Analyzing Winter Wheat (Triticum aestivum) Growth Pattern Using High Spatial Resolution Images: A Case Study at Lakehead University Agriculture Research Station, Thunder Bay, Canada
Abstract
1. Introduction
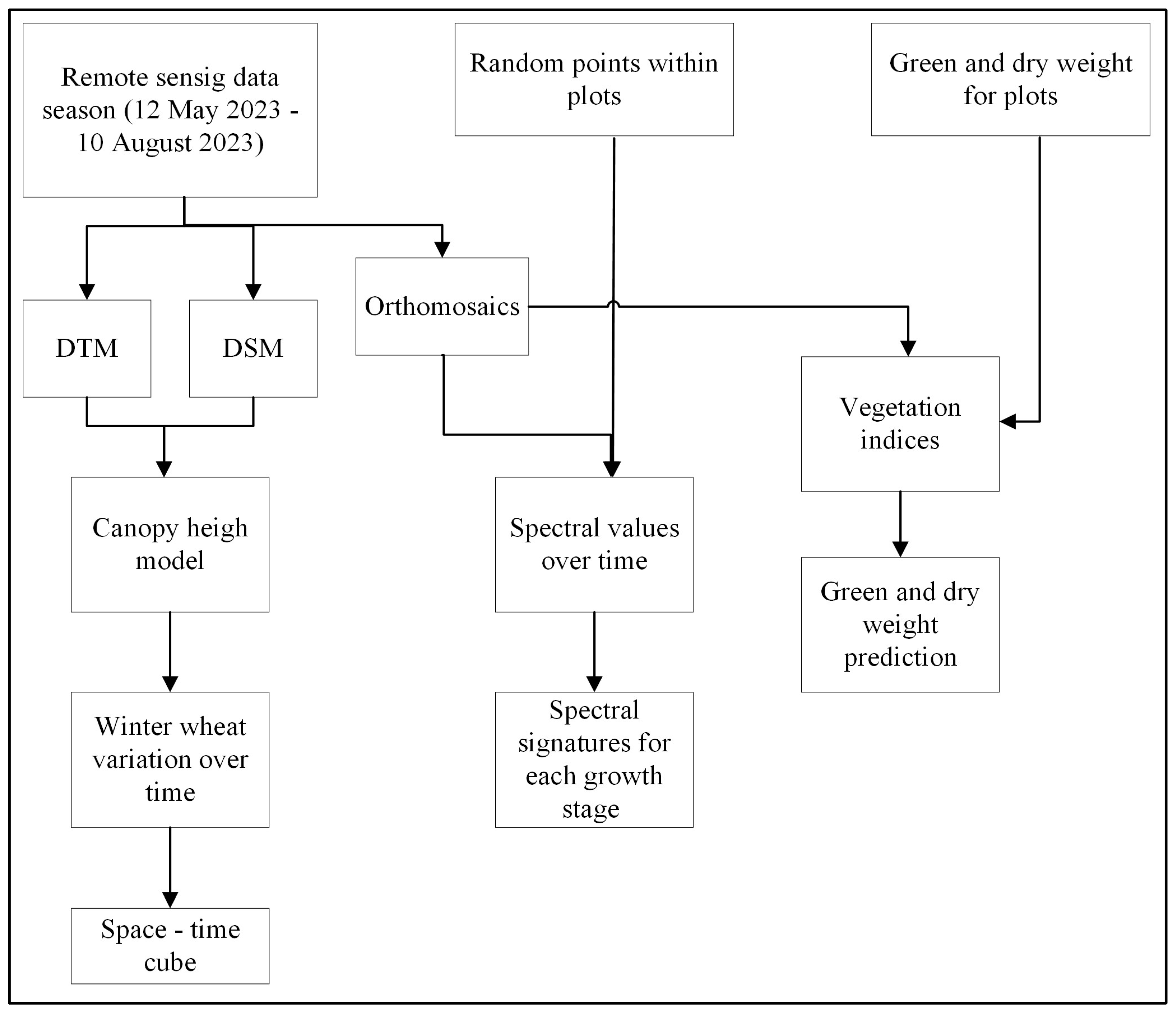
2. Materials and Methods
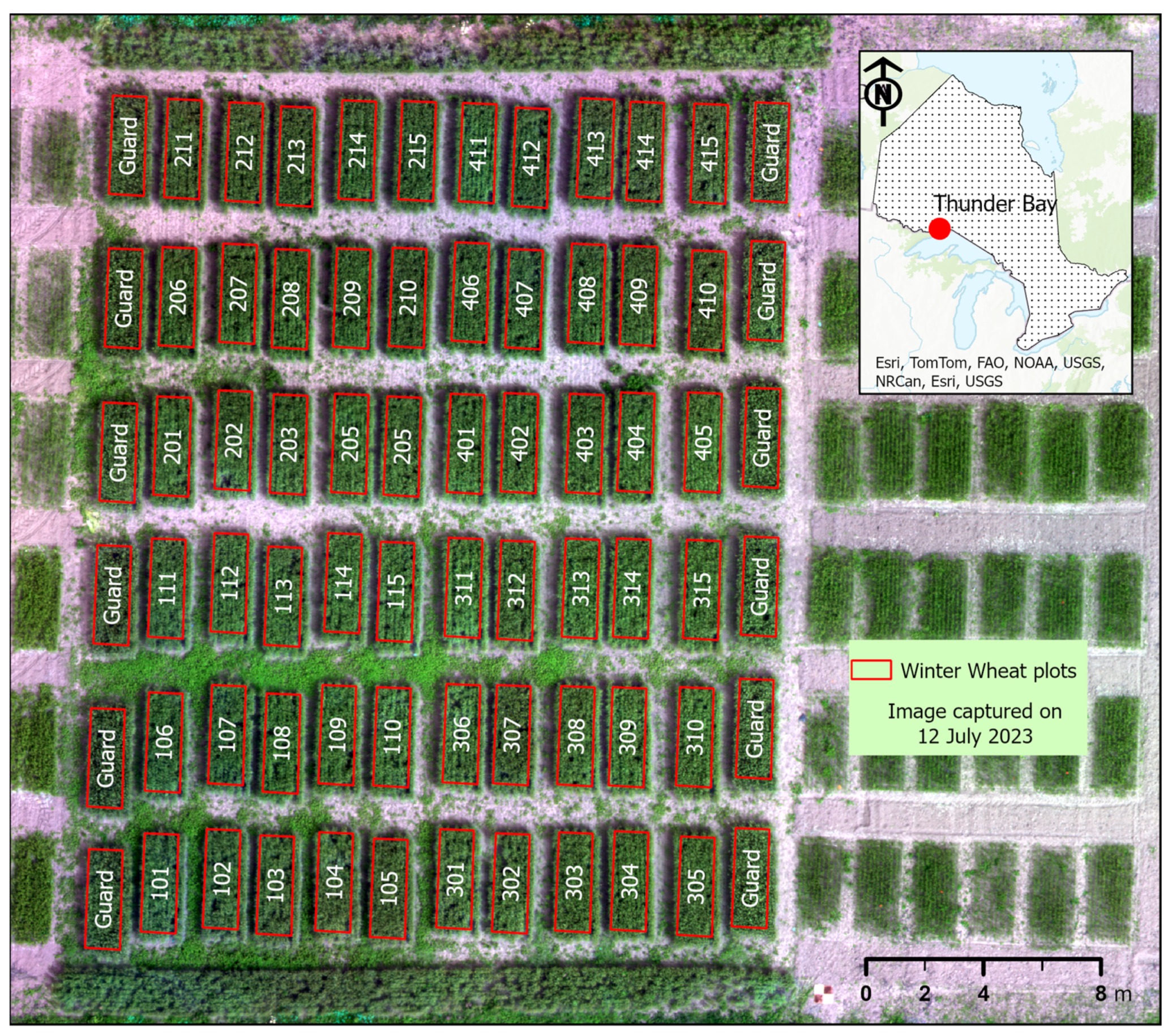
2.1. Study Area and Data
2.2. Spectral Signatures of Winter Wheat at Different Growing Stages
2.3. Analysis of Winter Wheat Height Variation over Time
Space–Time Cube
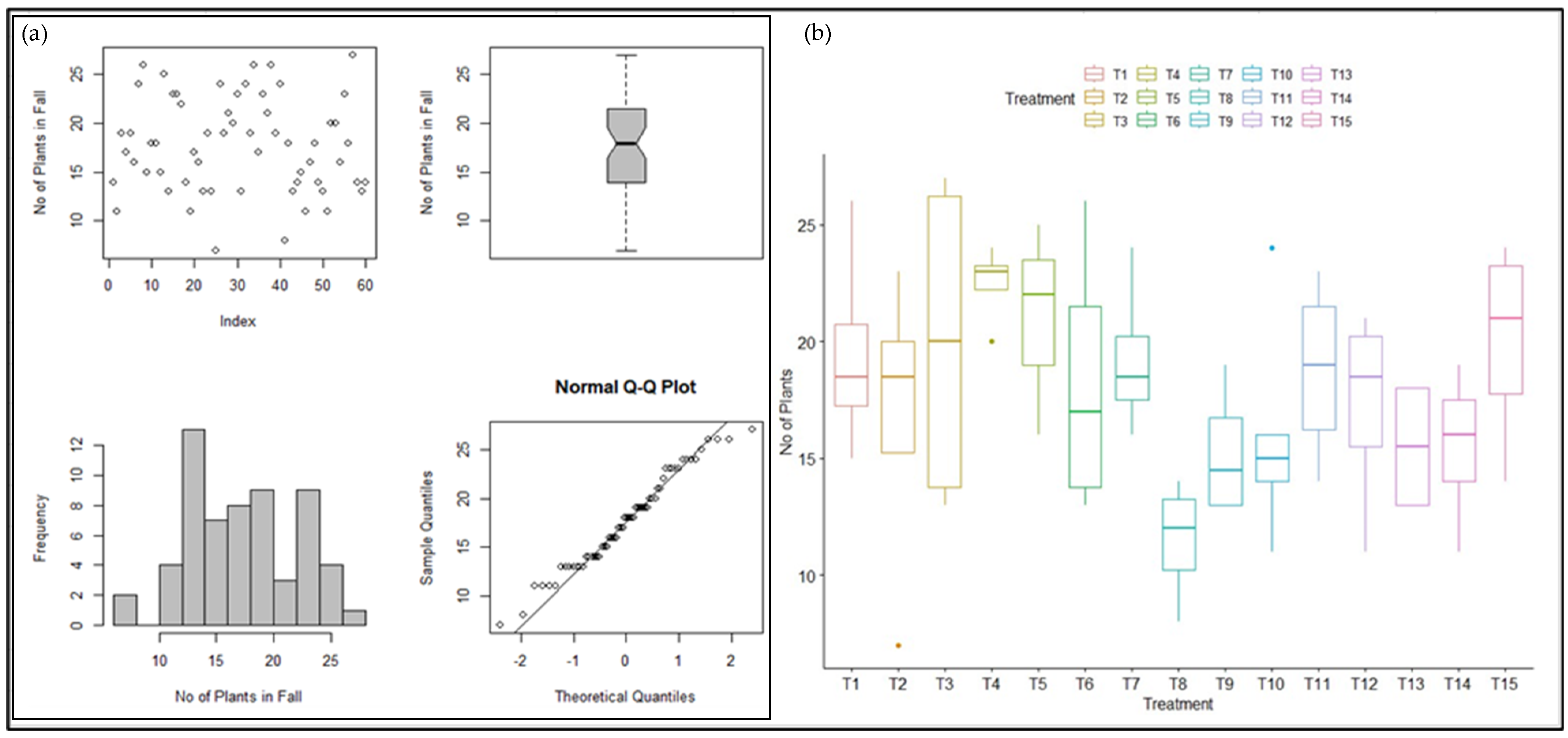
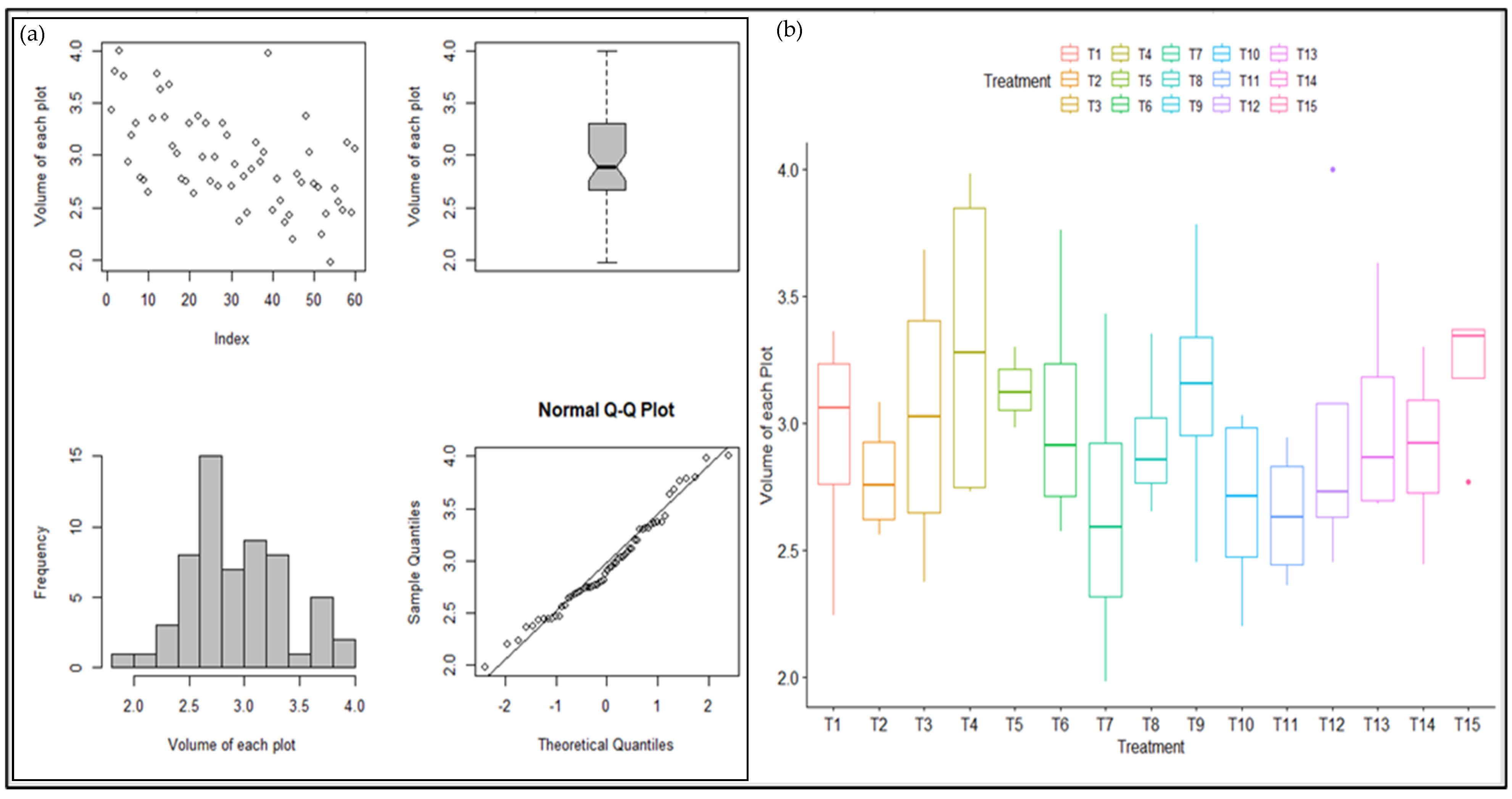
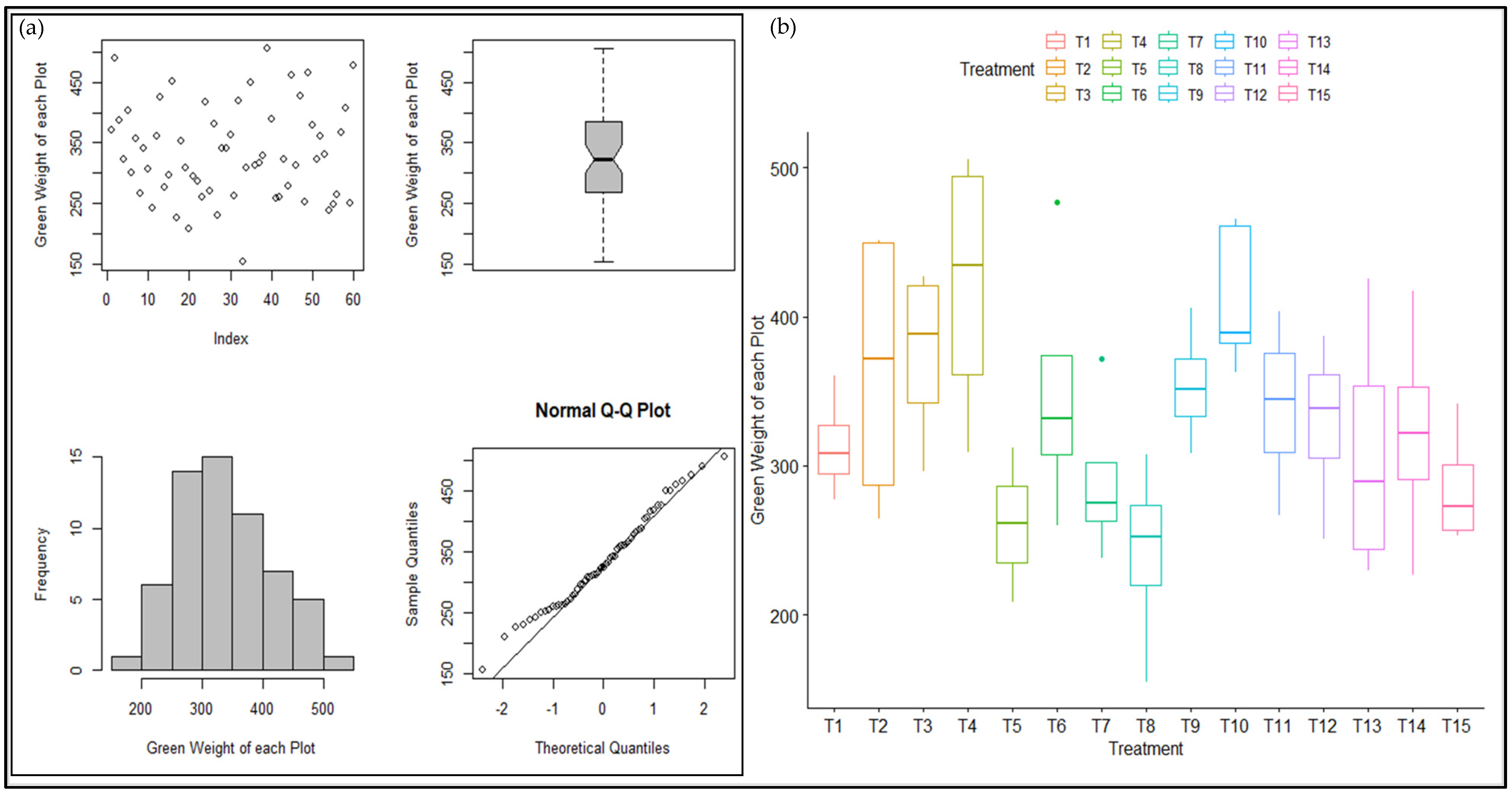
2.4. Statistical Analysis of Winter Wheat Growth Pattern
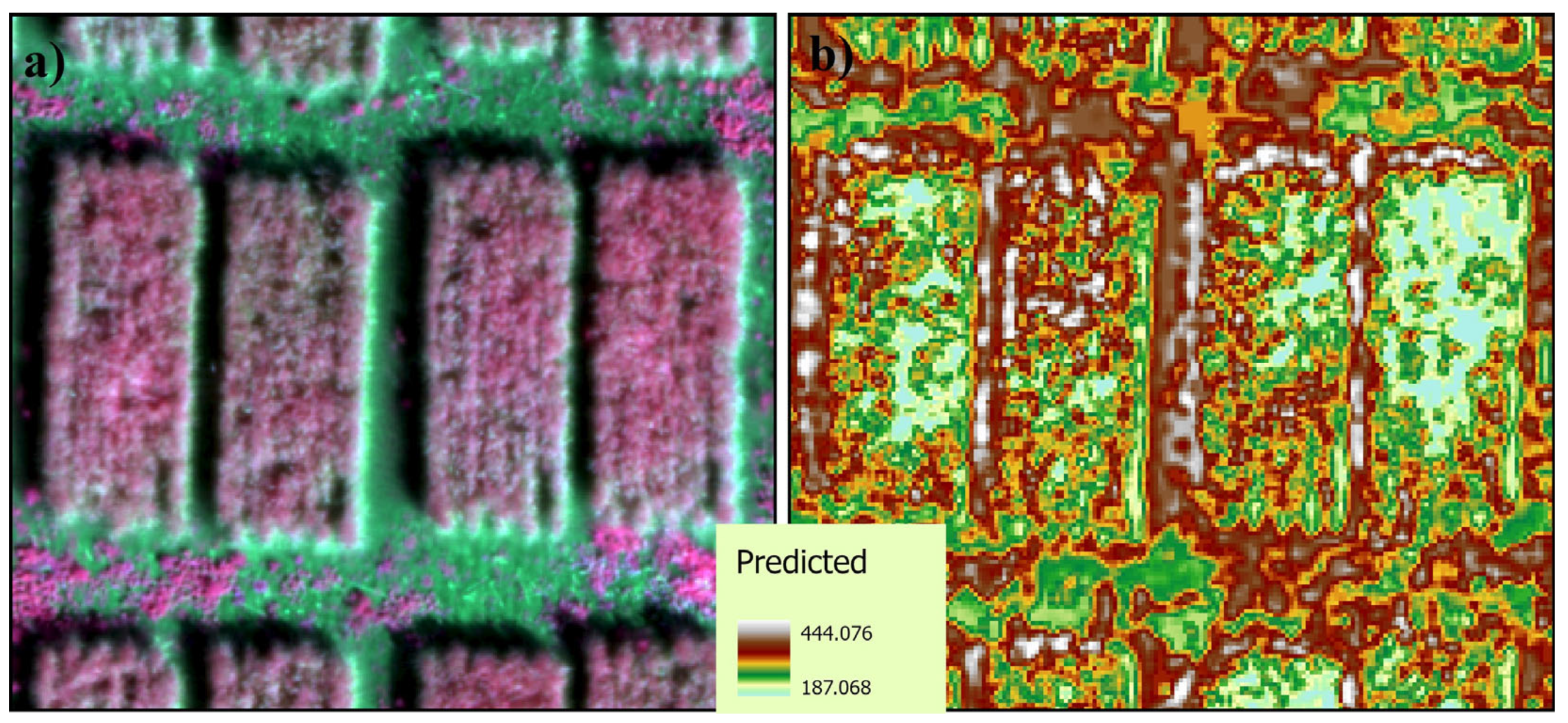
2.5. Estimating Winter Wheat Yield Using Remote Sensing
3. Results
3.1. Spectral Profiles
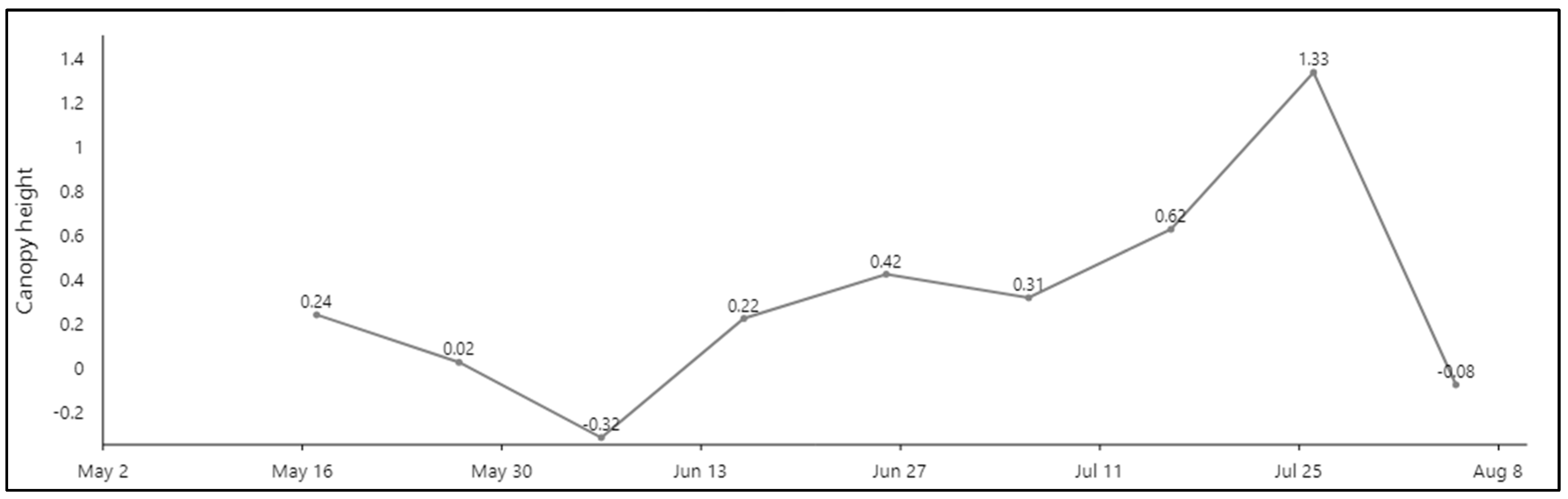
3.2. Analysis of Witner Wheat Hight Variation over Time
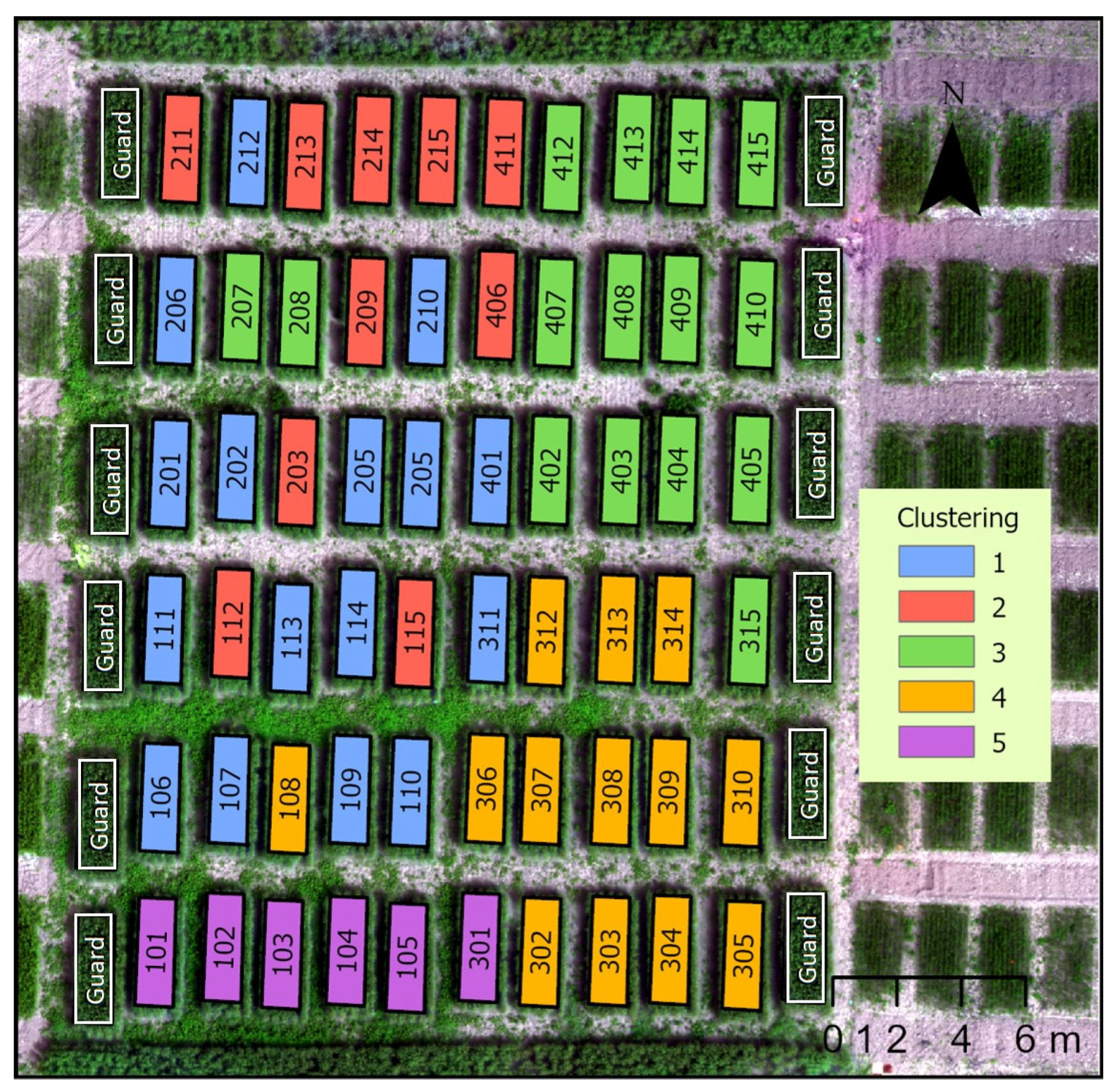
3.2.1. Times Series Clustering
3.2.2. Local Outlier Analysis
3.3. Statistical Analysis of Winter Wheat Growth Pattern
3.4. Yield Estimation
4. Discussion
4.1. Spectral Profiles
4.2. Analysis of Winter Wheat Height Variation over Time
4.3. Statistical Analysis of Winter Wheat Growth Pattern
4.4. Estimating Winter Wheat Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruane, A.C.; Rosenzweig, C. Climate Change Impacts on Agriculture. In Agriculture & Food Systems to 2050; World Scientific: Singapore, 2018; pp. 161–191. [Google Scholar] [CrossRef]
- Feng, X.; Tian, H.; Cong, J.; Zhao, C. A method review of the climate change impact on crop yield. Front. For. Glob. Chang. 2023, 6, 1198186. [Google Scholar] [CrossRef]
- Government of Canada. Climate Change Impacts on Agriculture. 2020. Available online: https://agriculture.canada.ca/en/environment/climate-change/climate-scenarios-agriculture (accessed on 28 October 2023).
- Government of Canada. The Biology of Triticum aestivum L. (Wheat). 2014. Available online: https://inspection.canada.ca/plant-varieties/plants-with-novel-traits/applicants/directive-94-08/biology-documents/triticum-aestivum-l-/eng/1330982915526/1330982992440 (accessed on 24 August 2023).
- Zong, J.; Ontario Is an Agricultural Powerhouse That Leads in Many Farming Categories. Canadian Agriculture at a Glance. Available online: https://www150.statcan.gc.ca/n1/pub/96-325-x/2021001/article/00006-eng.htm (accessed on 28 October 2023).
- Ontario Ministry of Agriculture Food and Rural Affairs. A Visual Guide to Winter Wheat Staging. Grain Farmers of Ontario. 2023. Available online: https://gfo.ca/wp-content/uploads/2023/02/Grain-Farmers-of-Ontario-Cereal-Staging-Guide-223on_compressed.pdf (accessed on 5 February 2024).
- Singh, P.; Pandey, P.C.; Petropoulos, G.P.; Pavlides, A.; Srivastava, P.K.; Koutsias, N.; Deng, K.A.K.; Bao, Y. Hyperspectral remote sensing in precision agriculture: Present status, challenges, and future trends. In Hyperspectral Remote Sensing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 121–146. [Google Scholar]
- Xie, Y.; Wang, C.; Yang, W.; Feng, M.; Qiao, X.; Song, J. Canopy hyperspectral characteristics and yield estimation of winter wheat (Triticum aestivum) under low temperature injury. Sci. Rep. 2020, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Sheppard, J.; Logan, R.; Shaw, J. Hyperspectral Band Selection for Multispectral Image Classification with Convolutional Networks. In Proceedings of the 2021 International Joint Conference on Neural Networks (IJCNN), Shenzhen, China, 18–22 July 2021; pp. 1–8. [Google Scholar] [CrossRef]
- Guarnera, C.; Multispectral Imaging in Precision Agriculture: An In-Depth Guide. Blue Falcon Aerial. Available online: https://www.bluefalconaerial.com/how-multispectral-drones-work/ (accessed on 28 October 2023).
- Ma, C.; Liu, M.; Ding, F.; Li, C.; Cui, Y.; Chen, W.; Wang, Y. Wheat growth monitoring and yield estimation based on remote sensing data assimilation into the SAFY crop growth model. Sci. Rep. 2022, 12, 5473. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Peng, D.; Zhang, B.; Chen, Z.; Yu, L.; Chen, J.; Pan, Y.; Zheng, S.; Hu, J.; Lou, Z.; et al. The Accuracy of Winter Wheat Identification at Different Growth Stages Using Remote Sensing. Remote Sens. 2022, 14, 893. [Google Scholar] [CrossRef]
- Han, X.; Wei, Z.; Chen, H.; Zhang, B.; Li, Y.; Du, T. Inversion of Winter Wheat Growth Parameters and Yield Under Different Water Treatments Based on UAV Multispectral Remote Sensing. Front. Plant Sci. 2021, 12, 609876. [Google Scholar] [CrossRef] [PubMed]
- Cintas, J.; Franch, B.; Van-Tricht, K.; Boogaard, H.; Degerickx, J.; Becker-Reshef, I.; Moletto-Lobos, I.; Mollà-Bononad, B.; Sobrino, J.A.; Gilliams, S.; et al. TRANCO: Thermo radiometric normalization of crop observations. Int. J. Appl. Earth Obs. Geoinf. 2023, 118, 103283. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Liu, J.-F.; Qin, Z.; Qin, F. Winter wheat identification by integrating spectral and temporal information derived from multi-resolution remote sensing data. J. Integr. Agric. 2019, 18, 2628–2643. [Google Scholar] [CrossRef]
- Cheng, E.; Zhang, B.; Peng, D.; Zhong, L.; Yu, L.; Liu, Y.; Xiao, C.; Li, C.; Li, X.; Chen, Y.; et al. Wheat yield estimation using remote sensing data based on machine learning approaches. Front. Plant Sci. 2022, 13, 1090970. [Google Scholar] [CrossRef] [PubMed]
- Badea, A.; Badea, G. An Overview on Space Time Cube Visualization in Gis; Researchgate: Berlin, Germany, 2018. [Google Scholar]
- ESRI. How Create Space Time Cube Works. 2023. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/space-time-pattern-mining/learnmorecreatecube.htm?ssp=1&darkschemeovr=1&setlang=en-CA&safesearch=moderate (accessed on 28 November 2023).
- Krishnan, P.; Aggarwal, P.; Mridha, N.; Bajpai, V. Spatio-Temporal Changes in Wheat Crop Cultivation in India. ISPRS—Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, XLII-3/W6, 385–395. [Google Scholar] [CrossRef]
- El Imanni, H.S.; El Harti, A.; El Iysaouy, L. Wheat Yield Estimation Using Remote Sensing Indices Derived from Sentinel-2 Time Series and Google Earth Engine in a Highly Fragmented and Heterogeneous Agricultural Region. Agronomy 2022, 12, 2853. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Liu, B.; Wu, Y.; Ma, J.; Zhang, W.; Wang, B.; Chen, Z. Estimation of Winter Wheat Yield Using Multiple Temporal Vegetation Indices Derived from UAV-Based Multispectral and Hyperspectral Imagery. Remote Sens. 2023, 15, 4800. [Google Scholar] [CrossRef]
- MicaSense. Comparison of MicaSense Cameras, Seattle, USA. Available online: https://support.micasense.com/hc/en-us/articles/1500007828482-Comparison-of-MicaSense-Cameras (accessed on 22 November 2023).
- PIX4D. PIX4Dmapper version 4.9. Available online: https://www.pix4d.com/product/pix4dmapper-photogrammetry-software/ (accessed on 10 December 2023).
- ESRI. Time Series Clustering (Space Time Pattern Mining). ArcGIS Pro. Available online: https://pro.arcgis.com/en/pro-app/3.1/tool-reference/space-time-pattern-mining/time-series-clustering.htm#:~:text=Time%20series%20can%20be%20clustered,by%20cluster%20membership%20and%20messages (accessed on 9 December 2023).
- Patil, P. What is Exploratory Data Analysis? Towards Data Science. 2018. Available online: https://towardsdatascience.com/exploratory-data-analysis-8fc1cb20fd15 (accessed on 26 November 2023).
- Moslemi, A. A tutorial-based survey on feature selection: Recent advancements on feature selection. Eng. Appl. Artif. Intell. 2023, 126, 107136. [Google Scholar] [CrossRef]
- Kawashima, S.; Nakatani, M. An Algorithm for Estimating Chlorophyll Content in Leaves Using a Video Camera. Ann. Bot. 1998, 81, 49–54. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E., III. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Third Earth Resources Technology Satellite-1 Symposium-Volume I: Technical Presentations; NASA: Washington, DC, USA, 1974. [Google Scholar]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the KDD ’16: 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, M.; Xu, Z.; Yang, T.; Su, Y.; Zhou, T.; Wang, H.; Wang, Y.; Lin, Y. Estimation of leaf nutrition status in degraded vegetation based on field survey and hyperspectral data. Sci. Rep. 2020, 10, 4361. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Alvino, A. Vegetation Indices Data Clustering for Dynamic Monitoring and Classification of Wheat Yield Crop Traits. Remote Sens. 2021, 13, 541. [Google Scholar] [CrossRef]
- Xie, Q.; Dash, J.; Huang, W.; Peng, D.; Qin, Q.; Mortimer, H.; Casa, R.; Pignatti, S.; Laneve, G.; Pascucci, S.; et al. Vegetation Indices Combining the Red and Red-Edge Spectral Information for Leaf Area Index Retrieval. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 1482–1493. [Google Scholar] [CrossRef]
- Qingxi, T.; Xia, Z.; Bing, Z.; Jihua, W. Crop growth monitoring study by using multi-temporal index image cube analysis. In Proceedings of the IEEE 2002 International Conference on Communications, Circuits and Systems and West Sino Expositions, Chengdu, China, 29 June–1 July 2002; pp. 1596–1601. [Google Scholar]
- Moso, J.C.; Cormier, S.; de Runz, C.; Fouchal, H.; Wandeto, J.M. Anomaly Detection on Data Streams for Smart Agriculture. Agriculture 2021, 11, 1083. [Google Scholar] [CrossRef]
- Yue, S.; Meng, Q.; Zhao, R.; Ye, Y.; Zhang, F.; Cui, Z.; Chen, X. Change in Nitrogen Requirement with Increasing Grain Yield for Winter Wheat. Agron. J. 2012, 104, 1687–1693. [Google Scholar] [CrossRef]
- Rohit, R.; Baranidharan, B. Crop Yield Prediction using XG Boost Algorithm. Int. J. Recent Technol. Eng. 2020, 5, 2277–3878. Available online: https://www.researchgate.net/publication/364028532_Crop_Yield_Prediction_using_XG_Boost_Algorithm (accessed on 2 February 2024).




| Band Name | Center Wavelength (NM) | Bandwidth (NM) |
|---|---|---|
| Blue | 475 | 32 |
| Green | 560 | 27 |
| Red | 668 | 14 |
| Red Edge | 717 | 12 |
| NIR | 842 | 57 |
| Stages | Image Date |
|---|---|
| Heading | 12 and 26 May 2023 |
| Flowering | 8, 16, 23 June 2023 |
| Milk | 4 and 12 July 2023 |
| Dough | 20 July 2023 |
| Ripening | 29 July and 10 August 2023 |
| Treatments | Nitrogen Content | Plots |
|---|---|---|
| T1 | No nitrogen | 411, 208, 112, 304 |
| T2 | 30 kg/ha at seeding and 90 kg/ha in early spring | 410, 105, 210, 312 |
| T3 | 120 kg/ha | 412,314, 209, 108 |
| T4 | 120 kg/ha (Entec Soil Nitrogen) | 211, 408, 115, 306 |
| T5 | 120 kg/ha (Urea SuperU) | 409, 202, 113, 310 |
| T6 | 100 kg/ha (Urea SuperU) | 214, 414, 101, 308 |
| T7 | 90 kg/ha (Urea) and 30 kg/ha (Entec Soil Nitrogen) | 212, 414, 101, 308 |
| T8 | 90 kg/ha (Urea) and 60 kg/ha (Entec Soil Nitrogen) | 415, 204, 114, 311 |
| T9 | 90 kg/ha (Urea) and 90 kg/ha (Entec Soil Nitrogen) | 206, 405, 313, 103 |
| T10 | 90 kg/ha (Urea) and 30 kg/ha (SuperU) | 203, 401, 315, 106 |
| T11 | 60 kg/ha (Urea) and 60 kg/ha (SuperU) | 215, 413, 104, 307 |
| T12 | 30 kg/ha (Urea) and 90 kg/ha (SuperU) | 213, 407, 102, 305 |
| T13 | 40 kg/ha, 40 kg/ha (Entec Soil Nitrogen) and 40 kg/ha (SuperU) | 207, 403, 111, 301 |
| T14 | 160 kg/ha (Urea) | 416, 215, 303, 109 |
| T15 | 120 kg/ha (treated with nitrogen stabilizer) | 201, 404, 107, 309 |
| Vegetation Index | Equation | Reference |
|---|---|---|
| Difference Vegetation Index | DVI = G − B | [27] |
| Modified Chlorophyll Absorption in Reflectance Index | MCARI = [(RE − R) − 0.2 × (RE − G)] × (RE/R) | [28] |
| Green Chlorophyll Index | GCI = (NIR/G) − 1 | [29] |
| Rededge Chlorophyll Index | RECI = (NIR/RE) − 1 | [29] |
| Normalized Difference Vegetation Index | NDVI = (NIR − R)/(NIR + R) | [30] |
| Green Normalized Difference Vegetation Index | GNDVI = (NIR − G)/(NIR + G) | [31] |
| Rededge Normalized Difference Vegetation Index | NDRE = (NIR − RE)/(NIR + RE) | [32] |
| Two-band Enhanced Vegetation Index | EVI2 = 2.5 × [(NIR − R)/(NIR + 2.4 × R + 1)] | [33] |
| 12 July 2023 | 20 July 2023 | 29 July 2023 |
|---|---|---|
| GCI, DVI | GCI, RECI, NDVI, NDRE, DVI | MCARI, GNDVI, NDRE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, M.V.B.; Heenkenda, M.K.; Sahota, T.S.; Serrano, L.S. Analyzing Winter Wheat (Triticum aestivum) Growth Pattern Using High Spatial Resolution Images: A Case Study at Lakehead University Agriculture Research Station, Thunder Bay, Canada. Crops 2024, 4, 115-133. https://doi.org/10.3390/crops4020009
Fuentes MVB, Heenkenda MK, Sahota TS, Serrano LS. Analyzing Winter Wheat (Triticum aestivum) Growth Pattern Using High Spatial Resolution Images: A Case Study at Lakehead University Agriculture Research Station, Thunder Bay, Canada. Crops. 2024; 4(2):115-133. https://doi.org/10.3390/crops4020009
Chicago/Turabian StyleFuentes, María V. Brenes, Muditha K. Heenkenda, Tarlok S. Sahota, and Laura Segura Serrano. 2024. "Analyzing Winter Wheat (Triticum aestivum) Growth Pattern Using High Spatial Resolution Images: A Case Study at Lakehead University Agriculture Research Station, Thunder Bay, Canada" Crops 4, no. 2: 115-133. https://doi.org/10.3390/crops4020009
APA StyleFuentes, M. V. B., Heenkenda, M. K., Sahota, T. S., & Serrano, L. S. (2024). Analyzing Winter Wheat (Triticum aestivum) Growth Pattern Using High Spatial Resolution Images: A Case Study at Lakehead University Agriculture Research Station, Thunder Bay, Canada. Crops, 4(2), 115-133. https://doi.org/10.3390/crops4020009






