The Potential of Cold Plasma-Based Seed Treatments in Legume–Rhizobia Symbiotic Nitrogen Fixation: A Review
Abstract
1. Introduction
2. Fundamentals of Cold Plasma Technology
3. Plasma Generation Devices
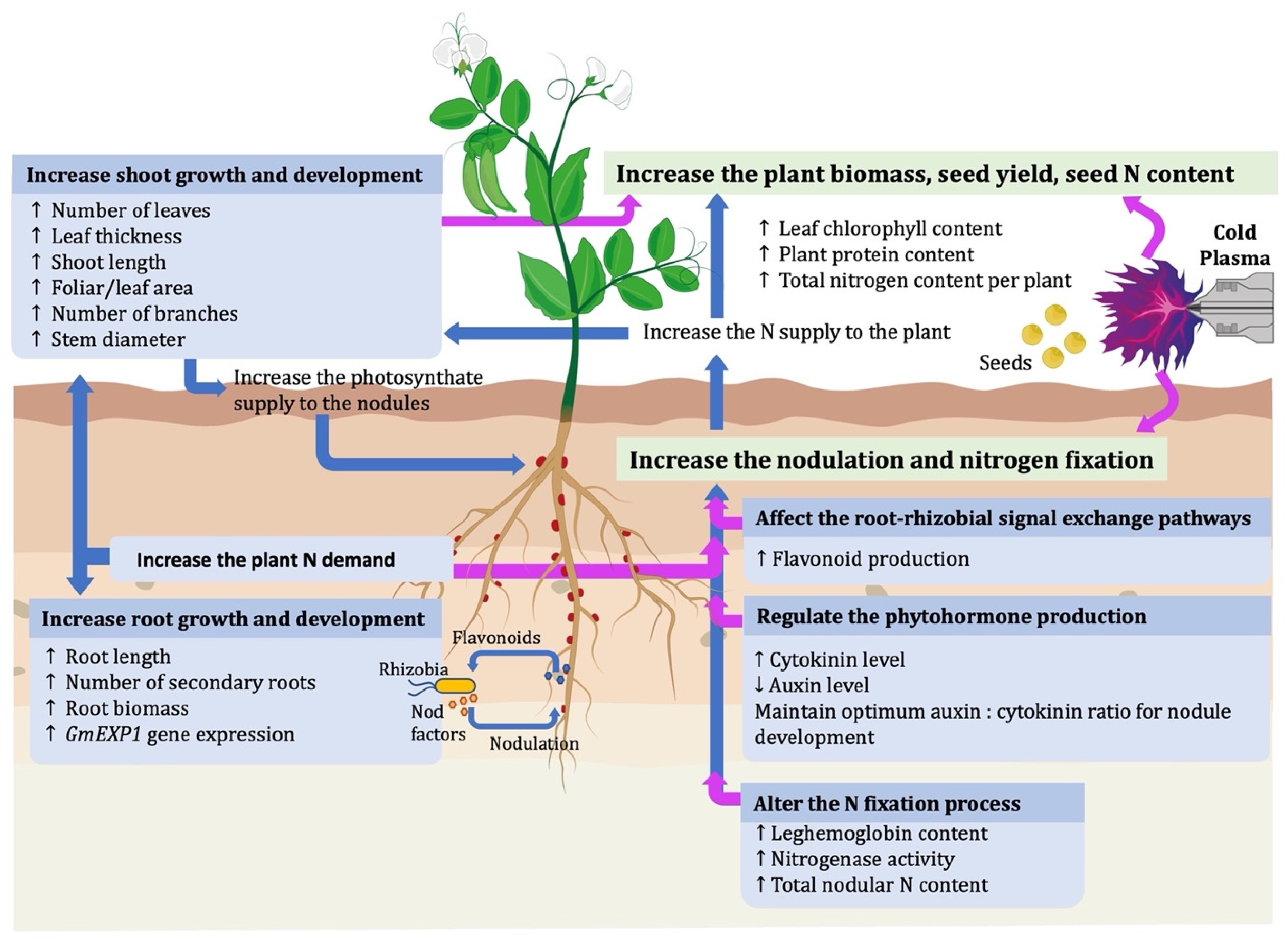
4. Effect of Cold Plasma on Nodulation and Biological Nitrogen Fixation in Legumes
5. Effect of Cold Plasma in Rhizobia Legume Roots Invasion
6. CP Induces Root/Shoot Growth
6.1. The Impact of CP on Seed Germination
6.2. The Impact of CP on Root Growth
6.3. The Impact of CP on Shoot Growth and Seed Yield
7. CP Alters the Phytohormone Production That Regulates Nodule Formation
8. CP Induces Leghemoglobin Production and Nitrogenase Activity
9. Potential Challenges of the Plasma Seed Treatments and Future Work
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The Rules of Engagement in the Legume-Rhizobial Symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, M.S.; McElroy, M.S.; Chapagain, T.; Papadopoulos, Y.A.; Raizada, M.N. Belowground Nitrogen Transfer from Legumes to Non-Legumes under Managed Herbaceous Cropping Systems. A Review. Agron. Sustain. Dev. 2016, 36, 58–74. [Google Scholar] [CrossRef]
- Pirhofer-Walzl, K.; Rasmussen, J.; Høgh-Jensen, H.; Eriksen, J.; Søegaard, K.; Rasmussen, J. Nitrogen Transfer from Forage Legumes to Nine Neighbouring Plants in a Multi-Species Grassland. Plant Soil 2012, 350, 71–84. [Google Scholar] [CrossRef]
- Yu, T.; Zhuang, Q. Modeling Biological Nitrogen Fixation in Global Natural Terrestrial Ecosystems. Biogeosciences 2020, 17, 3643–3657. [Google Scholar] [CrossRef]
- Herridge, D.F.; Giller, K.E.; Jensen, E.S.; Peoples, M.B. Quantifying Country-to-Global Scale Nitrogen Fixation for Grain Legumes II. Coefficients, Templates and Estimates for Soybean, Groundnut and Pulses. Plant Soil 2022, 474, 1–15. [Google Scholar] [CrossRef]
- Fritschi, F.B.; Ray, J.D. Soybean Leaf Nitrogen, Chlorophyll Content, and Chlorophyll a/b Ratio. Photosynthetica 2007, 45, 92–98. [Google Scholar] [CrossRef]
- Lea, P.J.; Azevedo, R.A. Nitrogen Use Efficiency. 2. Amino Acid Metabolism. Ann. Appl. Biol. 2007, 151, 269–275. [Google Scholar] [CrossRef]
- Ohyama, T. Nitrogen as a Major Essential Element of Plants. Nitrogen Assim. Plants 2010, 37, 1–17. [Google Scholar]
- Razon, L.F. Life Cycle Analysis of an Alternative to the Haber-Bosch Process: Non-Renewable Energy Usage and Global Warming Potential of Liquid Ammonia from Cyanobacteria. Environ. Prog. Sustain. Energy 2014, 33, 618–624. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and Future Role of Haber-Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Janzen, H.H.; Beauchemin, K.A.; Bruinsma, Y.; Campbell, C.A.; Desjardins, R.L.; Ellert, B.H.; Smith, E.G. The Fate of Nitrogen in Agroecosystems: An Illustration Using Canadian Estimates. Nutr. Cycl. Agroecosystems 2003, 67, 85–102. [Google Scholar] [CrossRef]
- Ladha, J.K.; Tirol-Padre, A.; Reddy, C.K.; Cassman, K.G.; Verma, S.; Powlson, D.S.; Van Kessel, C.; De Richter, D.B.; Chakraborty, D.; Pathak, H. Global Nitrogen Budgets in Cereals: A 50-Year Assessment for Maize, Rice, and Wheat Production Systems. Sci. Rep. 2016, 6, 19355. [Google Scholar] [CrossRef] [PubMed]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Liu, J.; Yu, X.; Qin, Q.; Dinkins, R.D.; Zhu, H. The Impacts of Domestication and Breeding on Nitrogen Fixation Symbiosis in Legumes. Front. Genet. 2020, 11, 00973. [Google Scholar] [CrossRef] [PubMed]
- Vlk, D.; Trněný, O.; Řepková, J. Genes Associated with Biological Nitrogen Fixation Efficiency Identified Using RNA Sequencing in Red Clover (Trifolium Pratense L.). Life 2022, 12, 1975. [Google Scholar] [CrossRef]
- Mendoza-Suárez, M.; Andersen, S.U.; Poole, P.S.; Sánchez-Cañizares, C. Competition, Nodule Occupancy, and Persistence of Inoculant Strains: Key Factors in the Rhizobium-Legume Symbioses. Front. Plant Sci. 2021, 12, 690567. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Hungria, M.; Prior, S.A.; Moura, M.C.; Poggere, G.; Motta, A.C.V. Improving Yield and Health of Legume Crops via Co-Inoculation with Rhizobia and Trichoderma: A Global Meta-Analysis. Appl. Soil Ecol. 2022, 176, 104493. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Raizada, M.N. Challenges in Using Precision Agriculture to Optimize Symbiotic Nitrogen Fixation in Legumes: Progress, Limitations, and Future Improvements Needed in Diagnostic Testing. Agronomy 2018, 8, 78. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic Nitrogen Fixation and the Challenges to Its Extension to Nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef]
- Geddes, B.A.; Paramasivan, P.; Joffrin, A.; Thompson, A.L.; Christensen, K.; Jorrin, B.; Brett, P.; Conway, S.J.; Oldroyd, G.E.D.; Poole, P.S. Engineering Transkingdom Signalling in Plants to Control Gene Expression in Rhizosphere Bacteria. Nat. Commun. 2019, 10, 3430. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Pauzaite, G.; Naucienė, Z.; Zukiene, R.; Degutyte-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V. Seed Treatment with Cold Plasma and Electromagnetic Field Induces Changes in Red Clover Root Growth Dynamics, Flavonoid Exudation, and Activates Nodulation. Plasma Process Polym. 2021, 18, 2000160. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of Soybean Nodulation by Seed Treatment with Non–Thermal Plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef] [PubMed]
- Ivankov, A.; Zukiene, R.; Nauciene, Z.; Degutyte-Fomins, L.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. The Effects of Red Clover Seed Treatment with Cold Plasma and Electromagnetic Field on Germination and Seedling Growth are Dependent on Seed Color. Appl. Sci. 2021, 11, 4676. [Google Scholar] [CrossRef]
- Reema; Khanikar, R.R.; Bailung, H.; Sankaranarayanan, K. Review of the Cold Atmospheric Plasma Technology Application in Food, Disinfection, and Textiles: A Way Forward for Achieving Circular Economy. Front. Phys. 2022, 10, 942–952. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. A Review on Recent Advances in Cold Plasma Technology for the Food Industry: Current Applications and Future Trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of Cold Radiofrequency Plasma with Seeds of Beans (Phaseolus Vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Park, G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.d.D.; et al. Can Cold Plasma be Used for Boosting Plant Growth and Plant Protection in Sustainable Plant Production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, J.H.; Sun, D.W. Cold Plasma-Mediated Treatments for Shelf Life Extension of Fresh Produce: A Review of Recent Research Developments. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1312–1326. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive Oxygen Species Generation and Signaling in Plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Motrescu, I.; Ciolan, M.A.; Calistru, A.E.; Jitareanu, G. Germination and Growth Improvement of Some Micro-Greens under the Influence of Reactive Species Produced in a Non-Thermal Plasma (NTP). Agronomy 2023, 13, 150. [Google Scholar] [CrossRef]
- Pauly, N.; Pucciariello, C.; Mandon, K.; Innocenti, G.; Jamet, A.; Baudouin, E.; Hérouart, D.; Frendo, P.; Puppo, A. Reactive Oxygen and Nitrogen Species and Glutathione: Key Players in the Legume-Rhizobium Symbiosis. J. Exp. Bot. 2006, 57, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold Plasma Seed Priming Modulates Growth, Redox Homeostasis and Stress Response by Inducing Reactive Species in Tomato (Solanum Lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current Advancements in the Molecular Mechanism of Plasma Treatment for Seed Germination and Plant Growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef] [PubMed]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Šerá, B.; Scholtz, V.; Jirešová, J.; Khun, J.; Julák, J.; Šerý, M. Effects of Non-Thermal Plasma Treatment on Seed Germination and Early Growth of Leguminous Plants—A Review. Plants 2021, 10, 1616. [Google Scholar] [CrossRef]
- Feizollahi, E.; Roopesh, M.S. Degradation of Zearalenone by Atmospheric Cold Plasma: Effect of Selected Process and Product Factors. Food Bioprocess Technol. 2021, 14, 2107–2119. [Google Scholar] [CrossRef]
- Cherif, M.M.; Assadi, I.; Khezami, L.; Ben Hamadi, N.; Assadi, A.A.; Elfalleh, W. Review on Recent Applications of Cold Plasma for Safe and Sustainable Food Production: Principles, Implementation, and Application Limits. Appl. Sci. 2023, 13, 2381. [Google Scholar] [CrossRef]
- Feizollahi, E.; Misra, N.N.; Roopesh, M.S. Factors Influencing the Antimicrobial Efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in Food Processing Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Martynenko, A.; Chemat, F.; Paniwnyk, L.; Barba, F.J.; Jambrak, A.R. Thermodynamics, Transport Phenomena, and Electrochemistry of External Field-Assisted Nonthermal Food Technologies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1832–1863. [Google Scholar] [CrossRef] [PubMed]
- Van Gaens, W.; Bogaerts, A. Kinetic Modelling for an Atmospheric Pressure Argon Plasma Jet in Humid Air. J. Phys. D Appl. Phys. 2013, 46, 275201. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of Atmospheric Pressure Plasmas on Isolated and Cellular DNA—A Review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. Cold Plasma in Food and Agriculture; Academic Press: London, UK, 2016; pp. 1–16. [Google Scholar]
- Gao, X.; Zhang, A.; Héroux, P.; Sand, W.; Sun, Z.; Zhan, J.; Wang, C.; Hao, S.; Li, Z.; Li, Z.; et al. Effect of Dielectric Barrier Discharge Cold Plasma on Pea Seed Growth. J. Agric. Food Chem. 2019, 67, 10813–10822. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, R.; Roulard, R.; Ramos, M.; Thiombiano, B.; Mesnard, F.; Gontier, E.; Jamali, A. Etching of the Seed Cuticle by Cold Plasma Shortens Imbibitional Leakage in Linum usitatissimum L. Ind. Crops Prod. 2021, 167, 113536. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of Plasma-Seed Treatments as a Potential Seed Processing Technology. Front. Plant Sci. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Billah, M.; Sajib, S.A.; Roy, N.C.; Rashid, M.M.; Reza, M.A.; Hasan, M.M.; Talukder, M.R. Effects of DBD Air Plasma Treatment on the Enhancement of Black Gram (Vigna Mungo L.) Seed Germination and Growth. Arch. Biochem. Biophys. 2020, 681, 108253. [Google Scholar] [CrossRef]
- Luu, D.T.; Maurel, C. Aquaporin Trafficking in Plant Cells: An Emerging Membrane-Protein Model. Traffic 2013, 14, 629–635. [Google Scholar] [CrossRef]
- Sun, P.; Wu, H.; Bai, N.; Zhou, H.; Wang, R.; Feng, H.; Zhu, W.; Zhang, J.; Fang, J. Inactivation of Bacillus subtilis Spores in Water by a Direct-Current, Cold Atmospheric-Pressure Air Plasma Microjet. Plasma Process Polym. 2012, 9, 157–164. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 Mediates the Regulation of ABA Catabolism and GA Biosynthesis in Arabidopsis Seed Dormancy and Germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The Hydroxyl Radical in Plants: From Seed to Seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Cullen, P.J.; Ostrikov, K. Atmospheric Pressure Nonthermal Plasma Sources. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Mehta, D.; Yadav, S.K. Recent Advances in Cold Plasma Technology for Food Processing. Food Eng. Rev. 2022, 14, 555–578. [Google Scholar] [CrossRef]
- Bárdos, L.; Baránková, H. Cold Atmospheric Plasma: Sources, Processes, and Applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Schnabel, U.; Niquet, R.; Schlüter, O.; Gniffke, H.; Ehlbeck, J. Decontamination and Sensory Properties of Microbiologically Contaminated Fresh Fruits and Vegetables by Microwave Plasma Processed Air (PPA). J. Food Process Preserv. 2015, 39, 653–662. [Google Scholar] [CrossRef]
- Foltin, V.; Leštinská, L.; Machala, Z. Spectroscopic Investigations of Atmospheric Pressure Microwave Torch Nitrogen Plasma Jet. Czech. J. Phys. 2006, 56, 712–720. [Google Scholar] [CrossRef]
- Shelar, A.; Singh, A.V.; Dietrich, P.; Maharjan, R.S.; Thissen, A.; Didwal, P.N.; Shinde, M.; Laux, P.; Luch, A.; Mathe, V.; et al. Emerging Cold Plasma Treatment and Machine Learning Prospects for Seed Priming: A Step towards Sustainable Food Production. RSC Adv. 2022, 12, 10467–10488. [Google Scholar] [CrossRef] [PubMed]
- Nehra, V.; Kumar, A.; Dwivedi, H.K. Atmospheric Non-Thermal Plasma Sources. Int. J. Eng. 2008, 2, 53–68. [Google Scholar]
- Lalor, J.; Scally, L.; Cullen, P.J.; Milosavljević, V. Impact of Plasma Jet Geometry on Residence Times of Radical Species. J. Vac. Sci. Technol. 2018, 36, 03E108. [Google Scholar] [CrossRef]
- Sprent, J.I.; James, E.K. Legume Evolution: Where Do Nodules and Mycorrhizas Fit In? Plant Physiol. 2007, 144, 575–581. [Google Scholar] [CrossRef]
- Xiao, T.T.; Schilderink, S.; Moling, S.; Deinum, E.E.; Kondorosi, E.; Franssen, H.; Kulikova, O.; Niebel, A.; Bisseling, T. Fate Map of Medicago Truncatula Root Nodules. Development 2014, 141, 3517–3528. [Google Scholar] [CrossRef]
- Guinel, F.C. Getting around the Legume Nodule: I. The Structure of the Peripheral Zone in Four Nodule Types. Botany 2009, 87, 1117–1138. [Google Scholar] [CrossRef]
- Nedved, H.; Kalatskaja, J.; Kopylova, N.; Herasimovich, K.; Rybinskaya, E.; Vusik, N.; Filatova, I.; Lyushkevich, V.; Laman, N. Short-Term and Long-Term Effects of Plasma and Radio Wave Seed Treatments on Red Clover Plants. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 937, p. 022137. [Google Scholar] [CrossRef]
- Perez-Piza, M.; Prevosto, L.; Grijalba, P.E.; Zilli, C.; Cejas, E.; Mancinelli, B.; Balestrasse, K. Improvement of Growth and Yield of Soybean Plants through the Application of Non-Thermal Plasmas to Seeds with Different Health Status. Heliyon 2019, 5, 1495. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Shen, M.; Hou, J.; Shao, H.; Dong, Y.; Jiang, J. Improving Seed Germination and Peanut Yields by Cold Plasma Treatment. Plasma Sci. Technol. 2016, 18, 1027–1033. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of Cold Plasma Treatment on Seed Germination and Seedling Growth of Soybean. Sci. Rep. 2014, 4, 5859–5866. [Google Scholar] [CrossRef]
- Lee, D.K.; Ahn, J.H.; Song, S.K.; Choi, Y.D.; Lee, J.S. Expression of an Expansin Gene is Correlated with Root Elongation in Soybean. Plant Physiol. 2003, 131, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Schaedel, M.; Hidrobo, G.; Grossman, J. From Microns to Meters: Exploring Advances in Legume Microbiome Diversity for Agroecosystem Benefits. Front. Sustain. Food Syst. 2021, 5, 668195. [Google Scholar] [CrossRef]
- Bani, M.; Pérez-de-Luque, A.; Rubiales, D.; Rispail, N. Physical and Chemical Barriers in Root Tissues Contribute to Quantitative Resistance to Fusarium Oxysporum f. Sp. Pisi in Pea. Front. Plant Sci. 2018, 9, 119. [Google Scholar] [CrossRef]
- Vasse, J.; De Billy, F.; Truchet, G. Abortion of Infection during the Rhizobium meliloti-Alfalfa Symbiotic Interaction Is Accompanied by a Hypersensitive Reaction. Plant J. 1993, 4, 555–566. [Google Scholar] [CrossRef]
- Zipfel, C.; Oldroyd, G.E.D. Plant Signalling in Symbiosis and Immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and Development of the Legume-Rhizobial Symbiotic Interface in Infection Threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Švubová, R.; Válková, N.; Bathoova, M.; Kyzek, S.; Gálová, E.; Medvecká, V.; Slováková, Ľ. Enhanced In Situ Activity of Peroxidases and Lignification of Root Tissues after Exposure to Non-Thermal Plasma Increases the Resistance of Pea Seedlings. Plasma Chem. Plasma Process 2021, 41, 903–922. [Google Scholar] [CrossRef]
- Feng, J.; Lee, T.; Schiessl, K.; Oldroyd, G.E.D. Processing of Nodule Inception Controls the Transition to Nitrogen Fixation in Root Nodules. Science 2021, 374, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.H.; Lin, Y.H.; Reid, D.E.; Gresshoff, P.M. Molecular Analysis of Legume Nodule Development and Autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Murray, J.D. The Role of Flavonoids in Nodulation Host-Range Specificity: An Update. Plants 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, M.S.; Cope, K.R. Split-Root Assays for Studying Legume-Rhizobia Symbioses, Rhizodeposition, and Belowground Nitrogen Transfer in Legumes. J. Exp. Bot. 2021, 72, 5285–5299. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.; Lagunas, B.; Gifford, M.L. Determinants of Host Range Specificity in Legume-Rhizobia Symbiosis. Front. Microbiol. 2020, 11, 585749. [Google Scholar] [CrossRef]
- Krönauer, C.; Radutoiu, S. Understanding Nod Factor Signalling Paves the Way for Targeted Engineering in Legumes and Non-legumes. Curr. Opin. Plant Biol. 2021, 62, 102026. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The Role of Flavonoids in Root-Rhizosphere Signalling: Opportunities and Challenges for Improving Plant-Microbe Interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Begum, A.A.; Leibovitch, S.; Migner, P.; Zhang, F. Specific Flavonoids Induced Nod Gene Expression and Pre-Activated Nod Genes of Rhizobium Leguminosarum Increased Pea (Pisum Sativum L.) and Lentil (Lens Culinaris L.) Nodulation in Controlled Growth Chamber Environments. J. Exp. Bot. 2001, 52, 1537–1543. [Google Scholar] [CrossRef]
- Concha, C.; Doerner, P.; Gutiérrez, R. The Impact of the Rhizobia-Legume Symbiosis on Host Root System Architecture. J. Exp. Bot. 2020, 71, 3902–3921. [Google Scholar] [CrossRef] [PubMed]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The Role of the Testa during Development and in Establishment of Dormancy of the Legume Seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.; Cheng, K.; Hsu, F.; Wu, J.S.; Ting, Y. Producing High Quality Mung Bean Sprout Using Atmospheric Cold Plasma Treatment: Better Physical Appearance and Higher γ-Aminobutyric Acid (GABA) Content. J. Sci. Food Agric. 2021, 101, 6463–6471. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Hairston, J.S.; Marshall, J.; Bookal, A.; Dholichand, A.; Patel, D. Plasma Seeds: Cold Plasma Accelerates Phaseolus vulgaris Seed Imbibition, Germination, and Speed of Seedling Growth. Plasma Med. 2020, 10, 139–158. [Google Scholar] [CrossRef]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of Low-Temperature Plasma on the Structure of Seeds, Growth and Metabolism of Fndogenous Phytohormones in Pea (Pisum Sativum L.). Plasma Chem. Plasma Process 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma Activated Water: The next Generation Eco-Friendly Stimulant for Enhancing Plant Seed Germination, Vigor and Increased Enzyme Activity, a Study on Black Gram (Vigna Mungo L.). Plasma Chem. Plasma Process 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Zhou, R.; Zhang, X.; Yang, S. Atmospheric-Pressure Plasma Treated Water for Seed Germination and Seedling Growth of Mung Bean and its Sterilization Effect on Mung Bean Sprouts. Innov. Food Sci. Emerg. Technol. 2019, 53, 36–44. [Google Scholar] [CrossRef]
- Le, T.Q.X.; Nguyen, L.N.; Nguyen, T.T.; Choi, E.H.; Nguyen, Q.L.; Kaushik, N.K.; Dao, N.T. Effects of Cold Plasma Treatment on Physical Modification and Endogenous Hormone Regulation in Enhancing Seed Germination and Radicle Growth of Mung Bean. Appl. Sci. 2022, 12, 10308. [Google Scholar] [CrossRef]
- Mudgil, Y.; Karve, A.; Teixeira, P.J.P.L.; Jiang, K.; Tunc-Ozdemir, M.; Jones, A.M. Photosynthate Regulation of the Root System Architecture Mediated by the Heterotrimeric G Protein Complex in Arabidopsis. Front. Plant Sci. 2016, 7, 1255. [Google Scholar] [CrossRef]
- Van Gelderen, K.; Kang, C.; Pierik, R. Light Signaling, Root Development, and Plasticity. Plant Physiol. 2018, 176, 1049–1060. [Google Scholar] [CrossRef]
- Streeter, J.G.; Streeter, J. Recent Developments in Carbon Transport and Metabolism in Symbiotic Systems. Symbiosis 1995, 19, 175–196. [Google Scholar]
- Udvardi, M.; Poole, P.S. Transport and Metabolism in Legume-Rhizobia Symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Kouchi, H.; Yoneyama, T. Dynamics of Carbon Photosynthetically Assimilated in Nodulated Soya Bean Plants under Steady-State Conditions 2. The Incorporation of 13 C into Carbohydrates, Organic Acids, Amino Acids and Some Storage Compounds. Ann. Bot. 1984, 53, 883–896. [Google Scholar] [CrossRef]
- Breakspear, A.; Liu, C.; Roy, S.; Stacey, N.; Rogers, C.; Trick, M.; Morieri, G.; Mysore, K.S.; Wen, J.; Oldroyd, G.E.D.; et al. The Root Hair “Infectome” of Medicago truncatula Uncovers Changes in Cell Cycle Genes and Reveals a Requirement for Auxin Signaling in Rhizobial Infection. Plant Cell 2014, 26, 4680–4701. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, E.E.; De Felipe, M.R.; Pueyo, J.J.; Lucas, M.M. Conformation of Cytoskeletal Elements during the Division of Infected Lupinus albus L. Nodule Cells. J. Exp. Bot. 2007, 58, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ortega, Y.; Carrasco-Castilla, J.; Juárez-Verdayes, M.A.; Toscano-Morales, R.; Fonseca-García, C.; Nava, N.; Cárdenas, L.; Quinto, C. Actin Depolymerizing Factor Modulates Rhizobial Infection and Nodule Organogenesis in Common Bean. Int. J. Mol. Sci. 2020, 21, 1907. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P.; Uchiumi, T.; Alunni, B.; Naë Lle Evanno, G.; Lique Cheron, A.; Catrice, O.; Mausset, A.-E.; Dé Rique Barloy-Hubler, F.; Galibert, F.; Kondorosi, A.; et al. Eukaryotic Control on Bacterial Cell Cycle and Differentiation in the Rhizobium-Legume Symbiosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5230–5235. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.E.; Kobayashi, H.; Walker, G.C. Molecular Determinants of a Symbiotic Chronic Infection. Ann. Rev. Gen. 2008, 42, 413–441. [Google Scholar] [CrossRef]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How Rhizobial Symbionts Invade Plants: The Sinorhizobium—Medicago Model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef]
- Lindström, K.; Mousavi, S.A. Effectiveness of Nitrogen Fixation in Rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Lepetit, M.; Brouquisse, R. Control of the Rhizobium–Legume Symbiosis by the Plant Nitrogen Demand Is Tightly Integrated at the Whole Plant Level and Requires Inter-Organ Systemic Signaling. Front. Plant Sci. 2023, 14, 1114840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-Inducing Effects of Argon Plasma on Soybean Sprouts via the Regulation of Demethylation Levels of Energy Metabolism-Related Genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Frank, M.; Reid, D. No Home without Hormones: How Plant Hormones Control Legume Nodule Organogenesis. Plant Commun. 2020, 1, 100104. [Google Scholar] [CrossRef] [PubMed]
- Gamas, P.; Brault, M.; Jardinaud, M.F.; Frugier, F. Cytokinins in Symbiotic Nodulation: When, Where, What For? Trends Plant Sci. 2017, 22, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.D.; Karas, B.J.; Sato, S.; Tabata, S.; Amyot, L.; Szczyglowski, K. A Cytokinin Perception Mutant Colonized by Rhizobium in the Absence of Nodule Organogenesis. Science 2007, 315, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Miri, M.; Janakirama, P.; Held, M.; Ross, L.; Szczyglowski, K. Into the Root: How Cytokinin Controls Rhizobial Infection. Trends Plant Sci. 2016, 21, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Tirichine, L.; Sandal, N.; Madsen, L.H.; Radutoiu, S.; Albrektsen, A.S.; Sato, S.; Asamizu, E.; Tabata, S.; Stougaard, J. A Gain-of-Function Mutation in a Cytokinin Receptor Triggers Spontaneous Root Nodule Organogenesis. Science 2007, 315, 104–107. [Google Scholar] [CrossRef]
- Gauthier-Coles, C.; White, R.G.; Mathesius, U. Nodulating Legumes are Distinguished by a Sensitivity to Cytokinin in the Root Cortex Leading to Pseudonodule Development. Front. Plant Sci. 2019, 9, 1901. [Google Scholar] [CrossRef]
- Suzaki, T.; Nishida, H. Autoregulation of Legume Nodulation by Sophisticated Transcriptional Regulatory Networks. Mol. Plant 2019, 12, 1179–1181. [Google Scholar] [CrossRef]
- Sasaki, T.; Suzaki, T.; Soyano, T.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Shoot-Derived Cytokinins Systemically Regulate Root Nodulation. Nat. Commun. 2014, 5, 4983. [Google Scholar] [CrossRef]
- Schulte, C.C.M.; Borah, K.; Wheatley, R.M.; Terpolilli, J.J.; Saalbach, G.; Crang, N.; de Groot, D.H.; George Ratcliffe, R.; Kruger, N.J.; Papachristodoulou, A.; et al. Metabolic Control of Nitrogen Fixation in Rhizobium-Legume Symbioses. Sci. Adv. 2021, 7, 2433. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Gresshoff, P.M. The Role of Symbiotic Nitrogen Fixation in Sustainable Production of Biofuels. Int. J. Mol. Sci. 2014, 15, 7380–7397. [Google Scholar] [CrossRef] [PubMed]
- Becana, M.; Moran, J.F.; Iturbe-Ormaetxe, I.; Gogorcena, Y.; Escuredo, P.R. Structure and Function of Leghemoglobins. An.Estac. Exp. Aula. Dei. 1994, 21, 203–208. [Google Scholar]
- Becana, M.; Klucas, R.V. Oxidation and Reduction of Leghemoglobin in Root Nodules of Leguminous Plants. Plant Physiol. 1992, 98, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.; van Dongen, J.T.; Günther, C.; Krusell, L.; Desbrosses, G.; Vigeolas, H.; Bock, V.; Czechowski, T.; Geigenberger, P.; Udvardi, M.K. Symbiotic Leghemoglobins are Crucial for Nitrogen Fixation in Legume Root Nodules but Not for General Plant Growth and Development. Current Biol. 2004, 15, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Siow, K.S.; Wee, M.F.M.R.; Patra, A. A Study to Examine the Ageing Behaviour of Cold Plasma-Treated Agricultural Seeds. Sci. Rep. 2023, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Ono, R.; Nakano, R.; Shiratani, M.; Tashiro, K.; Kuhara, S.; Yasuda, K.; Hagiwara, H. DNA Microarray Analysis of Plant Seeds Irradiated by Active Oxygen Species in Oxygen Plasma. Plasma Med. 2016, 6, 459–471. [Google Scholar] [CrossRef][Green Version]
- Tamošiūnė, I.; Gelvonauskienė, D.; Haimi, P.; Mildažienė, V.; Koga, K.; Shiratani, M.; Baniulis, D. Cold Plasma Treatment of Sunflower Seeds Modulates Plant-Associated Microbiome and Stimulates Root and Lateral Organ Growth. Front. Plant Sci. 2020, 11, 568924. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, S.B.; Ryu, S.; Oh, J.; Kim, D.S. Emerging Plasma Technology That Alleviates Crop Stress During the Early Growth Stages of Plants: A Review. Front. Plant Sci. 2020, 11, 988. [Google Scholar] [CrossRef]
- Mahanta, S.; Habib, M.R.; Moore, J.M. Effect of High-Voltage Atmospheric Cold Plasma Treatment on Germination and Heavy Metal Uptake by Soybeans (Glycine max). Int. J. Mol. Sci. 2022, 23, 1611. [Google Scholar] [CrossRef]

| Process | Reaction ⁑ |
|---|---|
| Ionization | e + M → M+ + 2e |
| Dissociative ionization | e + AB → A+ + B + 2e |
| Electron attachment | e + M → M− |
| Dissociative electron attachment | e + AB → A− + B |
| Excitation (electronic, vibrational or rotational) | e + M → M * + e |
| Dissociation (both products can be electronically excited) | e + AB → A + B |
| Symbiotic Nitrogen Fixation Related Traits | References |
|---|---|
| Early formation of nodules | [24] |
| Alter nodule number and dry weight | [22,23,24,65] |
| Increase nitrogenase activity in nodules | [23] |
| Increase nodule leghemoglobin content | [23] |
| More flavonoid/isoflavonoid production | [22,65] |
| Increase root length | [22,23,24,65,66,67,68] |
| Increase lateral root number | [22,65] |
| Increase root biomass | [23,24,65,67,68] |
| Increase GmEXP1 gene expression | [23,69] |
| Alter phytohormone levels (cytokinin and auxin) | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeysingha, D.N.; Dhaliwal, H.K.; Du, L.; De Silva, C.; Szczyglowski, K.; Roopesh, M.S.; Thilakarathna, M.S. The Potential of Cold Plasma-Based Seed Treatments in Legume–Rhizobia Symbiotic Nitrogen Fixation: A Review. Crops 2024, 4, 95-114. https://doi.org/10.3390/crops4010008
Abeysingha DN, Dhaliwal HK, Du L, De Silva C, Szczyglowski K, Roopesh MS, Thilakarathna MS. The Potential of Cold Plasma-Based Seed Treatments in Legume–Rhizobia Symbiotic Nitrogen Fixation: A Review. Crops. 2024; 4(1):95-114. https://doi.org/10.3390/crops4010008
Chicago/Turabian StyleAbeysingha, Dhanuja N., Harleen K. Dhaliwal, Lihui Du, Chathuranga De Silva, Krzysztof Szczyglowski, M. S. Roopesh, and Malinda S. Thilakarathna. 2024. "The Potential of Cold Plasma-Based Seed Treatments in Legume–Rhizobia Symbiotic Nitrogen Fixation: A Review" Crops 4, no. 1: 95-114. https://doi.org/10.3390/crops4010008
APA StyleAbeysingha, D. N., Dhaliwal, H. K., Du, L., De Silva, C., Szczyglowski, K., Roopesh, M. S., & Thilakarathna, M. S. (2024). The Potential of Cold Plasma-Based Seed Treatments in Legume–Rhizobia Symbiotic Nitrogen Fixation: A Review. Crops, 4(1), 95-114. https://doi.org/10.3390/crops4010008






