Pesticidal Potential and Selectivity of Soybean Extract on Pests and Non-Target Insects of Cocoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Samples
2.2. Laboratory Bioassays
2.2.1. Experiment I
2.2.2. Experiment II
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wessel, M.; Quist-Wessel, P.M.F. Cocoa production in West Africa, a review and analysis of recent developments. NJAS Wagening. J. Life Sci. 2015, 74–75, 1–7. [Google Scholar] [CrossRef]
- Adu-Acheampong, R.; Jiggins, J.; van Huis, A.; Cudjoe, A.R.; Johnson, V.; Sakyi-Dawson, O.; Ofori-Frimpong, K.; Osei-Fosu, P.; Tei-Quartey, E.; Jonfia-Essien, W.; et al. The cocoa mirid (Hemiptera: Miridae) problem: Evidence to support new recommendations on the timing of insecticide application on cocoa in Ghana. Int. J. Trop. Insect. Sci. 2014, 34, 58–71. [Google Scholar] [CrossRef]
- Avicor, S.W.; Awudzi, G.K.; Adu-Acheampong, R.; Boamah-Dankyi, P.; Adu-Acheampong, S. Contact toxicity and proximate effect of fipronil on insect pest and predatory ant community structure in cocoa agro-ecosystem. J. Agric. Food Res. 2023, 14, 100909. [Google Scholar] [CrossRef]
- Statista. Global Cocoa Bean Production from 2020/21 to 2023/24, by Country. Available online: https://www.statista.com/statistics/263855/cocoa-bean-production-worldwide-by-region/ (accessed on 7 February 2025).
- Awudzi, G.K.; Adu-Acheampong, R.; Ahadzi, S.K.; Avicor, S.W. Field Guide for Cocoa Insect Pests Identification, Damage Symptoms and Management; Cocoa Research Institute of Ghana Technical Bulletin No. 28; Cocoa Research Institute of Ghana: New Tafo-Akim, Ghana, 2019; 27p. [Google Scholar]
- Padi, B.; Owusu, G.K. Towards an integrated pest management for sustainable cocoa production in Ghana. In Paper from Workshop in Panama 1998; Smithsonian Institute: Washington, DC, USA, 2003; p. 16. Available online: http://nationalzoo.sci.edu/scbi./migratorybirds/research/cacao/padi.cfm (accessed on 21 March 2018).
- N’Guessan, K.F. Major pests and diseases, situations and damage assessment, protocols in Côte d’Ivoire. In Proceedings of the Regional Workshop on Integrated Management of Cocoa Pests and Pathogens in Africa, Accra, Ghana, 15–18 April 2013. [Google Scholar]
- Opoku, I.Y.; Appiah, A.A.; Akrofi, A.Y.; Owusu, G.K. Phytophthora megakarya: A potential threat to the cocoa industry in Ghana. Ghana J. Agric. Sci. 2000, 33, 237–248. [Google Scholar] [CrossRef]
- Akrofi, A.Y.; Amoako-Atta, I.; Assuah, M.; Asare, E.K. Black pod disease on cacao (Theobroma cacao, L.) in Ghana: Spread of Phytophthora megakarya and role of economic plants in the disease epidemiology. Crop Prot. 2015, 72, 66–75. [Google Scholar] [CrossRef]
- Dakwa, J.T. A serious outbreak of blackpod disease in a marginal area of Ghana. In Proceedings of the 10th International Cocoa Research Conference, Santo Domingo, Dominican Republic, 17–23 May 1987. [Google Scholar]
- Adu-Acheampong, R.; Sarfo, J.E.; Appiah, E.F.; Nkansah, A.; Awudzi, G.; Obeng, E.; Tagbor, P.; Sem, R. Strategy for insect pest control in cocoa. Am. J. Exp. Agric. 2015, 6, 416–423. [Google Scholar] [CrossRef]
- Opoku, I.Y.; Akrofi, A.Y.; Appiah, A.A. Assessment of sanitation and fungicide application directed at cocoa tree trunks for the control of Phytophthora black pod infections in pods growing in the canopy. Eur. J. Plant Pathol. 2007, 117, 167–175. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of ecofriendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Avicor, S.W.; Wajidi, M.F.F.; Achoribo, E.S.; Ong, M.T.; Hamzah, S.N. Tiger nut (Cyperus esculentus) as a potential phytoinsecticide: Larvicidal activity of crude extracts on Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Trop. Biomed. 2021, 38, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Gagman, H.A.; Ahmad, H.; Him, N.A.I.I.N.; Avicor, S.W. In vitro assessment of deworming potential of Guiera senegalensis in Nigerian ethnoveterinary industry using Caenorhabditis elegans. Bull. Natl. Res. Cent. 2022, 46, 3. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Khan, M.A.; El-Harty, E.H.; Ammar, M.H.; Farooq, M.; Migdadi, H.M. Comparative phytochemical profiling of different soybean (Glycine max (L.) Merr) genotypes using GC–MS. Saudi J. Biol. Sci. 2018, 25, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, M.D.C.; Serradilla, M.J.; Martin, S.; Ordiales, E.; Ruiz-Moyano, S.; Córdoba, M.D.G. Antioxidant and antimicrobial activity of natural phenolic extract from defatted soybean flour by-product for stone fruit postharvest application. J. Sci. Food Agric. 2016, 96, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, E.; Arwin, A. Phytochemical screening and proximate analysis of soybeans (Glycine max) variety Gamasugen 1 and Gamasugen 2 derived from gamma rays irradiation. J. Phys. Conf. Ser. 2019, 1402, 055023. [Google Scholar] [CrossRef]
- Kikuta, S. The cytotoxic effect of genistein, a soybean isoflavone, against cultured Tribolium cells. Insects 2020, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Igboabuchi, N.A.; Llodibia, C.V. A study on the antioxidant and antimicrobial activities of seed and leaf extracs of Glycine max (L) Merr. Asian J. Res. Bot. 2018, 1, 1–8. [Google Scholar]
- Bukari, Y.; Avicor, S.W.; Awudzi, G.; Ainooson, M.K.; Asare, E.K.; Amoako-Attah, I. In vitro activity of soybean extract on cocoa disease pathogens (Erythricium salmonicolor and Marasmiellus scandens) and termite pest (Microtermes subhyalinus). Cogent Food Agric. 2022, 8, 2147474. [Google Scholar] [CrossRef]
- Zakaria, N.; Norhisham, A.R.; Yasmin, I.; Yahya, M.S.; Sanusi, R.; Azhar, B. Insecticides may compromise the benefits of tree-crop diversification on arthropod biodiversity in cocoa agroforestry smallholdings. Agroecol. Sustain. Food Syst. 2024, 48, 1068–1093. [Google Scholar] [CrossRef]
- Entwistle, P.F. Pests of Cocoa; Longman Group Ltd.: London, UK, 1972; pp. 275–288. [Google Scholar]
- Bisseleua, D.H.B.; Begoude, D.; Tonnang, H.; Vidal, S. Ant-mediated ecosystem services and disservices on market yield in cocoa. Agric. Ecosyst. Environ. 2017, 247, 409–417. [Google Scholar] [CrossRef]
- Diamé, L.; Rey, J.-Y.; Vayssières, J.-F.; Grechi, I.; Chailleux, A.; Diarra, K. Ants: Major functional elements in fruit agro-ecosystems and biological control agents. Sustainability 2018, 10, 23. [Google Scholar] [CrossRef]
- Akesse-Ransford, G.; Owusu, E.O.; Kyerematen, R.; Adu-Acheampong, S. Arthropod diversity of cocoa farms under two management systems in the Eastern and Central regions of Ghana. Agrofor. Syst. 2021, 95, 791–803. [Google Scholar] [CrossRef]
- Ali, S.S.; Amoako-Attah, I.; Bailey, R.A.; Strem, M.D.; Schmidt, M.; Akrofi, A.Y.; Surujdeo-Maharaj, S.; Kolawole, O.O.; Begoude, B.A.D.; ten Hoopen, G.M.; et al. PCR-based identification of cacao black pod causal agents and identification of biological factors possibly contributing to Phytophthora megakarya’s field dominance in West Africa. Plant Pathol. 2016, 65, 1095–1108. [Google Scholar] [CrossRef]
- Opoku, I.Y. Field and Laboratory Characterization of Phytophthora palmivora and Phytophthora megakarya in Ghana; Cocoa Research Institute of Ghana Technical Bulletin No. 15; Cocoa Research Institute of Ghana: New Tafo-Akim, Ghana, 2004; 24p. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Lozano-Fuentes, S.; Saavedra-Rodriguez, K.; Black IV, W.C.; Eisen, L. QCal: A software application for the calculation of dose-response curves in insecticide resistance bioassays. J. Am. Mosq. Control Assoc. 2012, 28, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Hashemi, M.; Hosseini, M.S. Comparison of antifungal activities of various essential oils on the Phytophthora drechsleri, the causal agent of fruit decay. Iran. J. Microbiol. 2015, 7, 31–37. [Google Scholar] [PubMed]
- Hosseini Chaleshtori, S.A.; Ataie Kachouie, M.A.; Hashemi Jazi, S.M. Antibacterial effects of the methanolic extract of Glycine max (soybean). Microbiol. Res. 2017, 8, 7319. [Google Scholar] [CrossRef]
- Lim, L.; Ab Majid, A.H. Plant derived pesticides (Citrus hystrix DC, Mentha × piperita L., Ocimum basilicum L.) in controlling household ants (Tapinoma indicum (F.), Pheidole megacephala (F.), Monomorium pharaonis (L.)) (Hymenoptera: Formicidae). Pertanika J. Trop. Agric. Sci. 2019, 42, 1321–1342. [Google Scholar]
- Mahob, R.J.; Taliedje, D.M.; Mahot, H.C.; Mama Ngah, I.; Eteme Enama, S.; Cilas, C.; Fotso Toguem, Y.G.; Hanna, R.; Bilong Bilong, C.F. Biocontrol of the brown cocoa mirids using neem oil and an ethanolic extract from neem under laboratory conditions. Afr. Entomol. 2021, 29, 507–521. [Google Scholar] [CrossRef]
- Ayenor, G.K.; Van Huis, A.; Obeng-Ofori, D.; Padi, B.; Röling, N.G. Facilitating the use of alternative capsid control methods towards sustainable production of organic cocoa in Ghana. Int. J. Trop. Insect Sci. 2007, 27, 85–94. [Google Scholar] [CrossRef]
- Mboussi, S.B.; Ambang, Z.; Kakam, S.; Bagny Beilhe, L.; Tejada Moral, M. Control of cocoa mirids using aqueous extracts of Thevetia peruviana and Azadirachta indica. Cogent Food Agric. 2018, 4, 1430470. [Google Scholar] [CrossRef]
- Anikwe, J.C. Laboratory bioassay of selected plant extracts for the control of brown cocoa mirid, Sahlbergella singularis Haglund (Hemiptera: Miridae). J. Entomol. Nematol. 2013, 5, 29–32. [Google Scholar] [CrossRef]
- Ali, A.S.; Ali, M.F. Toxicity of some extracts of common plants towards three species of Pheidole ants under laboratory conditions. Egypt. Acad. J. Biol. Sci. 2019, 13, 185–194. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 2006, 99, 478–490. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef]
- Siyoun, T.; Basu, A.K.; Tihalun, G.; Kumsa, B. Study on the acaricidal effects of Azadirachta indica and Phytolacca dodecandra on Amblyomma ticks in Ethiopia. Ethiop. Vet. J. 2014, 18, 1–14. [Google Scholar]
- Ghahari, S.; Alinezhad, H.; Nematzadeh, G.; Tajbakhsh, M.; Baharfar, R. Chemical composition, antioxidant and biological activities of the essential oil and extract of the seeds of Glycine max (soybean) from North Iran. Curr. Microbiol. 2017, 74, 522–531. [Google Scholar] [CrossRef]
- Kim, H.J.; Suh, H.J.; Lee, C.H.; Kim, J.H.; Kang, S.C.; Park, S.; Kim, J.S. Antifungal activity of glyceollins isolated from soybean elicited with Aspergillus sojae. J. Agric. Food Chem. 2010, 58, 9483–9487. [Google Scholar] [CrossRef] [PubMed]
- Balanescu, F.; Busuioc, A.C.; Botezatu, A.V.D.; Gosav, S.; Avramescu, S.M.; Furdui, B.; Dinica, R.M. Comparative study of natural antioxidants from Glycine max, Anethum graveolens and Pimpinella anisum seed and sprout extracts obtained by ultrasound-assisted extraction. Separations 2022, 9, 152. [Google Scholar] [CrossRef]
- Hay, W.T.; Behle, R.W.; Berhow, M.A.; Miller, A.C.; Sterling, G.W. Biopesticide synergy when combining plant flavonoids and entomopathogenic baculovirus. Sci. Rep. 2020, 10, 6806. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as anti- microbial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Dahanukar, S.A.; Kulkarni, R.A.; Rege, N.N. Pharmacology of medicinal plants and natural products. Indian J. Pharmacol. 2000, 32, S81–S118. [Google Scholar]
- Wang, Q.; Wang, H.; Xie, M. Antibacterial mechanism of soybean isoflavone on Staphylococcus aureus. Arch. Microbiol. 2010, 192, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.K.S.; Bader, O.; Weig, M.; Oliveira, J.T.A.; Arantes, M.R.; Gomes, V.M.; Da Cunha, M.; Oliveira, D.H.; Sousa, O.B.D.; Lourencao, L.A.; et al. Soybean Toxin (SBTX) impairs fungal growth by interfering with molecular transport, carbohydrate/amino acid metabolism and drug/stress responses. PLoS ONE 2013, 8, e70425. [Google Scholar] [CrossRef] [PubMed]

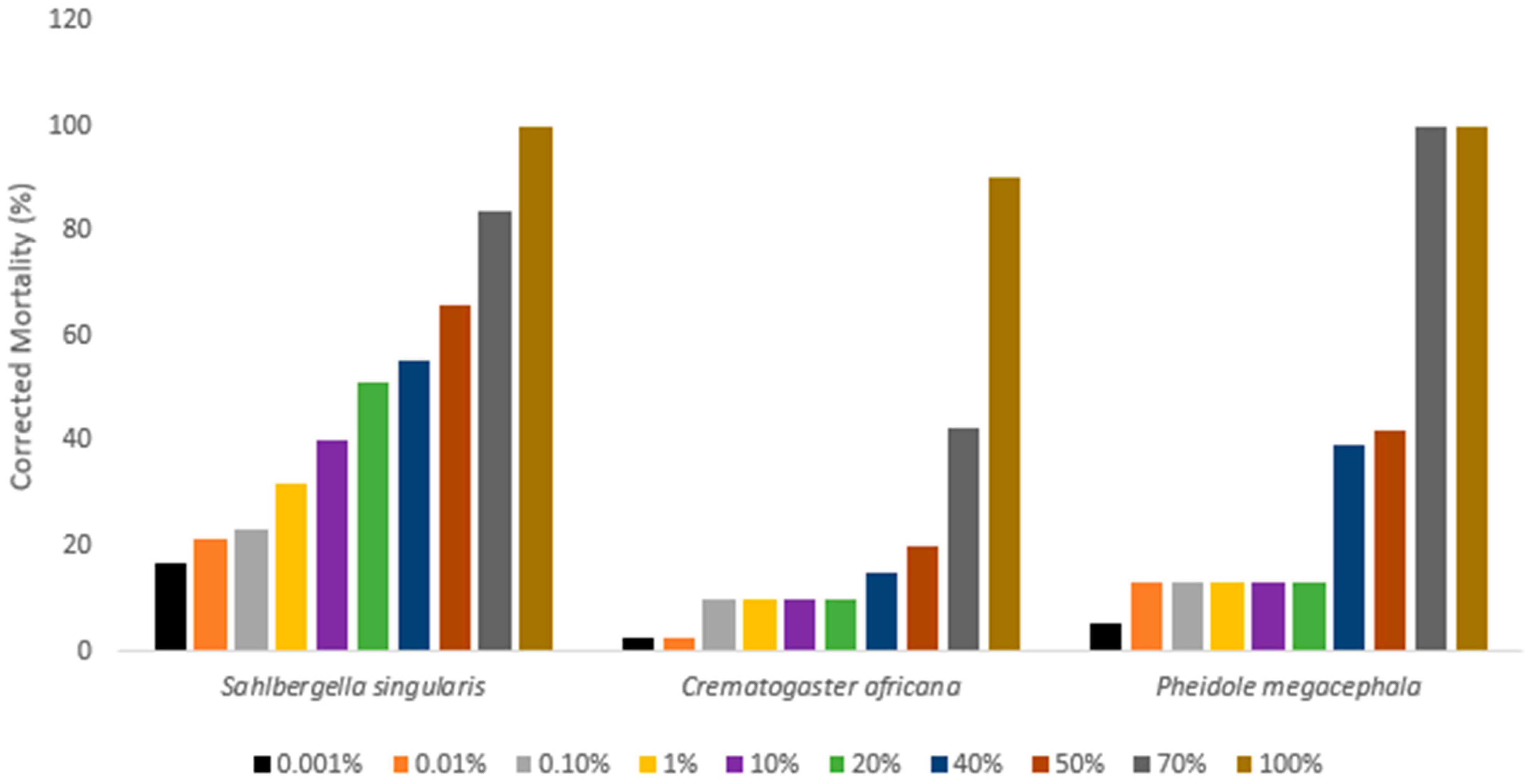
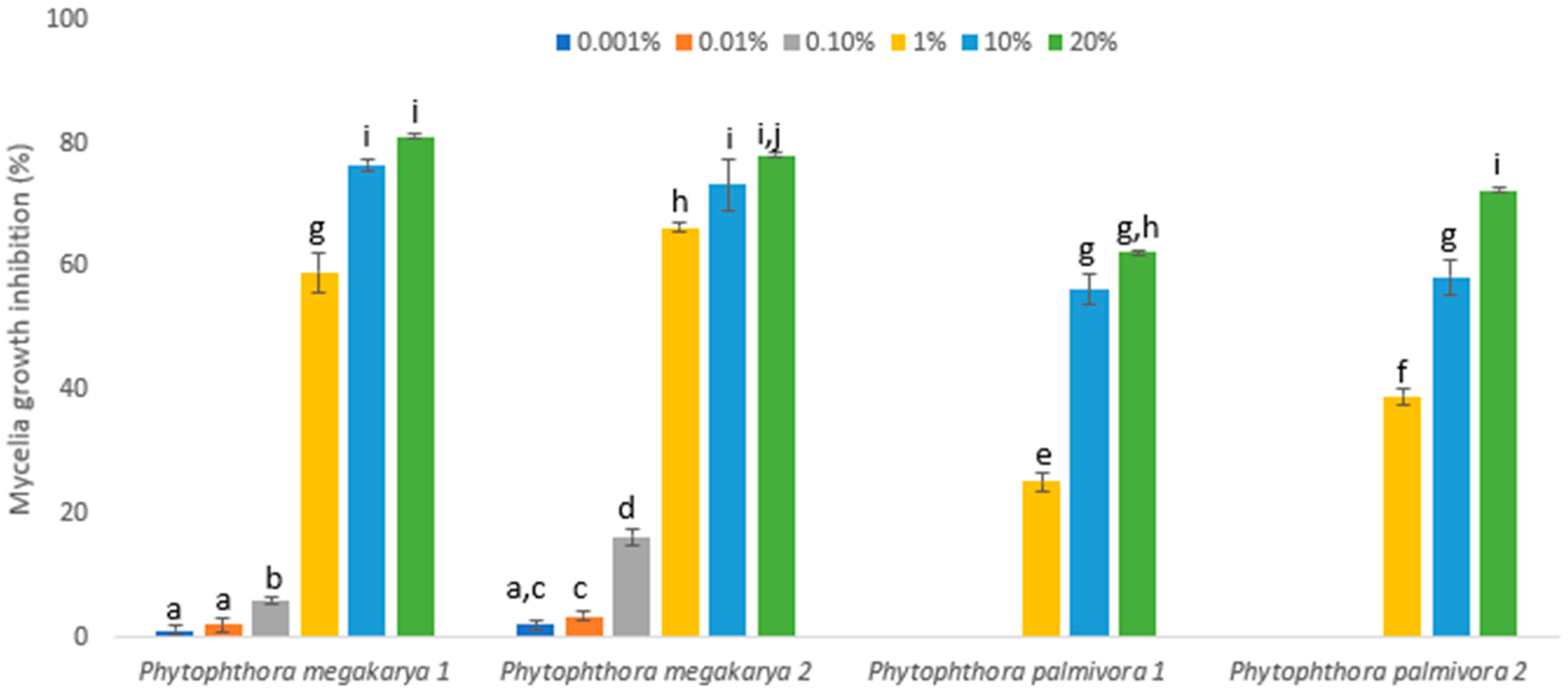
| Insect | LC50 (% w/v) | 95% CI | Slope ± SE | Intercept ± SE |
|---|---|---|---|---|
| Sahlbergella singularis | 3.5008 a | 2.1297–5.7540 | 0.2807 ± 0.0219 | −0.3517 ± 0.0787 |
| Crematogaster africana | 193.7304 b | 109.4207–342.9896 | 0.4471 ± 0.0502 | −2.3549 ± 0.1846 |
| Pheidole megacephala | - | - | - | - |
| Pathogen | IC50 (% w/v) | 95% CI | Slope ± SE | Intercept ± SE |
|---|---|---|---|---|
| Phytophthora megakarya 1 | 1.4843 a | 1.05253–2.09321 | 0.6867 ± 0.0539 | −0.2712 ± 0.1227 |
| Phytophthora megakarya 2 | 1.1256 a | 0.76277–1.66097 | 0.5612 ± 0.0437 | −0.0664 ± 0.1114 |
| Phytophthora palmivora 1 | 7.8737 b | 5.68198–10.91022 | 0.7605 ± 0.0758 | −1.5694 ± 0.1796 |
| Phytophthora palmivora 2 | 4.7038 b | 3.38922–6.527996 | 0.7323 ± 0.0660 | −1.1339 ± 0.1517 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avicor, S.W.; Bukari, Y.; Ainooson, M.K.; Awudzi, G.K.; Anyomi, W.E. Pesticidal Potential and Selectivity of Soybean Extract on Pests and Non-Target Insects of Cocoa. Crops 2025, 5, 7. https://doi.org/10.3390/crops5010007
Avicor SW, Bukari Y, Ainooson MK, Awudzi GK, Anyomi WE. Pesticidal Potential and Selectivity of Soybean Extract on Pests and Non-Target Insects of Cocoa. Crops. 2025; 5(1):7. https://doi.org/10.3390/crops5010007
Chicago/Turabian StyleAvicor, Silas Wintuma, Yahaya Bukari, Michael Kojo Ainooson, Godfred Kweku Awudzi, and Wisdom Edem Anyomi. 2025. "Pesticidal Potential and Selectivity of Soybean Extract on Pests and Non-Target Insects of Cocoa" Crops 5, no. 1: 7. https://doi.org/10.3390/crops5010007
APA StyleAvicor, S. W., Bukari, Y., Ainooson, M. K., Awudzi, G. K., & Anyomi, W. E. (2025). Pesticidal Potential and Selectivity of Soybean Extract on Pests and Non-Target Insects of Cocoa. Crops, 5(1), 7. https://doi.org/10.3390/crops5010007






