Assessing the Impact of King Coconut Husk Ash and Biochar, Combined with Chemical Fertilizer Application, on Enhancing Soil Fertility in Coconut Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Biochar/Ash Production
2.2. Treatments and Experimental Design
2.3. Data Collection and Analysis
3. Results and Discussion
3.1. Characterization of KCH Ash and Biochar
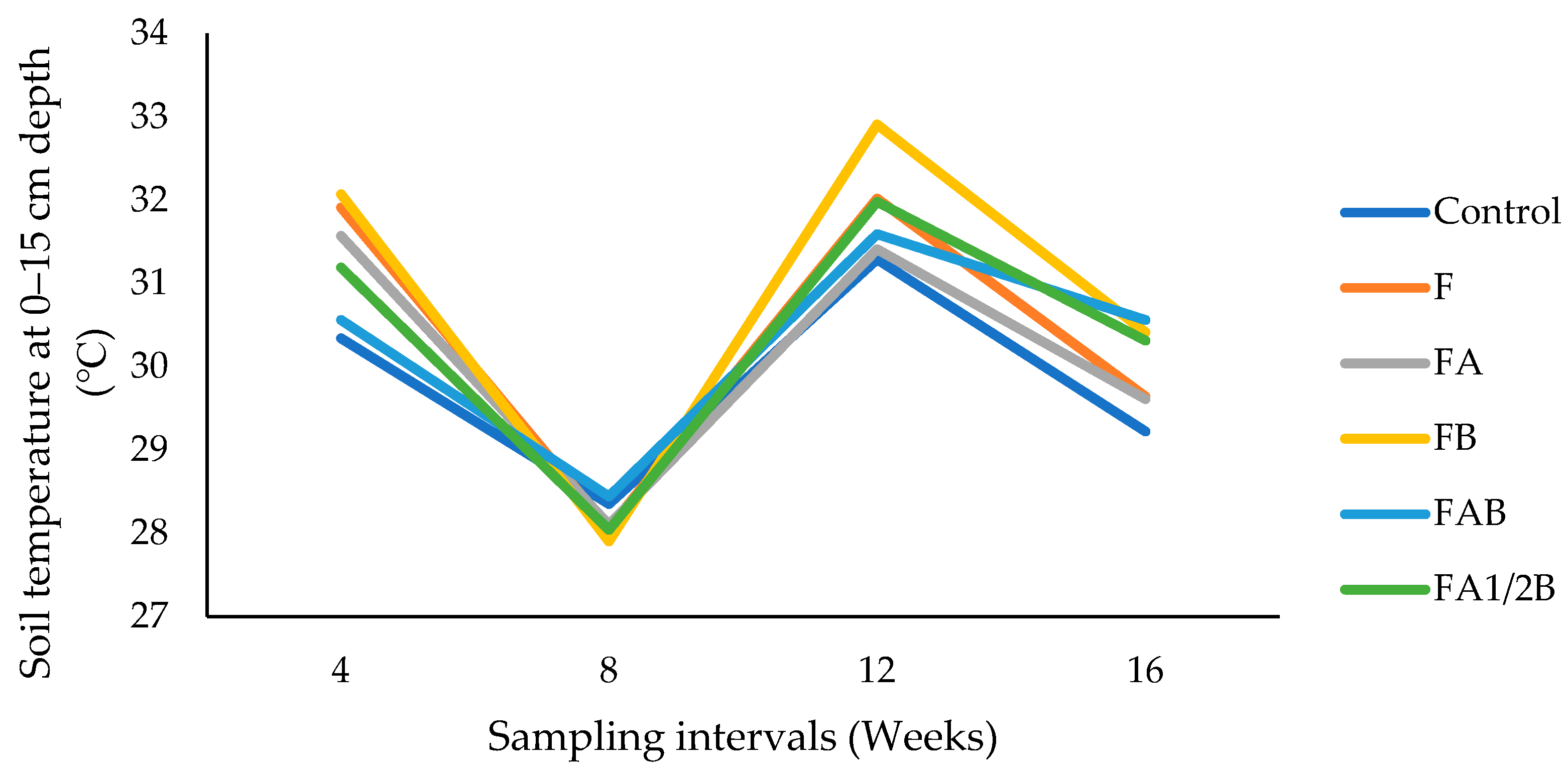
3.2. Soil Physical Properties
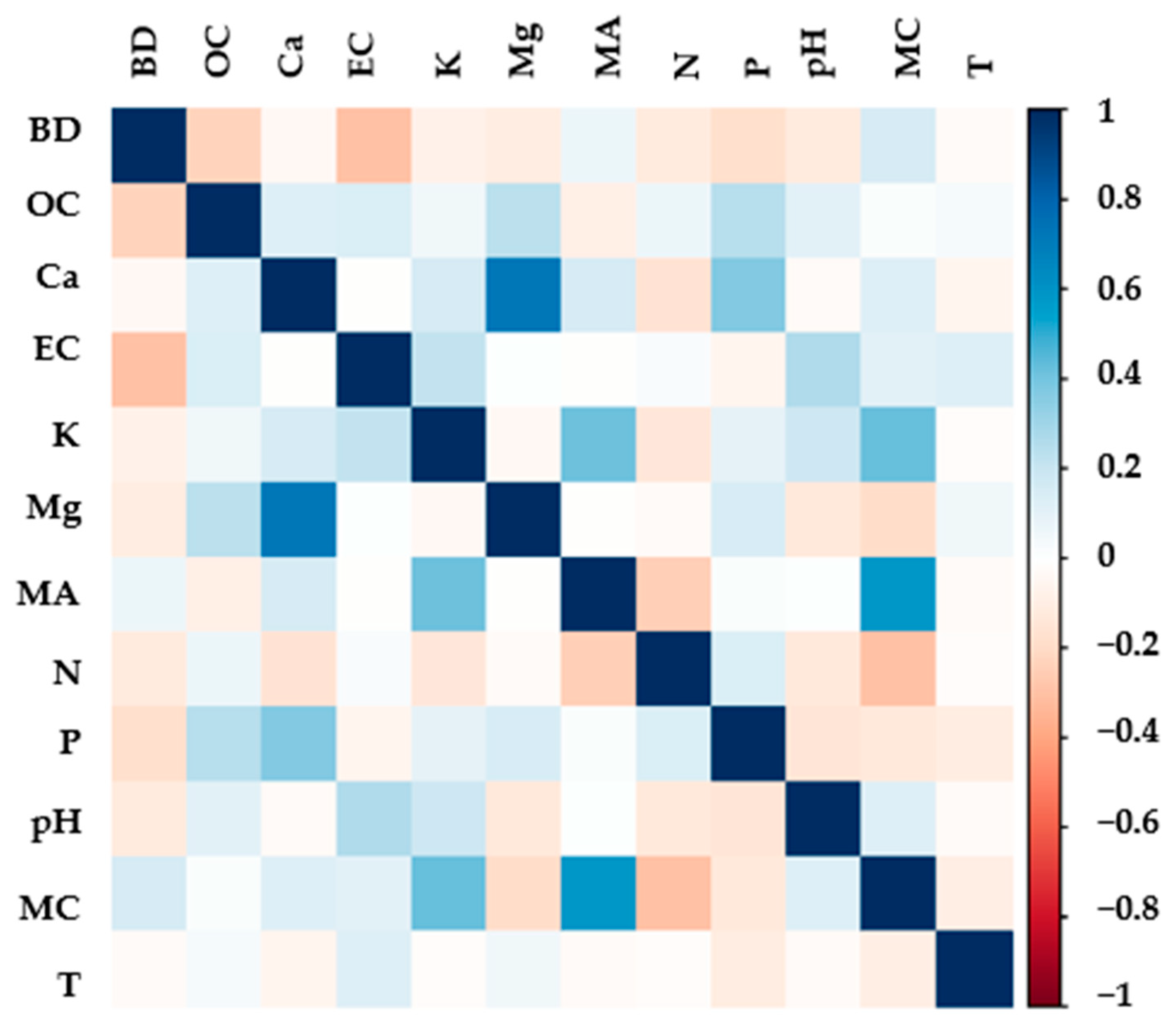
3.3. Soil Chemical Properties
3.3.1. Soil pH and EC
3.3.2. Available Nitrogen Content
3.3.3. Available Potassium Content
3.3.4. Available Phosphorus Content
3.3.5. Available Calcium and Magnesium Content
3.3.6. Organic Carbon Content
3.4. Soil Biological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dissanayaka, D.M.N.S.; Udumann, S.S.; Nuwarapaksha, T.D.; Atapattu, A.J. Effects of pyrolysis temperature on chemical composition of coconut-husk biochar for agricultural applications: A characterization study. Technol. Agron. 2023, 3, 13. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.N.S.; Nuwarapaksha, T.D.; Udumann, S.S.; Dissanayake, D.K.R.P.L.; Atapattu, A.J. A sustainable way of increasing productivity of coconut cultivation using cover crops: A review. Circ. Agric. Syst. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Alouw, J.C.; Wulandari, S. Present status and outlook of coconut development in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 418, 012035. [Google Scholar] [CrossRef]
- Adugna, G. A review on impact of compost on soil properties, water use and crop productivity. Acad. Res. J. Agric. Sci. Res. 2016, 4, 93–104. [Google Scholar]
- Dissanayaka, D.M.N.S.; Dissanayake, D.K.R.P.L.; Udumann, S.S.; Nuwarapaksha, T.D.; Atapattu, A.J. Agroforestry—A key tool in the climate-smart agriculture context: A review on coconut cultivation in Sri Lanka. Front. Agron. 2023, 5, 1162750. [Google Scholar] [CrossRef]
- Nuwarapaksha, T.D.; Udumann, S.S.; Dissanayaka, D.M.N.S.; Dissanayake, D.K.R.P.L.; Atapattu, A.J. Coconut based multiple cropping systems: An analytical review in Sri Lankan coconut cultivations. Circ. Agric. Syst. 2022, 2, 1–7. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, J.C.; Díaz-Flores, P.E.; Zavala-Sierra, D.; Preciado-Rangel, P.; Rodríguez-Fuentes, H.; Estrada-González, A.J.; Carballo-Méndez, F.J. Organic vs. conventional fertilization: Soil nutrient availability, production, and quality of tomato fruit. Water Air Soil Pollut. 2022, 233, 87. [Google Scholar] [CrossRef]
- Dissanayaka, N.S.; Dissanayake, L.; Dassanayake, S.D.; Udumann, S.S.; Keerthisinghe, J.P.; Jayalath, N.; Idirisinghe, S.K.; Silva, S.; Gammampila, J.; Janaka, R.; et al. Enhancing sustainable agriculture through king coconut husk ash: Investigating optimal processing parameters for high potassium content and efficient waste management. Biol. Life Sci. Forum 2023, 27, 17. [Google Scholar] [CrossRef]
- Ekanayaka, E.M.G.N.; Dissanayake, D.K.R.P.L.; Udumann, S.S.; Dissanayaka, D.M.N.S.; Nuwarapaksha, T.D.; Herath, H.M.S.K.; Atapattu, A.J. Sustainable utilization of king coconut husk as a feedstock in biochar production with the highest conversion efficiency and desirable properties. IOP Conf. Ser. Earth Environ. Sci. 2023, 1235, 012009. [Google Scholar] [CrossRef]
- Hossain, M.U.; Ng, S.T.; Antwi-Afari, P.; Amor, B. Circular economy and the construction industry: Existing trends, challenges and prospective framework for sustainable construction. Renew. Sustain. Energy Rev. 2020, 130, 109948. [Google Scholar] [CrossRef]
- Knoblauch, C.; Priyadarshani, S.R.; Haefele, S.M.; Schröder, N.; Pfeiffer, E.M. Impact of biochar on nutrient supply, crop yield and microbial respiration on sandy soils of northern Germany. Eur. J. Soil Sci. 2021, 72, 1885–1901. [Google Scholar] [CrossRef]
- Dissanayake, D.K.R.P.L.; Udumann, S.S.; Dissanayaka, D.M.N.S.; Nuwarapaksha, T.D.; Atapattu, A.J. Effect of biochar application rate on macronutrient retention and leaching in two coconut growing soils. Technol. Agron. 2023, 3, 5. [Google Scholar] [CrossRef]
- Islam, M.S.; Kwak, J.H.; Nzediegwu, C.; Wang, S.; Palansuriya, K.; Kwon, E.E.; Naeth, M.A.; El-Din, M.G.; Ok, Y.S.; Chang, S.X. Biochar heavy metal removal in aqueous solution depends on feedstock type and pyrolysis purging gas. Environ. Pollut. 2021, 281, 117094. [Google Scholar] [CrossRef]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Herath, H.M.I.K.; Wijebandara, D.M.D.I. Potential use of king coconut husk as a nutrient source for organic coconut cultivation. J. Food Agric. 2017, 10, 1–7. [Google Scholar] [CrossRef]
- Priyadarshani, J.; Seran, T.H. Paddy husk ash as a source of potassium for growth and yield of cowpea (Vigna unguiculata L.). J. Agric. Sci. 2006, 4, 67–76. Available online: http://www.sljol.info/index.php/JAS/article/view/1646/1402 (accessed on 10 January 2024). [CrossRef][Green Version]
- Pathan, S.M.; Aylmore, L.A.G.; Colmer, T.D. Properties of several fly ash materials in relation to use as soil amendments. J. Environ. Qual. 2003, 32, 687–693. [Google Scholar] [CrossRef]
- Rawat, J.; Sanwal, P.; Saxena, J. Potassium and its role in sustainable agriculture. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016; pp. 235–253. [Google Scholar] [CrossRef]
- Bakshi, S.; Fidel, R.; Banik, C.; Aller, D.; Brown, R.C. Retention of oxyanions on biochar surface. Sustain. Biochar Water Wastewater Treat. 2022, 233–276. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef]
- Fungo, B.; Lehmann, J.; Kalbitz, K.; Thionģo, M.; Okeyo, I.; Tenywa, M.; Neufeldt, H. Aggregate size distribution in a biochar-amended tropical Ultisol under conventional hand-hoe tillage. Soil Tillage Res. 2017, 165, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Y.L.L.; Ye, L.L.; Wang, C.H.; Zhou, H.; Sun, B. Temperature-and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 2011, 112, 159–166. [Google Scholar] [CrossRef]
- Jeffery, S.; Meinders, M.B.; Stoof, C.R.; Bezemer, T.M.; van de Voorde, T.F.; Mommer, L.; van Groenigen, J.W. Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma 2015, 251, 47–54. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Alkhasha, A.; Ibrahim, H.M. Effect of biochar particle size on water retention and availability in a sandy loam soil. J. Saudi Chem. Soc. 2020, 24, 1042–1050. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K.; Mierzwa–Hersztek, M. Biological effects of biochar and zeolite used for remediation of soil contaminated with toxic heavy metals. Sci. Rep. 2021, 11, 6998. [Google Scholar] [CrossRef] [PubMed]
- Maheswarappa, H.P.; Subramanian, P.; Dhanapal, R. Root distribution pattern of coconut (Cocos nucifera L.) in littoral sandy soil. J. Plant. Crops 2000, 28, 164–166. [Google Scholar]
- Dhanapal, R.; Maheswarappa, H.P.; Subramanian, P. Response of coconut roots to the methods of irrigation in littoral sandy soil. J. Plant. Crops 2000, 28, 208–211. [Google Scholar]
- Güereña, D.T.; Lehmann, J.; Thies, J.E.; Enders, A.; Karanja, N.; Neufeldt, H. Partitioning the contributions of biochar properties to enhanced biological nitrogen fixation in common bean (Phaseolus vulgaris). Biol. Fertil. Soils 2015, 51, 479–491. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis. Part 3. Chemical Methods, 5.3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 1021–1085. [Google Scholar] [CrossRef]
- Grace, C.; Hart, M.; Brookes, P.C. Laboratory Manual of the Soil Microbial Biomass Group; Rothamsted Experimental Station: Harpenden, UK, 2006; p. 65. [Google Scholar]
- Shi, R.; Li, J.; Jiang, J.; Mehmood, K.; Liu, Y.; Xu, R.; Qian, W. Characteristics of biomass ashes from different materials and their ameliorative effects on acid soils. J. Environ. Sci. 2017, 55, 294–302. [Google Scholar] [CrossRef]
- Park, B.B.; Yanai, R.D.; Sahm, J.M.; Lee, D.K.; Abrahamson, L.P. Wood ash effects on plant and soil in a willow bioenergy plantation. Biomass Bioenergy 2005, 28, 355–365. [Google Scholar] [CrossRef]
- Wang, X.; Lu, P.; Yang, P.; Ren, S. Effects of fertilizer and biochar applications on the relationship among soil moisture, temperature, and N2O emissions in farmland. PeerJ 2021, 9, e11674. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Šimanský, V.; Horák, J.; Igaz, D. Potential of biochar to alternate soil properties and crop yields 3 and 4 years after the application. Agronomy 2020, 10, 889. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties—New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef] [PubMed]
- Toková, L.; Igaz, D.; Horák, J.; Aydin, E. Effect of biochar application and re-application on soil bulk density, porosity, saturated hydraulic conductivity, water content and soil water availability in a silty loam Haplic Luvisol. Agronomy 2020, 10, 1005. [Google Scholar] [CrossRef]
- Luo, C.; Yang, J.; Chen, W.; Han, F. Effect of biochar on soil properties on the Loess Plateau: Results from field experiments. Geoderma 2020, 369, 114323. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Does biochar application alleviate soil compaction? Review and data synthesis. Geoderma 2021, 404, 115317. [Google Scholar] [CrossRef]
- Faye, A.; Stewart, Z.P.; Diome, K.; Edward, C.T.; Fall, D.; Ganyo, D.K.K.; Akplo, T.M.; Prasad, P.V. Single application of biochar increases fertilizer efficiency, C sequestration, and pH over the long-term in sandy soils of Senegal. Sustainability 2021, 13, 11817. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 183, 521512. [Google Scholar] [CrossRef]
- Dissanayake, D.K.R.P.L.; Dissanayaka, D.M.N.S.; Udumann, S.S.; Nuwarapaksha, T.D.; Atapattu, A.J. Is biochar a promising soil amendment to enhance perennial crop yield and soil quality in the tropics? Technol. Agron. 2023, 3, 4. [Google Scholar] [CrossRef]
- Cao, D.; Lan, Y.; Chen, W.; Yang, X.; Wang, D.; Ge, S.; Yang, J.; Wang, Q. Successive applications of fertilizers blended with biochar in the soil improve the availability of phosphorus and productivity of maize (Zea mays L.). Eur. J. Agron. 2021, 130, 126344. [Google Scholar] [CrossRef]
- Kumar, A.; Joseph, S.; Graber, E.R.; Taherymoosavi, S.; Mitchell, D.R.; Munroe, P.; Tsechansky, L.; Lerdahl, O.; Aker, W.; Sæbø, M. Fertilizing behavior of extract of organomineral-activated biochar: Low-dose foliar application for promoting lettuce growth. Chem. Biol. Technol. Agric. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Zhaoxiang, W.; Huihu, L.; Qiaoli, L.; Changyan, Y.; Faxin, Y. Application of bio-organic fertilizer, not biochar, in degraded red soil improves soil nutrients and plant growth. Rhizosphere 2020, 16, 100264. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Neupane, A.; Abdoulmoumine, N.; DeBruyn, J.M.; Walker, F.R.; Jagadamma, S. Co-application of biochar and nitrogen fertilizer reduced nitrogen losses from soil. PLoS ONE 2021, 16, e0248100. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Chang, S.X. Biochar decreases the efficacy of the nitrification inhibitor nitrapyrin in mitigating nitrous oxide emissions at different soil moisture levels. J. Environ. Manag. 2021, 295, 113080. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yan, B.; Zhong, L.; Zhang, R.; Guo, X.; Cui, X.; Lu, W.; Chen, G. Combustion ash addition promotes the production of K-enriched biochar and K release characteristics. J. Clean. Prod. 2021, 311, 127557. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K. Developing biochar-based slow-release NPK fertilizer for controlled nutrient release and its impact on soil health and yield. Biomass Convers. Biorefinery 2023, 13, 13051–13063. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K. Development and evaluation of biochar-based secondary and micronutrient enriched slow-release nano-fertilizer for reduced nutrient losses. Biomass Convers. Biorefinery 2024, 13, 12193–12204. [Google Scholar] [CrossRef]
- Ayeni, L.S. Cumulative effect of combined cocoa pod ash, poultry manure, NPK 20: 10: 10 fertilizer on major cations release for crop production in southwestern Nigeria. Int. Res. J. Agric. Sci. Soil Sci. 2011, 1, 248–253. Available online: http://interesjournals.org/IRJAS/Pdf/2011/September/Ayeni.pdf (accessed on 13 January 2024).
- Saletnik, B.; Zagula, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and biomass ash as a soil ameliorant: The effect on selected soil properties and yield of giant miscanthus (Miscanthus × giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef]
- Ullah, Z.; Ali, S.; Muhammad, N.; Khan, N.; Rizwan, M.; Khan, M.D.; Khan, N.; Khattak, B.; Alhaithloul, H.A.S.; Soliman, M.H.; et al. Biochar impact on microbial population and elemental composition of red soil. Arab. J. Geosci. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Ennis, C.J.; Evans, A.G.; Islam, M.; Ralebitso-Senior, T.K.; Senior, E. Biochar: Carbon sequestration, land remediation, and impacts on soil microbiology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2311–2364. [Google Scholar] [CrossRef]
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
- Bang-Andreasen, T.; Nielsen, J.T.; Voriskova, J.; Heise, J.; Rønn, R.; Kjøller, R.; Hansen, H.C.; Jacobsen, C.S. Wood ash induced pH changes strongly affect soil bacterial numbers and community composition. Front. Microbiol. 2017, 8, 1400. [Google Scholar] [CrossRef] [PubMed]



| Type of Fertilizer | Major Nutrient | Major Nutrient’s Percentage |
|---|---|---|
| Urea | Nitrogen | 46 |
| ERP | Phosphorus pentoxide | 28–30 |
| MOP | Potassium oxide | 60 |
| Dolomite | Magnesium oxide | 20 |
| Treatments | Abbreviations | Chemical Fertilizer (kg) | Biochar (kg) | Ash (kg) | |||
|---|---|---|---|---|---|---|---|
| Urea | ERP * | MOP * | Dolomite | ||||
| 1 | Control | - | - | - | - | - | - |
| 2 | F | 0.8 | 0.9 | 0.9 | 1.0 | - | - |
| 3 | FA | 0.8 | 0.9 | - | 1.0 | - | 3.2 |
| 4 | FB | 0.8 | 0.9 | 0.9 | 1.0 | 30 | - |
| 5 | FAB | 0.8 | 0.9 | - | 1.0 | 30 | 3.2 |
| 6 | FA1/2B | 0.8 | 0.9 | - | 1.0 | 30 | 1.6 |
| Material | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Available | Total | Available | Total | Available | Total | Available | Total | Available | |
| KCH biochar | 1.57 | 0.22 | 0.93 | 0.58 b,* | 3.61 b | 0.18 b | 0.40 | 0.36 | 0.35 | 0.02 |
| KCH ash | 0.95 | 0.02 | 1.30 | 1.28 a | 16.01 a | 2.10 a | 1.38 | 1.35 | 1.39 | 1.35 |
| Parameter | Sampling Intervals (Weeks) | Treatments | |||||
|---|---|---|---|---|---|---|---|
| Control | F | FA | FB | FAB | FA½B | ||
| Moisture content (%) | 4 | 2.94 b,* | 5.50 ab | 7.82 a | 4.97 b | 7.88 a | 3.86 b |
| 8 | 13.23 | 11.45 | 13.93 | 15.64 | 14.14 | 13.45 | |
| 12 | 3.43 b | 4.12 b | 4.05 b | 4.03 b | 6.14 a | 3.50 b | |
| 16 | 3.63 | 3.78 | 4.52 | 3.89 | 4.27 | 4.92 | |
| Bulk density (g cm−3) | 4 | 1.30 a | 0.94 ab | 1.09 ab | 1.08 ab | 1.02 ab | 0.70 b |
| 8 | 1.47 | 1.24 | 1.26 | 1.3 | 0.97 | 1.27 | |
| 12 | 1.32 | 1.09 | 1.12 | 1.12 | 1.04 | 1.12 | |
| 16 | 1.21 | 1.09 | 0.96 | 0.95 | 1.03 | 1.06 | |
| Parameter | Sampling Intervals (Weeks) | Treatments | |||||
|---|---|---|---|---|---|---|---|
| Control | F | FA | FB | FAB | FA1/2B | ||
| pH | 4 | 7.63 | 7.42 | 8.13 | 7.76 | 7.99 | 8.28 |
| 8 | 6.74 | 7.31 | 7.63 | 7.28 | 7.58 | 7.43 | |
| 12 | 6.69 | 6.32 | 6.05 | 6.46 | 6.77 | 6.82 | |
| 16 | 6.98 | 7.09 | 7.38 | 7.31 | 7.81 | 8.01 | |
| EC (μS/cm) | 4 | 62.75 c,* | 258.49 b | 245.34 b | 219.31 bc | 390.60 ab | 432.33 a |
| 8 | 29.34 b | 70.14 b | 57.60 b | 216.78 a | 43.70 b | 190.91 a | |
| 12 | 70.24 b | 79.36 b | 58.79 b | 108.36 ab | 149.11 ab | 175.19 b | |
| 16 | 43.11 c | 44.13 c | 73.43 bc | 173.33 a | 93.94 abc | 107.06 b | |
| Parameter | Treatments | |||||
|---|---|---|---|---|---|---|
| Control | F | FA | FB | FAB | FA1/2B | |
| Available Ca (%) | 0.17 | 0.18 | 0.21 | 0.12 | 0.13 | 0.15 |
| Available Mg (%) | 0.016 | 0.027 | 0.029 | 0.012 | 0.009 | 0.021 |
| Organic carbon (%) | 4.59 b,* | 5.02 ab | 6.87 a | 4.78 b | 4.43 b | 5.29 ab |
| Treatments | P Index | R Index | Score Index | Combined Index | Rank |
|---|---|---|---|---|---|
| Control | 0.220 | 0.000 | 0.000 | 0.220 | 6 |
| F | 0.158 | 0.579 | 0.457 | 1.194 | 4 |
| FA | 0.446 | 0.310 | 0.393 | 1.150 | 5 |
| FB | 0.000 | 1.000 | 1.000 | 2.000 | 2 |
| FAB | 0.157 | 0.641 | 0.619 | 1.417 | 3 |
| FA1/2B | 1.000 | 0.644 | 0.839 | 2.483 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamila, S.K.; Udumann, S.S.; Dissanayaka, N.S.; Rajaratnam, K.; Atapattu, A.J. Assessing the Impact of King Coconut Husk Ash and Biochar, Combined with Chemical Fertilizer Application, on Enhancing Soil Fertility in Coconut Plantations. Crops 2024, 4, 227-241. https://doi.org/10.3390/crops4020017
Shamila SK, Udumann SS, Dissanayaka NS, Rajaratnam K, Atapattu AJ. Assessing the Impact of King Coconut Husk Ash and Biochar, Combined with Chemical Fertilizer Application, on Enhancing Soil Fertility in Coconut Plantations. Crops. 2024; 4(2):227-241. https://doi.org/10.3390/crops4020017
Chicago/Turabian StyleShamila, Selvaraja Kaushalya, Shashi S. Udumann, Nuwandhya S. Dissanayaka, Kowshalya Rajaratnam, and Anjana J. Atapattu. 2024. "Assessing the Impact of King Coconut Husk Ash and Biochar, Combined with Chemical Fertilizer Application, on Enhancing Soil Fertility in Coconut Plantations" Crops 4, no. 2: 227-241. https://doi.org/10.3390/crops4020017
APA StyleShamila, S. K., Udumann, S. S., Dissanayaka, N. S., Rajaratnam, K., & Atapattu, A. J. (2024). Assessing the Impact of King Coconut Husk Ash and Biochar, Combined with Chemical Fertilizer Application, on Enhancing Soil Fertility in Coconut Plantations. Crops, 4(2), 227-241. https://doi.org/10.3390/crops4020017







