must address plant phenology and physiology to accelerate generation times [40].
Certain species encounter intricate challenges when employing the double haploid technique, involving factors like haploid production and chromosome doubling, as documented [41]. In these species, inadequate responses to tissue culture result in reduced haploid induction rates and significant associated costs [42,43]. Furthermore, there is a notable prevalence of plant mortality and abnormal development. For species with suboptimal tissue culture performance and no established chromosome doubling methods, these challenges persist. This scenario is exemplified by rye, watermelon, other secondary cucurbit species, tomato, and leguminous species [44,45,46,47].
In a harmonious synergy that expands the horizons of agricultural innovation and high-throughput phenotyping, genetic improvement, gene editing, and genotyping, as well as genomic selection can be integrated with emerging methodologies. This collaborative approach not only leverages the capabilities of each technique but also fosters a comprehensive framework for advancing the frontiers of modern plant breeding and crop enhancement. SB helps to reduce the cost and space requirements of HDP [48]. The use of recombinant inbred lines developed through self-fertilization can be better for genetic mapping than DH as they have a higher recombination frequency [49,50].
SSD methods hasten the progression and assessment of segregation generations within a condensed time frame under the accelerated conditions of SB. To increase the turnover rate of plant generations, various approaches include shuttle breeding, physiological stress, embryo rescue, and increasing CO2 concentration. Shuttle breeding involves growing two generations of wheat per year at different altitudes, latitudes, and climates, making it more accessible and less labor-intensive [8,36,51]. Overall, the techniques of SB hold the potential to substantially enhance genetic advancement by facilitating the creation and introduction of innovative cultivars.
4. Potential Advantages of SB Techniques
In order to quickly create homozygous lines following first crosses, SB approaches entail the modification of growing variables like photoperiod, temperature, soil moisture, population density, and carbon dioxide levels Table 1 [52,53]. These adjustments facilitate the generation of multiple breeding cycles annually, making it well-suited for accelerating breeding processes and assessing populations in diverse environments using a range of selection techniques. Ultimately, these adaptations contribute to enhanced genetic gain [54,55]. Enhancing growth, development, flowering, and seed set in many crop species and genotypes requires manipulating the photoperiod regime. According to reports, certain photoperiod regimes can cause early blooming in crops like wheat, barley [56,57], chickpeas, grain amaranth [58], and groundnut [59]. For indoor SB in nations with unstable electricity supplies, low-energy LEDs and solar power systems can be used to adjust the photoperiod [9,22].

Table 1.
Aspects, advantages, and limitations of speed breeding.
Modulating soil and air temperatures is vital for promoting favorable plant progression and maturation. Extreme temperatures, whether excessively low or high, can significantly impact the timing of key transitions along the growth process [61]. It is important to modulate temperature within required ranges to allow plants to have the environmental factors to promote flowering, maturity, and seed set [71]. Certain major crops, including maize, wheat, barley, canola, cotton, and soybean, exhibit specific temperature requirements for various growth stages. For instance, they typically require temperatures of 12–30 °C for seed germination and 25–30 °C for flowering and overall growth [19,72,73]. In contrast, cool-season crops like broccoli thrive at a maximum temperature of 25 °C. However, during severe weather events, maize can experience maximum temperatures as high as 38 °C. These elevated temperatures have adverse effects on crop health, including decreased pollen viability, as observed in corn and rice. In these instances, daytime temperatures ranging from 30 to 35 °C resulted in reduced seed set in male-sterile soybean [74,75].
Previous studies have highlighted that light-responsive genotypes observed in various crop species display a wide array of reactions when subjected to temperature fluctuations in all growth stages of plant development [9,62]. These intricate and often nuanced temperature-dependent responses have garnered considerable attention from researchers due to their significance in optimizing crop yields and productivity. In the pursuit of more efficient crop development, particularly in developing nations where conventional methods may face limitations, the significance of temperature regulation cannot be emphasized enough. To address this challenge, innovative technologies are being integrated into agricultural practices. Notably, the application of solar- or battery-powered air-conditioning systems has emerged as a promising solution. This approach offers the potential to create a controlled and stable environment while remaining cost-effective, aligning with the overarching goal of optimizing resource utilization in agricultural contexts [63].
The modulation of soil moisture is crucial in crop development, due to its direct and indirect influence on plant biometric development. After flowering, reducing soil moisture content has been shown to facilitate rapid grain filling and maturation in wheat, barley, canola, and chickpea [16]. Effective soil moisture management strategies are versatile and can be adapted for use in a variety of settings, whether it is open fields or controlled indoor environments. Both water scarcity and excess water have been observed to trigger early flowering and maturation in wheat, pearl millet, pea, and barley [40,76,77]. These approaches hold immense potential for optimization, leading to a more streamlined and efficient generation turnover process [65]. Moreover, indoor cultivation offers a unique advantage in terms of soil moisture control. Within the controlled parameters of indoor facilities, growers have greater precision and flexibility in managing moisture levels to meet the specific needs of crops [66].
Pioneering studies set the stage for accelerating crop breeding cycles. These studies focused on wheat, barley, and canola, utilizing a combination of 22 h of light and temperatures from 22/17 °C, along with immature seed harvesting techniques. These interventions significantly reduced generation times to 65 days for wheat, 68 days for barley, and 98 days for canola, enabling approximately 4 to 6 generations per year [78]. However, other studies found that canola, under a photoperiod of 20 h, achieved generation times ranging from 62 to 71 days, allowing approximately 5.9 generations per year [79], whereas barley genotypes, such as Franklin, Gairdner, Gimmett, Commander, Fleet, Baudin, and Lockyer, experienced early flowering and increased generation cycles per year (7.3 to 9.3) using a 16/8 hr light/dark photoperiod and a light intensity of 500 μmol/m2/s [80]. In contrast, conventional methods typically take 70 days, resulting in 6 generation cycles per year. Building on this approach, chickpeas subjected to 22 h of light and a temperature of 25 ± 1 °C, along with immature seed harvest, reduced generation times to 50–52.7 days for early maturing accessions and 55.4–58.6 days for medium maturity accessions, achieving around 6.2 to 7 generations per year [61]. These studies suggest that photoperiod and temperature have a great impact on crop generation time and have been tested for different crops. The effect of photoperiod and temperature studied on some of the important crops is listed in Table 2.

Table 2.
Speed Breeding methods and implication in major crops.
HDP is a low-cost SB strategy that involves cultivating crops with a higher quantity of plants in proximity, surpassing conventional practices for peak yield. This innovative approach not only promotes early flowering and faster maturation but also reduces the length of a crop cycle, effectively accelerating the breeding process. Additionally, HDP enables the maintenance of the large population size required for advanced selections, making it a valuable tool in modern agriculture [66,67,68]. Modifying carbon dioxide levels can enhance rapid plant growth and speed up the transition from the vegetative to the reproductive stage in some plants, but different crop species and genotypes within a species have varying responses [69]. Modifying CO2 levels requires the appropriate facilities and safety protocols [70]. Plant nutrition and hormonal therapies, together with methods like organ tissue culture, have been successfully used to accelerate development, as well as trigger flowering and seed formation in a variety of crop species [8,60]. In controlled environments, the observation of diverse responses to plant growth regulators (PGRs) can be significant. Remarkably, certain crops, including faba beans and lentils, have showcased an extraordinary ability to complete up to eight generations annually when subjected to such controlled conditions [88].
Moreover, to further enhance the breeding process, specific methods involving the drying and chilling of seeds can be employed. These techniques prove especially valuable as they effectively break seed dormancy in immature seeds, leading to remarkable germination rates [8].
5. Applications of SB beyond Crop Improvement
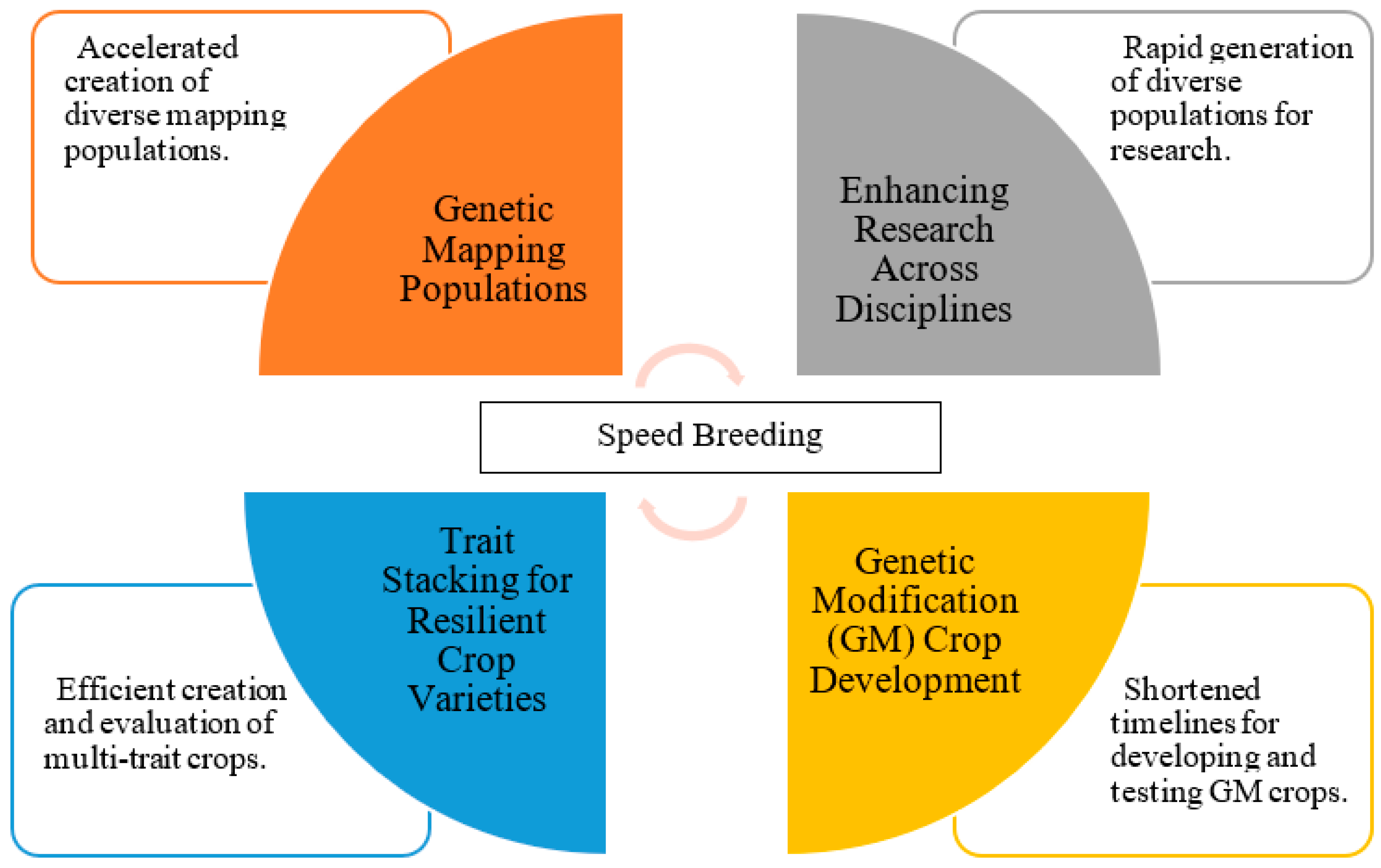
SB techniques have indeed revolutionized the field of crop improvement, but their impact extends far beyond this primary domain. These techniques have found diverse and innovative applications in various aspects of plant research and development, unlocking new possibilities and efficiencies in the realm of agriculture and biotechnology (Figure 3; Table 3) [9,10].
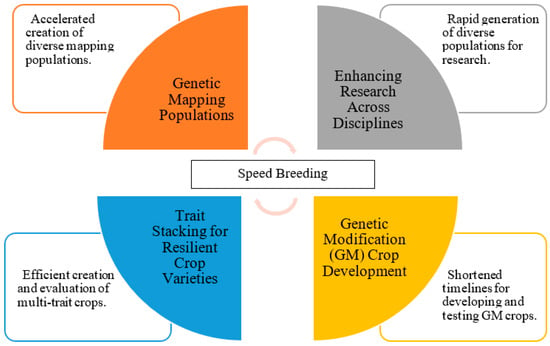
Figure 3.
Applications of speed breeding techniques in plant research and crop improvement.

Table 3.
Advantages and limitations of different aspects of speed breeding.
5.1. Genetic Mapping Populations
SB has emerged as a game-changer in the creation of mapping populations. Traditionally, generating diverse segregating populations for genetic mapping was a time-consuming process [89]. However, SB’s ability to rapidly cycle through generations has transformed this area [53]. Researchers can now efficiently produce large and genetically diverse mapping populations, providing them with the necessary resources to pinpoint genes associated with key traits [15]. This breakthrough has significantly accelerated the field of quantitative trait locus (QTL) analysis and MAS [4,12]. Studies have demonstrated the use of a mapping population developed through a biotron SB system to enhance salinity stress tolerance in rice. This involved introgressing the hst1 gene from Kaijin into high-yielding Yukinko-mai rice using SNP marker-assisted selection [63]. In just six generations over 17 months, the BC3F2 hst1 homozygous line was developed, demonstrating salinity tolerance at both seedling and reproductive stages. In response to climate challenges in Australian wheat regions, a multi-trait approach, including QTL analysis, was used to enhance wheat yield and stability under water limitation and heat. Novel phenotyping techniques, along with a nested association mapping strategy, aid in identifying valuable traits such as stay-green and root characteristics. The University of Queensland’s “SB” method accelerates line generation, and genotyping with DArTseq markers helps validate known QTL and discover new ones for these traits [90]. Thus, a rapid breeding approach, coupled with novel marker technologies and MAS, exemplifies the powerful integration of genomics and SB for the development of improved crop lines, overcoming traditional limitations [91].
5.2. Genetic Modification (GM) Crop Development
In the realm of GM, SB offers a valuable tool for biotechnologists. GM crops play a pivotal role in modern agriculture by addressing critical challenges such as pest resistance, disease resilience, and environmental adaptability [92]. SB facilitates the development and testing of these crops by dramatically shortening the time required for their creation. Controlled environments and accelerated generation times enable researchers to advance the entire GM crop development process from gene insertion to field trials [34,93]. This not only reduces the time to market for GM crops but also empowers researchers to respond swiftly to emerging agricultural issues. SB, therefore, acts as a catalyst for innovation in the biotechnology sector, fostering advancements in crop biotechnology that can benefit both farmers and consumers [21]. ExpressEdit is a promising approach that integrates gene editing and SB, sidestepping traditional tissue culture. It introduces “preassembled Cas9-sgRNA ribonucleoproteins” into plant shoot apical meristems using techniques like particle bombardment or biolistic DNA delivery. Cas9-lacking plants with the desired trait can be bred using Marker-Assisted Backcrossing (MABC). These “CRISPR-ready” plants can undergo further modifications with targeted sgRNA, advancing crop breeding and agricultural productivity [92]. Furthermore, the use of CRISPR/Cas9 technology has proven highly effective in enhancing yield-related traits by disrupting negative regulators that influence factors determining crop yield. This includes improvements in grain size, grain quantity, grain weight, panicle size, and tiller numbers in crops like rice and wheat through the targeting of genes such as OsGS3, OsGn1a, OsGW5, TaGW2, TaGASR7, OsGLW2, TaDEP1, OsDEP1, and OsAAP3 [94,95] Additionally, the disruption of the Waxy gene has improved nutritional quality and led to the development of high-yield waxy corn cultivars. Meanwhile, the knockout of weight-related genes (GW5, GW2, TGW6) in rice has resulted in increased grain weight [1,96].
5.3. Trait Stacking for Resilient Crop Varieties
Trait stacking, the practice of introducing multiple desirable traits into a single crop variety, has gained prominence in modern agriculture [93]. SB plays a pivotal role in streamlining this process. With the ability to rapidly generate and assess multiple generations of plants, breeders can efficiently create and evaluate multi-trait combinations [6]. This efficiency not only saves time but also conserves resources. As a result, crop breeders can develop highly adaptable and resilient varieties more effectively. These multi-trait crops can withstand diverse challenges, ranging from changing climatic conditions to evolving pest pressures. The accelerated trait stacking made possible by SB contributes to the development of crop varieties capable of meeting the demands of a dynamically changing world. This involved studies in rice concentrated on stacking genes associated with cellular detoxification, osmolyte accumulation, antioxidant mechanisms, and signaling pathways to boost tolerance against abiotic stresses like drought and salinity [97]. Meanwhile, in the case of the European two-rowed barley cultivar Scarlett, the study showcased the utilization of innovative techniques, such as rapid trait introgression and SB, to enhance disease resistance. This was achieved by integrating multiple disease-resistance genes from four donor lines through a modified backcross approach, leading to the creation of 87 introgression lines in a remarkably short span of two years [20]. Early-generation selection was used to enhance the population with desirable allelic combinations for multiple traits in wheat breeding. This innovative multi-trait phenotyping method incorporates root system architecture, leaf rust resistance, and plant height for swift selection of favorable allelic combinations. This approach aligns seamlessly with speed breeding, enabling up to four consecutive screens each year and significantly boosting breeding efficiency [39].
5.4. Enhancing Research across Disciplines
Speed breeding’s unique capabilities transcend traditional crop improvement and genetic research. It finds applications in diverse fields, including ecology, physiology, and agronomy [53]. Researchers can use these techniques to speed up experiments and gather data more efficiently. This accelerates progress in the understanding of plant responses to environmental stressors, optimizing cultivation practices, and exploring plant physiology [12]. The rapid generation cycles allow for quicker hypothesis testing and the collection of critical data, enabling researchers to make informed decisions and develop innovative solutions to address global agricultural challenges [7,22].
Developing new and improved breeding cultivars for optimal yield in field environments is a complex task, often hindered by the intricate interplay between genetic traits and environmental conditions. However, the integration of systems biology with speed breeding techniques has emerged as a powerful solution to this challenge. Systems biology provides a holistic understanding of plant growth processes and, when coupled with speed breeding, offers the precise control over-growth conditions through computational modeling. This synergy allows researchers to fine-tune environmental factors, such as light intensity, to accelerate plant growth while maintaining optimal conditions. Additionally, systems biology unravels the intricate genetic regulatory networks governing plant development, enabling the identification of key genes like HIGH RESPONSE TO PHOTOPERIOD (HR) and LATE FLOWERING (LF) in crops like winter peas, facilitating the precise manipulation of critical developmental stages like flowering [98]. Predictive models further enhance the breeding process by estimating the effects of genetic modifications on crop traits, streamlining the design of plants with desired characteristics. Cloning flowering key genes in peas, such as LF and LATE1, has provided valuable insights into the molecular mechanisms governing flowering [99,100].
Furthermore, SB supports advancements in high-throughput phenotyping. The rapid generation of diverse plant populations allows for extensive phenotypic screening, enabling the identification of superior genotypes with precision. This enhances the efficiency of breeding programs, leading to the accelerated release of improved crop varieties.
As we look to the future, SB holds promise in a range of emerging applications. From harnessing the potential of gene editing technologies to integrating genotyping and genomic selection, the synergy between SB and cutting-edge methodologies is driving innovation. This collaborative approach not only leverages the strengths of each technique but also establishes a comprehensive framework for modern plant breeding and crop enhancement.
6. Limitations Associated with SB
Although SB is a potent technique for boosting the pace of genetic gain in various plant species, it has several limitations (Table 3). One major obstacle is the lack of advanced controlled environment facilities, which can raise the cost of establishing controlled environment that is ideal for the target species’ rapid cycling. In addition, maintaining a steady supply of electricity and maintaining a comfortable temperature can be difficult, especially in resource-poor nations with inadequate infrastructures and continual financial help from foreign organizations [9].
Due to the rigorous growth circumstances, SB can lead to a low seed output and genotypic variations in plant species [15]. These variations may affect the stability and uniformity of crops, raising concerns about the consistency of crop performance across different environments. Additionally, an excessive photoperiod can slow down plant growth and cause stress hormone levels to rise [73]. Flowering in Amaranth, rice, and soybean treated with 10 hr. photoperiods using blue-enriched and far-red deprived light had no impact on soybean flowering but in some genotypes of amaranth and rice, flowering time was reduced by 20 and 10 days, respectively. However, some rice genotypes demonstrated flowering variations due to light intensity [34]. Striking the right balance between accelerating growth and avoiding stress-induced responses is a nuanced challenge that breeders must navigate. To achieve an accurate trait expression, field crop phenotyping must be validated because phenotyping in controlled environments might be biased. Due to crossover interactions and differences in growth environments, phenotypic consistency in oats has been restricted in terms of plant height and flowering time [84]. Without careful management, growing plants beyond their physiological limits can be harmful and result in catastrophic losses of priceless breeding stock. Wheat, durum wheat, barley, and Brachypodium distachyon all showed faster growth and blooming under extended photoperiod conditions of 22 h of light and 2 h of darkness, finishing their life cycles in just half the time as they would under natural conditions. However, under these circumstances, the number of seeds per spike of wheat decreased [63]. To prevent genetic loss, mitigation measures include adjusting environmental factors, establishing temperature and photoperiod saturation limits, and preserving backup seeds from everyone. The control of pests and diseases, as well as the tracking of individuals for gene-discovery purposes, are other significant difficulties in SB [101].
SB in the public sector is hindered by a lack of skilled plant breeders and technologists in developing nations, staff turnover, and inadequate legal and administrative structures. Developing nations need to invest in plant breeding education, research, and employee retention to support long-term crop improvement projects and scientific advancements [79]. SB requires advanced infrastructure to regulate environmental factors, which is lacking in many developing countries due to limited institutional support and a lack of specialized equipment. In developing countries, agriculture accounts for the largest share of water usage, representing 70–90% of all water withdrawals. The study of agricultural water is comprehensive, encompassing various related issues such as the connection between agricultural water use, food security [102], and water management in the agricultural sector [103]. It has been reported that farmers in low-income developing countries receive limited government support, with many African countries allocating only 3% of their budget to agriculture, despite its significant role in employment and economic activity [104]. Collaboration between national and regional organizations is needed to build infrastructure and encourage knowledge exchange. The cost of infrastructure can be reduced by using innovative local technology, such as retrofitted shipping containers with solar-powered temperature and lighting controls [81]. Atlanta-based startup PodPonics and the indoor farming company Cropbox are at the forefront of indoor urban agriculture innovation. PodPonics converts old shipping containers into hydroponic farms, producing an acre’s worth of produce in just 320 square feet with minimal water use and no pesticides. Cropbox specializes in the year-round cultivation of leafy greens and herbs, boasting 8 annual crop cycles, guaranteed production, high yields, and resource efficiency with 80% less fertilizer and 27,000 gallons of water annually [105,106]. Both initiatives offer fresh, sustainable, and efficient solutions to indoor urban food production [12]. The lack of reliable water and electricity supply is a major challenge for SB in public plant breeding programs in developing countries [107]. Alternative solutions, such as semi-controlled field-based systems and the use of sustainable solar power should be explored. Solar-powered temperature controls could be a cost-effective solution to maintain a comfortable temperature [59]. The lack of advanced controlled environment facilities and skilled personnel in developing countries decreases genetic gain through SB by limiting the ability to create ideal growth conditions, resulting in low seed output, genotypic variations, and potential genetic loss, and hindering the development of skilled personnel needed to support long-term crop improvement projects.
7. Conclusions
SB emerges as a promising solution to address the pressing challenges of food security in the context of a growing global population and climate uncertainties. This review has shed light on the historical development of SB and its applications, selection methods, advantages, and limitations. The journey of SB, from early experiments with artificial lighting to the utilization of cutting-edge technologies like LED lights and solar power, highlights its potential for revolutionizing crop breeding. By fine-tuning environmental variables such as photoperiod, temperature, soil moisture, and population density, SB enables the rapid generation of crops, significantly shortening traditional breeding cycles. The benefits of SB are substantial. It not only accelerates the development of crops with desired traits but also contributes to the vital goals of food security and sustainability. Speed breeding’s capacity to create diverse mapping populations and promote GM and streamline trait stacking opens unprecedented avenues for enhancing crop quality and resilience. Additionally, it facilitates interdisciplinary research, empowering researchers to address critical agricultural and plant physiology challenges. Nonetheless, SB presents its share of challenges. The demand for advanced controlled environment facilities and a skilled workforce can be daunting, particularly in developing nations. Managing genotypic variations and navigating ethical considerations underscore the importance of responsible SB practices. Furthermore, with the involvement of organizations from different fields, SB has evolved as a competent tool to global food demand under changing environmental conditions.
Author Contributions
J.P. wrote the draft manuscript. S.J., V.N.M. and X.W. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Andrus, C.F. Plant breeding systems. Euphytica 1963, 12, 205–228. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirb.-Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Bailey, L.H. Some Preliminary Studies of the Influence of the Electric Arc Lamp upon Greenhouse Plants; Cornell University: Ithaca, NY, USA, 1891; Volume 30. [Google Scholar]
- Begna, T. Speed breeding to accelerate crop improvement. Int. J. Agric. Sc. Food Technol. 2022, 8, 178–186. [Google Scholar] [CrossRef]
- Bermejo, C.; Gatti, I.; Cointry, E. In vitro embryo culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell Tiss. Organ. Cult. 2016, 127, 585–590. [Google Scholar] [CrossRef]
- Bhatta, M.; Sandro, P.; Smith, M.R.; Delaney, O.; Voss-Fels, K.P.; Gutierrez, L.; Hickey, L.T. Need for speed: Manipulating plant growth to accelerate breeding cycles. Curr. Opin. Plant Biol. 2021, 60, 101986. [Google Scholar] [CrossRef]
- Bonea, D. Speed breeding and its importance for the improvement of agricultural crops. AAMC 2022, 52, 59–66. [Google Scholar] [CrossRef]
- Bugbee, B.; Koerner, G. Yield comparisons and unique characteristics of the dwarf wheat cultivar ‘USU-Apogee’. Adv. Space Res. 1997, 20, 1891–1894. [Google Scholar] [CrossRef]
- Byerlee, D.; Fischer, K. Accessing modern science: Policy and institutional options for agricultural biotechnology in developing countries. World Dev. 2002, 30, 931–948. [Google Scholar] [CrossRef]
- Cazzola, F.; Bermejo, C.J.; Guindon, M.F.; Cointry, E. Speed breeding in pea (Pisum sativum L.), an efficient and simple system to accelerate breeding programs. Euphytica 2020, 216, 178. [Google Scholar] [CrossRef]
- Chiurugwi, T.; Kemp, S.; Powell, W.; Hickey, L.T. Speed breeding orphan crops. Theor. Appl. Genet. 2019, 132, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Phil. Trans. R. Soc. B 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Ferrie, A.M.R. Doubled haploid production in nutraceutical species: A review. Euphytica 2007, 158, 347–357. [Google Scholar] [CrossRef]
- Gaba, Y.; Pareek, A.; Singla-Pareek, S.L. Raising climate-resilient crops: Journey from the conventional breeding to new breeding approaches. Curr. Genom. 2021, 22, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef]
- Gilland, B. World population and food supply. Food Policy 2002, 27, 47–63. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Hickey, L.T.; Germán, S.E.; Pereyra, S.A.; Diaz, J.E.; Ziems, L.A.; Fowler, R.A.; Platz, G.J.; Franckowiak, J.D.; Dieters, M.J. Speed breeding for multiple disease resistance in barley. Euphytica 2017, 213, 64. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M.; et al. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Mahrukh; Nisa, R.T.; Zaid, A.; Mushtaq, M. The utilization of speed breeding and genome editing to achieve zero hunger. In Sustainable Agriculture in the Era of the OMICs Revolution; Prakash, C.S., Fiaz, S., Nadeem, M.A., Baloch, F.S., Qayyum, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–15. ISBN 978-3-031-15567-3. [Google Scholar]
- Jacquier, N.M.; Gilles, L.M.; Martinant, J.-P.; Rogowsky, P.M.; Widiez, T. Maize in Planta haploid inducer lines: A cornerstone for doubled haploid technology. In Doubled Haploid Technology; Volume 2: Hot Topics, Apiaceae, Brassicaceae, Solanaceae; Humana: New York, NY, USA, 2021; Volume 2288, pp. 25–48. [Google Scholar] [CrossRef]
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.; Barta, D.; Ignatius, R.; Martin, T. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef]
- Hoenecke, M.; Bula, R.; Tibbitts, T. Importance of blue photon levels for lettuce seedlings grown under red-light-emitting diodes. HortScience 1992, 27, 427–430. [Google Scholar] [CrossRef]
- Zhou, W. Advanced ASTROCULTURETM Plant Growth Unit: Capabilities and Performances; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2005. [Google Scholar] [CrossRef]
- Morrow, R.C.; Duffie, N.A.; Tibbitts, T.W.; Bula, R.J.; Barta, D.J.; Ming, D.W.; Wheeler, R.M.; Porterfield, D.M. Plant Response in the ASTROCULTURETM Flight Experiment Unit; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1995. [Google Scholar] [CrossRef]
- Link, B.; Durst, S.; Zhou, W.; Stankovic, B. Seed-to-seed growth of Arabidopsis thaliana on the International Space Station. Adv. Space Res. 2003, 31, 2237–2243. [Google Scholar] [CrossRef]
- Croxdale, J.; Cook, M.; Tibbitts, T.W.; Brown, C.S.; Wheeler, R.M. Structure of Potato tubers formed during spaceflight. J. Exp. Bot. 1997, 48, 2037–2043. [Google Scholar] [CrossRef]
- Wu, B.-S.; Mansoori, M.; Trumpler, K.; Addo, P.W.; MacPherson, S.; Lefsrud, M. Effect of amber (595 Nm) light supplemented with narrow blue (430 Nm) light on tomato biomass. Plants 2023, 12, 2457. [Google Scholar] [CrossRef]
- Guo, X.; Xue, X.; Chen, L.; Li, J.; Wang, Z.; Zhang, Y. Effects of LEDs light spectra on the growth, yield, and quality of winter wheat (Triticum aestivum L.) cultured in plant factory. J. Plant Growth Regul. 2023, 42, 2530–2544. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Bahuguna, R.N.; Djanaguiraman, M.; Gamuyao, R.; Prasad, P.V.V.; Craufurd, P.Q. Implications of high temperature and elevated CO2 on flowering time in plants. Front. Plant Sci. 2016, 7, 913. [Google Scholar] [CrossRef]
- Jähne, F.; Hahn, V.; Würschum, T.; Leiser, W.L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342. [Google Scholar] [CrossRef]
- Croser, J.S.; Pazos-Navarro, M.; Bennett, R.G.; Tschirren, S.; Edwards, K.; Erskine, W.; Creasy, R.; Ribalta, F.M. Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell Tiss. Organ. Cult. 2016, 127, 591–599. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Wright, G.C.; Dieters, M.J.; George, D.L.; Hunter, M.N.; Tatnell, J.R.; Fleischfresser, D.B. Development and application of speed breeding technologies in a commercial peanut breeding program. Peanut Sci. 2013, 40, 107–114. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, K.; Shah, M.P.; Singh, A.K.; Kumar, A.; Kumar, Y. Application of omics technologies for microbial community structure and function analysis in contaminated environment. In Wastewater Treatment: Cutting-Edge Molecular Tools, Techniques and Applied Aspects; Shah, M.P., Sarkar, A., Mandal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–40. [Google Scholar] [CrossRef]
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both multi-segment light intensity and extended photoperiod lighting strategies, with the same daily light integral, promoted Lactuca sativa L. growth and photosynthesis. Agronomy 2019, 9, 857. [Google Scholar] [CrossRef]
- Alahmad, S.; Dinglasan, E.; Leung, K.M.; Riaz, A.; Derbal, N.; Voss-Fels, K.P.; Able, J.A.; Bassi, F.M.; Christopher, J.; Hickey, L.T. Speed breeding for multiple quantitative traits in durum wheat. Plant Methods 2018, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Gudi, S.; Kumar, P.; Singh, S.; Tanin, M.J.; Sharma, A. Strategies for accelerating genetic gains in crop plants: Special focus on speed breeding. Physiol. Mol. Biol. Plants 2022, 28, 1921–1938. [Google Scholar] [CrossRef] [PubMed]
- Shariatpanahi, M.E.; Niazian, M.; Ahmadi, B. Methods for chromosome doubling. Methods Mol. Biol. 2021, 2287, 127–148. [Google Scholar]
- Melchinger, A.E.; Molenaar, W.S.; Mirdita, V.; Schipprack, W. Colchicine alternatives for chromosome doubling in maize haploids for doubled-haploid production. Crop Sci. 2016, 56, 559–569. [Google Scholar] [CrossRef]
- Watts, A.; Sankaranarayanan, S.; Raipuria, R.K.; Watts, A. Production and application of doubled haploid in brassica improvement. In Brassica Improvement; Wani, S., Thakur, A., Jeshima Khan, Y., Eds.; Springer: Cham, Switzerland, 2020; pp. 67–84. [Google Scholar] [CrossRef]
- Croser, J.S.; Lülsdorf, M.M.; Davies, P.A.; Clarke, H.J.; Bayliss, K.L.; Mallikarjuna, N.; Siddique, K.H.M. Toward doubled haploid production in the Fabaceae: Progress, constraints, and opportunities. Crit. Rev. Plant Sci. 2006, 25, 139–157. [Google Scholar] [CrossRef]
- Forster, B.P.; Heberle-Bors, E.; Kasha, K.J.; Touraev, A. The resurgence of haploids in higher plants. Trends Plant Sci. 2007, 12, 368–375. [Google Scholar] [CrossRef]
- Seguí-Simarro, J.M.; Corral-Martínez, P.; Parra-Vega, V.; González-García, B. Androgenesis in recalcitrant Solanaceous crops. Plant Cell Rep. 2011, 30, 765–778. [Google Scholar] [CrossRef]
- Dong, Y.-Q.; Zhao, W.-X.; Li, X.-H.; Liu, X.-C.; Gao, N.-N.; Huang, J.-H.; Wang, W.-Y.; Xu, X.-L.; Tang, Z.-H. Androgenesis, gynogenesis, and parthenogenesis haploids in cucurbit species. Plant Cell Rep. 2016, 35, 1991–2019. [Google Scholar] [CrossRef]
- Massel, K.; Lam, Y.; Wong, A.C.; Hickey, L.T.; Borrell, A.K.; Godwin, I.D. Hotter, Drier, CRISPR: The latest edit on climate change. Theor. Appl. Genet. 2021, 134, 1691–1709. [Google Scholar] [CrossRef]
- Mobini, S.H.; Warkentin, T.D. A simple and efficient method of in vivo rapid generation technology in pea (Pisum sativum L.). In Vitro Cell Dev. Biol.-Plant 2016, 52, 530–536. [Google Scholar] [CrossRef]
- Naqvi, R.Z.; Siddiqui, H.A.; Mahmood, M.A.; Najeebullah, S.; Ehsan, A.; Azhar, M.; Farooq, M.; Amin, I.; Asad, S.; Mukhtar, Z.; et al. Smart breeding approaches in post-genomics era for developing climate-resilient food crops. Front. Plant Sci. 2022, 13, 972164. [Google Scholar] [CrossRef]
- Pandey, S.; Singh, A.; Parida, S.K.; Prasad, M. Combining speed breeding with traditional and genomics-assisted breeding for crop improvement. Plant Breed. 2022, 141, 301–313. [Google Scholar] [CrossRef]
- Pfeiffer, N.E. Microchemical and morphological studies of effect of light on plants. Bot. Gaz. 1926, 81, 173–195. [Google Scholar] [CrossRef]
- Radha, T.; Mathew, L. Fruit Crops; New India Publishing: New Delhi, India, 2007; Volume 3. [Google Scholar]
- Rajan, S.; Singh, G. Approaches and Strategies for Precision Farming in Mango. In Precision Farming in Horticulture; Singh, H.P., Singh, G., Samuel, J.C., Pathak, R.K., Eds.; Central Institute of Subtropical Horticulture: Lucknow, India, 2003; pp. 124–144. [Google Scholar]
- Shamshiri, R.R.; Kalantari, F.; Ting, K.C.; Thorp, K.R.; Hameed, I.A.; Weltzien, C.; Ahmad, D.; Shad, Z.M. Advances in greenhouse automation and controlled environment agriculture: A transition to plant factories and urban agriculture. Int. J. Agric. Biol. Eng. 2018, 11, 1–22. [Google Scholar] [CrossRef]
- Riaz, A.; Athiyannan, N.; Periyannan, S.; Afanasenko, O.; Mitrofanova, O.; Aitken, E.A.B.; Lagudah, E.; Hickey, L.T. Mining Vavilov’s treasure chest of wheat diversity for adult plant resistance to Puccinia triticina. Plant Dis. 2017, 101, 317–323. [Google Scholar] [CrossRef]
- Ribalta, F.M.; Pazos-Navarro, M.; Nelson, K.; Edwards, K.; Ross, J.J.; Bennett, R.G.; Munday, C.; Erskine, W.; Ochatt, S.J.; Croser, J.S. Precocious floral initiation and identification of exact timing of embryo physiological maturity facilitate germination of immature seeds to truncate the lifecycle of pea. Plant Growth Regul. 2017, 81, 345–353. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Samantara, K.; Bohra, A.; Mohapatra, S.R.; Prihatini, R.; Asibe, F.; Singh, L.; Reyes, V.P.; Tiwari, A.; Maurya, A.K.; Croser, J.S.; et al. Breeding more crops in less time: A perspective on speed breeding. Biology 2022, 11, 275. [Google Scholar] [CrossRef]
- Tripp, R.; Louwaars, N.; Eaton, D. Plant variety protection in developing countries. a report from the field. Food Policy 2007, 32, 354–371. [Google Scholar] [CrossRef]
- Samineni, S.; Sen, M.; Sajja, S.B.; Gaur, P.M. Rapid generation advance (RGA) in Chickpea to produce up to seven generations per year and enable speed breeding. Crop J. 2020, 8, 164–169. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, A.; Dhakte, P.; Raturi, G.; Vishwakarma, G.; Barbadikar, K.M.; Das, B.K.; Shivaraj, S.M.; Sonah, H.; Deshmukh, R. Speed breeding opportunities and challenges for crop improvement. J. Plant Growth Regul. 2023, 42, 46–59. [Google Scholar] [CrossRef]
- Slafer, G.A.; Rawson, H.M. Phyllochron in wheat as affected by photoperiod under two temperature regimes. Funct. Plant Biol. 1997, 24, 151–158. [Google Scholar] [CrossRef]
- Shaw, H.J. The Consuming Geographies of Food: Diet, Food Deserts and Obesity; Routledge: London, UK, 2014; p. 210. [Google Scholar]
- Sinha, P.; Singh, V.K.; Bohra, A.; Kumar, A.; Reif, J.C.; Varshney, R.K. Genomics and breeding innovations for enhancing genetic gain for climate resilience and nutrition traits. Theor. Appl. Genet. 2021, 134, 1829–1843. [Google Scholar] [CrossRef]
- Stam, P. Construction of Integrated genetic linkage maps by means of a new computer package: Join Map. Plant J. 1993, 3, 739–744. [Google Scholar] [CrossRef]
- Steinwand, M.A.; Ronald, P.C. Crop biotechnology and the future of food. Nat. Food 2020, 1, 273–283. [Google Scholar] [CrossRef]
- Stetter, M.G.; Zeitler, L.; Steinhaus, A.; Kroener, K.; Biljecki, M.; Schmid, K.J. Crossing methods and cultivation conditions for rapid production of segregating populations in three grain Amaranth species. Front. Plant Sci. 2016, 7, 816. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.J.; Biswas, S.; Tsakirpaloglou, N.; Septiningsih, E.M. Functional allele validation by gene editing to leverage the wealth of genetic resources for crop improvement. Int. J. Mol. Sci. 2022, 23, 6565. [Google Scholar] [CrossRef]
- Schneider, K. Mapping populations and principles of genetic mapping. In The Handbook of Plant Genome Mapping: Genetic and Physical Mapping; Meksem, K., Kahl, G., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–21. [Google Scholar] [CrossRef]
- McClung, C.R.; Lou, P.; Hermand, V.; Kim, J.A. The importance of ambient temperature to growth and the induction of flowering. Front. Plant Sci. 2016, 7, 1266. [Google Scholar] [CrossRef]
- Wanga, M.A.; Shimelis, H.; Mashilo, J.; Laing, M.D. Opportunities and challenges of speed breeding: A review. Plant Breed. 2021, 140, 185–194. [Google Scholar] [CrossRef]
- Dupuis, I.; Dumas, C. Influence of temperature stress on in vitro fertilization and heat shock protein synthesis in maize (Zea mays L.) reproductive tissues. Plant Physiol. 1990, 94, 665–670. [Google Scholar] [CrossRef]
- Kim, H.Y.; Horie, T.; Nakagawa, H.; Wada, K. Effects of elevated CO2 concentration and high temperature on growth and yield of rice: II. The effect on yield and its components of Akihikari rice. Jpn. J. Crop Sci. 1996, 65, 644–651. [Google Scholar] [CrossRef]
- Vadez, V.; Hash, T.; Bidinger, F.R.; Kholova, J. Phenotyping pearl millet for adaptation to drought. Front. Physiol. 2012, 3, 386. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early Flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Hatta, M.A.M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding: A powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, P.; Wang, H.; Lu, Z.; Liu, C.J.; Liu, H.; Yan, G.J. How to advance up to seven generations of canola (Brassica napus L.) per annum for the production of pure line populations? Euphytica 2016, 209, 113–119. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, H.B.; Chen, G.D.; Yan, G.J.; Liu, C.J. A procedure allowing up to eight generations of wheat and nine generations of barley per annum. Euphytica 2013, 191, 311–316. [Google Scholar] [CrossRef]
- Schoen, A.; Wallace, S.; Holbert, M.F.; Brown-Guidera, G.; Harrison, S.; Murphy, P.; Sanantonio, N.; Van Sanford, D.; Boyles, R.; Mergoum, M.; et al. Reducing the generation time in winter wheat cultivars using speed breeding. Crop Sci. 2023, 63, 2079–2090. [Google Scholar] [CrossRef]
- Mobini, S.; Khazaei, H.; Warkentin, T.D.; Vandenberg, A. Shortening the generation cycle in Faba bean (Vicia faba) by application of cytokinin and cold stress to assist speed breeding. Plant Breed. 2020, 139, 1181–1189. [Google Scholar] [CrossRef]
- Lulsdorf, M.M.; Banniza, S. Rapid Generation cycling of an F2 population derived from a cross between Lens vulinaris Medik. and Lens ervoides (Brign.) grande after aphanomyces root rot selection. Plant Breed. 2018, 137, 486–491. [Google Scholar] [CrossRef]
- Liu, H.; Zwer, P.; Wang, H.; Liu, C.; Lu, Z.; Wang, Y.; Yan, G. A fast generation cycling system for oat and triticale breeding. Plant Breed. 2016, 135, 574–579. [Google Scholar] [CrossRef]
- González-Barrios, P.; Bhatta, M.; Halley, M.; Sandro, P.; Gutiérrez, L. Speed breeding and early panicle harvest accelerates oat (Avena sativa L.) breeding cycles. Crop Sci. 2021, 61, 320–330. [Google Scholar] [CrossRef]
- Edet, O.U.; Ishii, T. Cowpea speed breeding using regulated growth chamber conditions and seeds of oven-dried immature pods potentially accommodates eight generations per year. Plant Methods 2022, 18, 106. [Google Scholar] [CrossRef]
- Saxena, K.; Saxena, R.; Hickey, L.; Varshney, R. Can a speed breeding approach accelerate genetic gain in pigeonpea? Euphytica 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation efforts will not fully alleviate the increase in water scarcity occurrence probability in wheat-producing areas. Sci. Adv. 2019, 5, eaau2406. [Google Scholar] [CrossRef]
- Uğur, T.; GÖREN, H.K. Speed breeding: An innovative approach for accelerated genetic improvement in agricultural crops and integrating with molecular-based approaches. In Pioneer and Contemporary Studies in Agriculture, Forest and Water Issues; Duvar Yayınları: Izmir, Turkey, 2023; pp. 241–264. [Google Scholar] [CrossRef]
- Christopher, J.; Richard, C.; Chenu, K.; Christopher, M.; Borrell, A.; Hickey, L. Integrating rapid phenotyping and speed breeding to improve stay-green and root adaptation of wheat in changing, water-limited, Australian environments. Procedia Environ. Sci. 2015, 29, 175–176. [Google Scholar] [CrossRef]
- Rana, M.M.; Takamatsu, T.; Baslam, M.; Kaneko, K.; Itoh, K.; Harada, N.; Sugiyama, T.; Ohnishi, T.; Kinoshita, T.; Takagi, H.; et al. Salt tolerance improvement in rice through efficient SNP marker-assisted selection coupled with speed-breeding. Int. J. Mol. Sci. 2019, 20, 2585. [Google Scholar] [CrossRef]
- Varshney, R.K.; Bohra, A.; Yu, J.; Graner, A.; Zhang, Q.; Sorrells, M.E. Designing future crops: Genomics-assisted breeding comes of age. Trends Plant Sci. 2021, 26, 631–649. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Cooper, M.; Hayes, B.J. Accelerating crop genetic gains with genomic selection. Theor. Appl. Genet. 2019, 132, 669–686. [Google Scholar] [CrossRef]
- Waltz, E. CRISPR-edited crops free to enter market, skip regulation. Nat. Biotechnol. 2016, 34, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Eş, I.; Gavahian, M.; Marti-Quijal, F.J.; Lorenzo, J.M.; Khaneghah, A.M.; Tsatsanis, C.; Kampranis, S.C.; Barba, F.J. The application of the CRISPR-Cas9 Genome editing machinery in food and agricultural science: Current status, future perspectives, and associated challenges. Biotechnol. Adv. 2019, 37, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, Y.; Qin, R.; Li, H.; Qiu, C.; Li, L.; Wei, P.; Yang, J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genom. 2016, 43, 529–532. [Google Scholar] [CrossRef]
- Shailani, A.; Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Stacking for future: Pyramiding genes to improve drought and salinity tolerance in rice. Physiol. Plant 2021, 172, 1352–1362. [Google Scholar] [CrossRef]
- Wenden, B.; Rameau, C. Systems biology for plant breeding: The example of flowering time in pea. Comptes Rendus Biol. 2009, 332, 998–1006. [Google Scholar] [CrossRef]
- Foucher, F.; Morin, J.; Courtiade, J.; Cadioux, S.; Ellis, N.; Banfield, M.J.; Rameau, C. Determinate and Late Flowering are two Terminal Flower1/Centroradialis homologs that control two distinct phases of flowering initiation and development in pea. Plant Cell 2003, 15, 2742–2754. [Google Scholar] [CrossRef] [PubMed]
- Hecht, V.; Knowles, C.L.; Vander Schoor, J.K.; Liew, L.C.; Jones, S.E.; Lambert, M.J.; Weller, J.L. Pea LATE BLOOMER1 is a GIGANTEA ortholog with roles in photoperiodic flowering, deetiolation, and transcriptional regulation of circadian clock gene homologs. Plant Physiol. 2007, 144, 648–661. [Google Scholar] [CrossRef]
- Warnasooriya, S.N.; Brutnell, T.P. Enhancing the productivity of grasses under high-density planting by engineering light responses: From model systems to feedstocks. J. Exp. Bot. 2014, 65, 2825–2834. [Google Scholar] [CrossRef]
- FAO. Water at a Glance—The Relationship between Water, Agriculture, Food Security and Poverty; FAO: Rome, Italy, 2008. [Google Scholar]
- Srivastav, A.L.; Dhyani, R.; Ranjan, M.; Madhav, S.; Sillanpää, M. Climate-resilient strategies for sustainable management of water resources and agriculture. Environ. Sci. Pollut. Res. 2021, 28, 41576–41595. [Google Scholar] [CrossRef] [PubMed]
- United Nations Conference on Trade and Development May. 2011. Available online: https://unctad.org/system/files/official-document/tdr2011_en.pdf (accessed on 10 September 2023).
- Saenz, A. Transforming Shipping Containers into Local Farms—Podponics Brings Produce to the City. Singularity Hub. 2011. Available online: https://singularityhub.com/2011/08/30/transforming-shipping-containers-into-local-farms-podponics-brings-produce-to-the-city/#sm.000100v3z66e9fdyt062k15j3s238 (accessed on 10 September 2023).
- CropBox. Available online: https://cropbox.co/ (accessed on 10 September 2023).
- Ribaut, J.; De Vicente, M.; Delannay, X. Molecular breeding in developing countries: Challenges and perspectives. Curr. Opin. Plant Biol. 2010, 13, 213–218. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).