Abstract
Abiotic stresses, such as drought, salinity, and heat, exacerbated by climate change, pose significant challenges to global agriculture. These stresses negatively impact crop physiology, leading to yield losses and complicating efforts to breed resilient varieties. While advancements in molecular biology and genomics have identified stress-resistance genes, their effective utilization in breeding programs depends on precise phenotypic evaluation under diverse stress conditions. High-throughput phenotyping (HTP) technologies have emerged as indispensable tools, enabling non-destructive, rapid assessment of critical traits like root architecture, chlorophyll content, and canopy temperature in controlled and field environments. Unlike existing reviews, this manuscript critically addresses technological barriers such as cost scalability, field adaptability, and the integration of artificial intelligence for real-time data analysis. Additionally, it provides a fresh perspective on multi-omics integration in phenomics to bridge the genotype–phenotype gap, ensuring a more holistic approach to precision agriculture. This review bridges gaps in crop improvement by identifying practical solutions to enhance the adoption of HTP in breeding programs. It ensures food security amidst the escalating impacts of climate change.
1. Introduction
The growing impacts of climate change have significantly intensified the threats posed by abiotic stresses, such as drought, heat, salinity, and waterlogging, on global agriculture. Recent studies have shown that these environmental stressors continue to reduce crop yields and threaten food security globally [1]. Developing crop varieties that can withstand these conditions is crucial. To address these challenges, breeding for climate-resilient crops has become an urgent need, with modern tools such as high-throughput phenotyping (HTP) playing a central role in accelerating crop improvement efforts [2].
Traditional phenotyping methods have mainly been labor-intensive and time-consuming. However, advances in high-throughput phenotyping technologies have allowed researchers to overcome these bottlenecks, enabling precise, rapid phenotypic assessments in both controlled and field environments [3]. These technologies significantly improve breeding programs to enhance crop resilience to abiotic stresses by enabling the detailed, non-destructive measurement of critical traits like root and shoot architecture, chlorophyll content, and canopy temperature [4].
Over the past decade, HTP has evolved with innovations in imaging systems, sensor technologies, robotics, and artificial intelligence (AI)-driven data analytics [5,6]. Researchers have developed various innovative tools integrating spectroscopy, automated imaging, and high-performance computing [7,8]. HTP refers to the automated and rapid collection of large-scale plant trait data using advanced imaging, sensor technology, and computational tools to assess plant responses under various environmental conditions. These advanced technologies enable rapid, precise, and non-destructive assessment of plant traits across diverse environments in laboratory-controlled and real-world field settings [9]. By enabling non-destructive, continuous monitoring throughout the plant life cycle, these technologies have dramatically increased the speed and accuracy of phenotypic data collection. As a result, sophisticated high-throughput systems, including automated greenhouses, glass chambers, and hydroponic and aeroponic systems, now offer greater precision in data recording while significantly reducing the need for field replications [10,11]. These advancements have given rise to phenomics, which involves the large-scale, high-dimensional collection, analysis, and integration of phenotypic data with other omics approaches, enabling researchers to better understand plant responses to abiotic stresses [12]. Phenomics bridges the gap between genotyping and phenotyping, allowing for a more integrated approach to breeding climate-resilient crops [13,14]. Despite these technological advancements, significant challenges remain, including the high cost of implementation, scalability limitations, and the need for improved AI-driven analytical tools. Addressing these challenges is crucial for ensuring that phenotyping technologies become more accessible and widely adopted in breeding programs.
The objectives of this review are threefold: (1) to evaluate HTP techniques and their applications in managing abiotic stresses, including recent advancements in AI-driven data processing and multi-omics integration; (2) to critically analyze the limitations and challenges of current platforms, particularly regarding cost scalability, field adaptability, and data processing bottlenecks; and (3) to propose innovative strategies for improving the adoption of HTP technologies in breeding programs, with an emphasis on cost-effective, field-deployable solutions. By offering a comprehensive assessment, this review aims to provide actionable insights to drive advancements in climate-resilient agriculture while highlighting phenomics significant opportunities for addressing global agricultural challenges. These include enhancing breeding efficiency, accelerating genetic gain, and developing sustainable solutions to mitigate the impacts of climate change. By serving as a valuable resource for researchers, breeders, and policymakers, this review underscores the pivotal role that advancements in high-throughput phenotyping technologies play in developing crop varieties better suited to cope with the growing impacts of climate change.
2. Phenomics
Phenomics is the study of the phenome—the complete set of physical and biochemical traits expressed by an organism in response to genetic and environmental factors [15]. Unlike genomics, which explores an organism’s fixed genetic composition, phenomics captures the dynamic and variable nature of trait expression, influenced by environmental contexts [16]. This makes phenomics an essential yet challenging tool for improving crop performance under abiotic stresses such as drought, salinity, and heat [17,18].
The term “phenome” mirrors the “genome,” emphasizing the link between genetic potential and observable traits [19]. In plant science, phenomics offers critical insights into developmental, physiological, and structural traits across a plant’s life cycle, thereby elucidating plant responses to abiotic stressors [20,21]. Phenomics employs two primary approaches: forward phenomics, which identifies desirable genotypes from extensive germplasm collections using HTP to enhance breeding efficiency and reverse phenomics, which investigates the biological mechanisms underlying known desirable traits, enabling targeted breeding strategies and refined selection criteria [2,10,14,22].
Together, forward and reverse phenomics bridge the genotype-to-phenotype gap, fostering the development of stress-resilient crops. This section further reclassifies phenomics methodologies by proximity (proximal vs. remote) and the environments in which they are applied (controlled vs. field), offering a structured perspective on HTP techniques [20]
2.1. Proximal Sensing Phenomics in Controlled Environments
Proximal sensing refers to the use of technologies close to the plant, usually within controlled environments like greenhouses or growth chambers [22]. These methods allow for high-resolution, precise monitoring of physiological and morphological traits that are sensitive indicators of stress responses [10,21]. Studies demonstrate that proximal sensing can be particularly effective in early-stage breeding trials and in-depth physiological analyses due to its non-invasive nature and adaptability for longitudinal studies [23]. Shoot and root phenomics represent critical components of plant phenotyping. Shoot phenomics focuses on above-ground traits, such as leaf area and canopy structure, directly impacted by environmental conditions. Root phenomics examines below-ground traits like root architecture and water uptake, providing insights into stress tolerance mechanisms. Together, they enable a comprehensive understanding of plant responses to abiotic stresses (Table 1, Figure 1).

Table 1.
Applications of High-Throughput Phenotyping (HTP) Across Different Platforms.
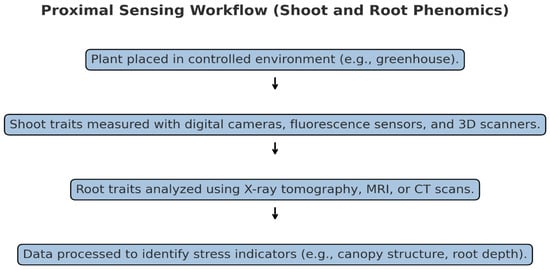
Figure 1.
Proximal sensing workflow.
2.1.1. Shoot Phenomics
Shoot phenotyping focuses on above-ground traits, which are critical indicators of plant health under stress conditions. Recent studies have utilized digital imaging systems, chlorophyll fluorescence sensors, and high-resolution 3D scanners to capture essential metrics like plant height, leaf area, canopy structure, and chlorophyll content. For instance, Yang et al. (2020) [20] employed 3D imaging to analyze canopy architecture and biomass in wheat, finding correlations between canopy traits and drought resilience. The plant-to-sensor approach, where plants are transported to a fixed imaging station, is often favored in controlled environments for its high accuracy and repeatability [23]. In addition, hyperspectral imaging has advanced shoot phenomics by allowing precise measurement of pigment composition, stress-induced changes in chlorophyll fluorescence, and other physiological markers. For example, Rahaman et al. (2015) [27] showed that combined 2D and 3D imaging techniques increased accuracy in assessing drought tolerance across rice genotypes (Table 1).
2.1.2. Root Phenomics
Root systems play a crucial role in water and nutrient uptake, making root phenotyping essential for understanding stress resilience. However, root traits are notoriously challenging to measure due to their subterranean nature. Advances in imaging technologies like Magnetic Resonance Imaging (MRI), Computed Tomography (CT), and X-ray tomography have transformed root phenotyping, allowing for non-invasive observations of root architecture. Nagel et al. (2012) [32] developed the GROWSCREEN-Rhizo platform, which combines automated image capture and data processing to measure root traits, such as depth, lateral root distribution, and root angle, under simulated drought conditions (Table 1). These technologies have been further enhanced with 3D modeling, enabling a complete reconstruction of root growth patterns, as shown in a study by Atkinson et al. (2019) [35], which used CT scans to evaluate root depth and density in wheat and barley under nutrient deficiency stress (Table 1, Figure 1).
2.2. Ground-Based Phenomics in Field Environments
Ground-based phenotyping platforms bring high-throughput data collection to the field, bridging the gap between controlled laboratory studies and real-world conditions. Ground-based systems allow researchers to collect trait data in situ, withstanding the environmental variability typical of field trials. These systems are crucial for capturing large datasets over multiple growing seasons, providing insights into plant responses in realistic settings.
2.2.1. Ground-Based Mobile Platforms
Mobile ground-based phenotyping units, such as carts and vehicles fitted with multi-sensor arrays, facilitate data capture for large-scale field studies. They often integrate digital cameras, thermal imaging, and spectrometers to measure traits such as plant height, biomass, nitrogen content, and canopy structure. Busemeyer et al. (2013) [38] demonstrated that the BreedVision platform, a mobile cart fitted with multiple sensors, provided reliable biomass and nitrogen-use efficiency data in wheat and soybean, significantly advancing breeding programs for nutrient-use efficiency. Similarly, Harris et al. (2021) [47] used ground-based platforms to monitor grapevine leaf phenotypes and correlate them with root traits, showing how vegetative and reproductive traits can be simultaneously assessed to optimize water and nutrient usage in vineyards (Table 2).

Table 2.
Image analysis programs and phenotyping platforms.
2.2.2. Fixed Ground-Based Platforms
Fixed phenotyping installations in field plots also provide essential data on plant development and stress responses. These setups often consist of stationary sensors arranged around the crop plot, capturing high-frequency measurements of temperature, humidity, leaf color, and growth rate. The use of fixed platforms in phenotyping wheat for canopy temperature under drought conditions by Maes et al. (2019) [39] illustrated how canopy temperature can indicate transpiration and stomatal conductance, which are both critical for drought resilience (Table 2) [60].
2.3. Remote Sensing Phenomics for Large-Scale Field Trials
Remote sensing enables large-scale phenotypic assessments through technologies like drones unmanned aerial vehicles(UAVs), and satellites. This approach is essential for monitoring extensive crop fields, as it provides scalable and rapid data collection across varied terrains and environmental conditions. By capturing spatial and temporal data over broad areas, remote sensing offers a practical solution for large breeding programs targeting abiotic stress tolerance (Figure 2).
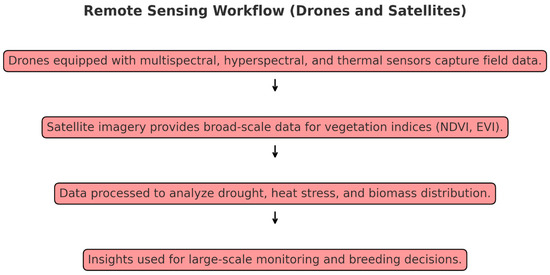
Figure 2.
Remote sensing workflow.
2.3.1. Drone-Based Phenotyping
UAVs equipped with multispectral, hyperspectral, and thermal sensors have become indispensable in phenotyping due to their high spatial resolution and flexibility. Drone-based phenotyping allows researchers to assess drought and heat stress responses by measuring canopy temperature, vegetation indices, and leaf area index (LAI). For example, Maes and Steppe (2019) [39] used UAVs to monitor drought stress in maize, detecting changes in canopy temperature and transpiration rate that correlated with water use efficiency. Another study by Yao et al. (2017) [61] applied UAV-based remote sensing to estimate LAI and biomass across various crops, illustrating the potential for this technology in monitoring large breeding populations.
2.3.2. Satellite-Based Phenotyping
Although satellites offer lower spatial resolution than UAVs, their broad coverage makes them ideal for large-scale and regional monitoring. Multispectral satellite images provide data for indices such as Normalized Difference Vegetation Index (NDVI) and Enhanced Vegetation Index (EVI), which serve as indicators of crop health, biomass, and stress levels. Satellite-based phenotyping has been particularly useful in regions facing water scarcity, where monitoring changes in vegetation indices can help guide irrigation management and identify drought-tolerant genotypes [44]. The recent study by Zhang et al. (2024) [40] illustrated the utility of satellite-derived NDVI and EVI in assessing maize and wheat fields across drought-prone areas, helping breeders select genotypes resilient to water scarcity.
2.4. Integrated Phenomics Platforms
Integrated phenotyping systems combine multiple imaging and sensing modalities to capture a comprehensive profile of plant physiological and structural traits. By concurrently measuring traits such as biomass, chlorophyll fluorescence, and canopy temperature, these platforms provide an all-encompassing view of plant health, resilience, and performance under stress conditions. These platforms leverage multi-modal imaging systems, integrating technologies like positron emission tomography (PET), MRI, and hyperspectral cameras to explore real-time plant responses (Table 2). Additionally, integrated drone-ground platforms enhance phenotyping by combining aerial and ground-based data collection for a more detailed assessment of plant traits. Another critical advancement in integrated phenomics is Light Detection and Ranging (LiDAR) technology, which enables precise three-dimensional modeling of plant architecture, offering improved insights into canopy structure and biomass distribution.
2.4.1. Multi-Modal Imaging Systems
Advanced phenomics platforms that integrate imaging modalities such as PET, MRI, and hyperspectral cameras have shown exceptional utility in exploring the real-time dynamics of plant responses. For example, Jahnke et al. (2009) [34] combined MRI and PET to visualize carbon and water transport within sugar beet taproots under drought stress (Table 1). This integration provided insights into how plants manage internal resources during water scarcity. Another example by Williamson et al. (2022) [62] illustrated the use of multispectral imaging combined with 3D structure reconstruction to track biomass accumulation and leaf area in wheat, enabling breeders to assess growth rates and stress resilience with greater accuracy.
2.4.2. Integrated Drone-Ground Platforms
Some systems combine drone and ground-based data collection for a multi-scale view of phenotypes, enhancing the resolution and depth of data across field plots. Integrated platforms like these allow for synchronized capture of canopy temperature, root architecture, and plant height, creating a full profile of plant responses in varying field conditions. A study by Rahaman et al. (2015) [27] showed how integrating drone and ground-based phenotyping provided robust estimates of crop water status and soil moisture, optimizing breeding selection for drought-tolerant traits.
2.4.3. Applications of LiDAR in Integrated Phenomics
Recent advancements in LiDAR have expanded phenomics capabilities in capturing 3D plant architecture and biomass in dense crop canopies [23]. LiDAR’s ability to penetrate dense foliage and create high-resolution, three-dimensional models makes it invaluable for monitoring structural traits in crops. LiDAR imaging has shown promise in enhancing plant density estimations and identifying canopy architecture traits correlated with drought resilience and nutrient-use efficiency, as demonstrated by Khadka et al. (2020) [22] in wheat trials.
2.4.4. Multi-Omics Data Integration for Enhanced Insights
Beyond imaging and sensor-based assessments, modern phenomics increasingly relies on multi-omics data integration, which combines genomics, transcriptomics, proteomics, and metabolomics with phenotypic data to uncover deeper biological insights [63]. This approach allows researchers to identify the genetic and molecular mechanisms underlying plant responses to abiotic stresses. For example, genotype-to-phenotype (G2P) mapping enables breeders to link genomic variations with observable traits, improving marker-assisted selection (MAS) strategies. Transcriptomic data further refine these models by revealing gene expression patterns in response to environmental stress, providing dynamic insights into stress-adaptive traits [56]. Additionally, multi-omics analyses can help uncover novel quantitative trait loci (QTLs) and their regulatory networks, accelerating the development of stress-resilient crops. The integration of multi-omics datasets through machine learning (ML) pipelines enhances phenotype predictions and improves breeding efficiency [64]. AI-driven approaches such as deep learning and predictive modeling further refine phenotyping accuracy by correlating omics data with complex phenotypic traits. This synergy between multi-omics, HTP, and AI-driven analytics transforms crop breeding, enabling the selection of genotypes better adapted to climate change and environmental variability.
3. Imaging Techniques
Advanced phenotyping relies on various imaging techniques to analyze how plants interact with light, whether it is transmitted, reflected, or absorbed. These imaging methods help capture detailed quantitative data about plant traits with remarkable precision [62]. The most commonly used imaging techniques in phenotyping include visible-light imaging, infrared and thermal-based imaging, fluorescence imaging, spectroscopy imaging, and integrated imaging systems [65]. These methods are essential for accurate and precise phenotyping under various environmental conditions [19,66].
3.1. Visible Light Imaging
Visible light imaging has been a long-standing tool in phenotyping, extensively used to assess traits linked to abiotic stress responses. This technique captures traits like biomass, shoot elongation, root architecture, leaf morphology, and seed or panicle structure [67]. Traits are typically evaluated using 2D digital images, but advancements have moved from 2D to 3D imaging, improving precision in analyzing complex traits [68]. Neilson et al. (2015) [69] used 2D digital images to study biomass under drought conditions, while Rahaman et al. (2015) [27] showed that combining 2D and 3D imaging technologies enhances accuracy in assessing phenotypic variation across crops [27]. Visible light imaging sensors, such as Charge-Coupled Device (CCD), and Complementary Metal-Oxide-Semiconductor (CMOS) arrays, accurately capture phenotypic data under stress conditions that convert light into electrical signals. CCDs transfer charges sequentially for high-quality, noise-free imaging, making them ideal for precision applications like scientific research, though they are slower and more power-intensive. CMOS sensors process signals directly at each pixel, enabling faster, energy-efficient imaging with lower costs, suitable for real-time and dynamic applications (Figure 3). While CCDs excel in uniformity and precision, modern CMOS advancements have improved their sensitivity, making both technologies vital for diverse imaging needs [70] (Figure 3). These sensors are widely used across various crops, making them a fundamental tool in research and breeding programs, enabling genetic studies to uncover the mechanisms behind stress tolerance.
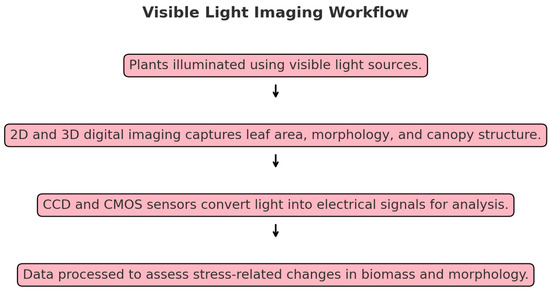
Figure 3.
Visible light imaging workflow.
3.2. Infrared and Thermal-Based Imaging
Infrared and thermal-based imaging provide critical insights into the physiological responses of plants, particularly to water content and temperature regulation [71]. These techniques use radiation principles, such as the Stefan–Boltzmann equation, to record and visualize data. The Stefan–Boltzmann law states that the total energy emitted by a surface is proportional to the fourth power of its absolute temperature. This principle is fundamental in thermal imaging for plant phenotyping, where infrared thermography is used to measure the heat emitted by plant surfaces. Since plant leaves lose heat through transpiration, variations in leaf temperature can indicate water stress, stomatal conductance, and overall plant health. By applying this equation, researchers can assess drought responses, optimize irrigation strategies, and detect early stress symptoms in crops, making thermal imaging a powerful tool in high-throughput phenotyping [70]. This combination of infrared and visible-light imaging offers detailed phenotyping of traits related to water use efficiency, drought tolerance, and gas exchange [62]. For example, Rahaman et al. (2015) [27] demonstrated that infrared imaging is practical in assessing stomatal conductance and canopy temperature in drought-stressed crops. Similarly, thermal cameras have been widely used to evaluate osmotic tolerance and sodium exclusion in plants exposed to salinity stress [71,72,73]. These technologies allow for real-time measurement of stress responses, making them indispensable in breeding programs targeting abiotic stress resistance [40].
3.3. Fluorescence Imaging
Fluorescence imaging is a powerful tool for measuring chlorophyll fluorescence, providing insights into photosynthetic efficiency under stress conditions [25]. When plants absorb low-wavelength light (e.g., blue light), they emit fluorescence in the red spectrum (600–750 nm), which is captured and analyzed using fluorescence sensors [74]. Chlorophyll fluorescence imaging (ChlF) is particularly valuable in assessing photosynthetic performance, as shown by Hairmansis et al. (2014) [75] in rice and Awlia et al. (2016) [25] in Arabidopsis. This technique effectively identifies early responses to abiotic stresses like drought and salinity, indirectly measuring plant health and metabolic activity [76]. Fluorescence imaging has been used to assess a range of physiological traits, including stomatal conductance, metabolite transport, and phloem loading [77]. The ability to monitor photosynthesis under stress conditions makes fluorescence imaging a vital tool for identifying and selecting stress-tolerant genotypes in breeding programs [78].
3.4. Spectroscopy Imaging
Spectroscopy imaging leverages the interaction of solar radiation with plants, captured through hyperspectral and multispectral cameras, to assess plant health and stress responses [8]. Hyperspectral imaging divides the light spectrum into multiple bands, creating a detailed electromagnetic spectrum that can be used to identify physiological and biochemical changes [79]. Vegetation indices such as NDVI and PRI are calculated using spectral data, providing insights into traits like chlorophyll content, water status, and active biomass [44]. Zarco-Tejada et al. (2012) demonstrated the utility of vegetation indices in assessing pigment content and biomass in crops under stress [80]. Hyperspectral imaging offers a non-invasive method for detecting stress responses, making it a critical tool in field phenotyping for precision agriculture [80,81].
3.5. Integrated Imaging Techniques
Integrated imaging systems combine modalities to capture a more comprehensive picture of plant physiology and structure [34]. Techniques such as PET and MRI assess stress-related physiological changes [81]. Borisjuk et al. (20120 employed MRI to visualize root architecture and water transport in various crops) [81]. Jahnke et al. (2009) combined PET and MRI to investigate carbon fluxes in sugar beet under drought stress [34]. Advanced imaging techniques like Forster resonance energy transfer (FRET) are used for molecular phenotyping, providing insights into real-time dynamics of elements like calcium and zinc in plant roots [82]. The combination of PET, MRI, and FRET techniques allows researchers to capture real-time plant function and structure changes, making these tools invaluable in studying abiotic stress responses [83].
4. Plant Phenomics Data Management
The advent of HTP technologies has led to the generation of vast and complex datasets, making efficient data collection, storage, processing, and management crucial in agricultural research [84,85]. Phenomics involves capturing, analyzing, and interpreting high-dimensional phenotypic data to understand the relationship between plant traits and their genetic and environmental influences. As the volume and complexity of data increases, big data analytics, AI, and ML are playing an increasingly important role in improving data integration, accuracy, and predictive power [63,86]. The key components of plant phenomics data management include automated data collection, cloud-based storage, metadata documentation, AI-powered data processing, and computational modeling, all of which enhance the efficiency and scalability of phenotypic studies (Table 3 and Table 4) [87,88].

Table 3.
Models Used in Phenomics.

Table 4.
Tools and Technologies Used in Phenomics Data Management.
4.1. Automated Data Collection and Sensor Fusion
Automated data collection in plant phenotyping relies on integrating multiple imaging and sensor technologies to capture plant traits efficiently. AI-driven sensor fusion combines these diverse imaging sources, improving data accuracy, noise reduction, and stress detection [88]. For example, combining RGB imaging with near-infrared (NIR) sensors allows early detection of water stress by correlating leaf reflectance patterns with transpiration efficiency [89]. Deep learning algorithms, including convolutional neural networks (CNNs) and random forest classifiers, further refine phenotype classification and stress response predictions (Table 3). These automated platforms enhance trait assessment by eliminating human bias and increasing throughput. ML plays a crucial role in integrating and analyzing sensor data. AI-based segmentation models can distinguish between plant organs and background noise, ensuring accurate feature extraction. Furthermore, predictive modeling techniques such as support vector machines (SVMs) and random forest regression models help in early stress detection and phenotypic trait classification [22]. Automated data pipelines process large datasets in real time, reducing the need for manual intervention and streamlining data interpretation. The integration of edge computing allows for on-site analysis, ensuring rapid phenotype assessments and facilitating immediate breeding decisions [64].
4.2. Data Storage, Cloud Computing, and Computational Advances
Managing large-scale phenotypic datasets requires robust cloud computing platforms and high-performance computing (HPC) clusters. Cloud-based solutions offer scalable data storage, parallel processing capabilities, and cross-institutional accessibility, allowing researchers to analyze and retrieve data seamlessly [85]. Platforms such as Google Cloud, AWS, and Microsoft Azure provide centralized storage for high-throughput phenotyping data, ensuring easy collaboration and real-time processing (Table 4). Advancements in HPC clusters enable the rapid processing of large-scale datasets, accelerating G2P mapping, QTL identification, and multi-trait prediction modeling. Federated databases facilitate cross-institutional data sharing, ensuring that datasets remain standardized and accessible for large-scale breeding programs (Table 4). Additionally, blockchain-based data security frameworks enhance data integrity by ensuring traceability and preventing unauthorized alterations in phenotypic records [63,84].
4.3. Multi-Omics Data Integration and AI-Driven Annotation
Modern phenomics increasingly integrates multi-omics approaches, combining genomics, transcriptomics, proteomics, and metabolomics with phenotypic information to enhance trait discovery, breeding efficiency, and stress resilience studies [63,87]. G2P mapping allows researchers to link genetic variations with plant traits, improving MAS and accelerating breeding programs [86]. AI-based multi-omics analysis utilizes deep learning frameworks such as long short-term memory (LSTM) networks to uncover complex relationships between genetic and phenotypic data [64]. To improve data management, AI-powered metadata annotation ensures that phenotypic datasets are structured, indexed, and searchable for long-term research applications. Natural language processing (NLP) models automate metadata tagging, improving retrieval and interoperability of large datasets. Standardized metadata frameworks, such as the FAIR Data Principles (Findable, Accessible, Interoperable, Reusable), ensure data usability and reproducibility, facilitating better integration across research studies (Table 3) [84].
4.4. Data Quality Management and AI-Enhanced Phenotype Predictions
Ensuring data quality and consistency in phenomics is critical for obtaining reliable biological insights. AI-powered data cleaning, anomaly detection, and predictive analytics techniques enhance the precision of phenotypic datasets [90]. Big data analytics enables the identification of outliers, missing values, and sensor inconsistencies, ensuring that phenotyping datasets maintain high reliability. AI-driven dynamic filtering techniques iteratively refine datasets by removing low-confidence measurements and improving data accuracy. Advanced machine learning models, such as random forest regression and CNNs, improve phenotype prediction by integrating environmental and genetic factors [88]. Automated image processing tools extract features from large phenotypic datasets, ensuring efficient trait classification. Additionally, G2P mapping and deep learning-based multi-omics integration enable precise MAS, optimizing breeding efficiency [22].
4.5. Practical Applications in Breeding and Sustainable Agriculture
The advancements in AI-driven phenomics have revolutionized plant breeding programs, optimizing the selection of stress-resilient and high-yielding genotypes [86]. AI-based phenomic selection models integrate genomic and phenotypic data, improving the accuracy of breeding selection processes. Deep learning algorithms such as recurrent neural networks (RNNs) and attention-based models analyze multi-trait datasets, facilitating faster and more accurate trait selection [64]. UAVs equipped with multispectral and thermal sensors provide large-scale field phenotyping, allowing for efficient stress assessment across different environments [89]. AI-powered smart agriculture tools integrate real-time phenotyping data with agronomic decision support systems, enabling farmers to optimize irrigation, fertilization, and pest management strategies based on predictive modeling [40].
The integration of big data analytics, machine learning, and cloud-based phenomics platforms enhances the efficiency of crop health monitoring, resource utilization, and yield prediction. By leveraging AI-driven multi-sensor platforms, agricultural research can develop sustainable, climate-resilient breeding solutions that improve global food security [63].
5. Current Advancements and Impact of Phenomics Studies on Abiotic Stress Management
Phenomics has become central to modern plant science, particularly in addressing the growing need for crops that can withstand abiotic stresses such as drought, salinity, heat, and waterlogging [62]. HTP technologies, which integrate advanced imaging systems, sensors, and data analysis tools, have enabled researchers to capture plant responses to stress at unprecedented scales and resolutions [91]. Recent studies highlight the critical role that phenomics is playing in breeding programs and agricultural research, helping us better understand plant physiology and stress adaptation mechanisms [84].
Drought and heat stress are among the most critical abiotic stressors affecting global agriculture, and phenomics has been instrumental in advancing our understanding of plant responses to these conditions [36,86]. Thermal infrared imaging has revolutionized drought tolerance screening by enabling researchers to measure canopy temperature depression a reliable indicator of drought resilience [68,88]. This non-invasive method allowed researchers to rapidly screen large populations of maize genotypes, identifying those that maintained cooler canopies under drought stress [88]. Canopy temperature depression is now widely regarded as a reliable indicator of drought resilience, and similar methods are being applied in other crops, including wheat and rice. Fahlgren et al. (2015) expanded on this work by employing thermal imaging in a field-based study of wheat genotypes exposed to heat stress [68]. They demonstrated that thermal imaging could detect subtle differences in stomatal conductance and transpiration rates, critical for understanding heat tolerance. These studies provided actionable data for breeding programs and demonstrated the versatility of thermal imaging as a tool for large-scale stress phenotyping. Integrating thermal imaging into phenomics platforms has significantly sped up the identification of stress-tolerant genotypes, enabling faster crop improvement [90,91].
Salinity stress is a growing concern in many parts of the world, where soil salinization threatens agricultural productivity [44]. Traditional methods of salinity tolerance screening were slow and often lacked the precision needed to differentiate between stress responses at early stages. Hyperspectral imaging has addressed this gap by offering a non-invasive way to monitor physiological changes in plants exposed to salt stress. Romer et al. (2012) [92] utilized hyperspectral imaging to track changes in leaf reflectance in wheat under salt stress. By capturing spectral signatures corresponding to water content, chlorophyll levels, and pigment composition, hyperspectral imaging allowed the early detection of salt stress before visible symptoms appeared [91]. The study showed that specific wavelengths in the near-infrared region were highly correlated with salt-induced physiological changes, making hyperspectral imaging an invaluable tool for early stress detection [72].
The benefits of hyperspectral imaging extend beyond early detection the researchers used hyperspectral data to calculate vegetation indices such as the NDVI and PRI. These indices were directly linked to pigment concentration and biomass, helping identify salt-tolerant genotypes in large breeding populations [88]. The capacity to differentiate stress responses at the physiological level has made hyperspectral imaging one of the most powerful tools in phenomics, enabling more accurate and faster phenotyping of stress responses across different environments.
While aboveground phenotyping has received significant attention, developing high-throughput root phenotyping platforms has brought a much-needed focus on how root systems respond to abiotic stress. Studying root system architecture (RSA) is crucial for understanding water and nutrient uptake under drought and nutrient-deficient conditions [32].
In one of the pivotal studies on root phenomics, Nagel et al. (2012) [32] used the GROWSCREEN-Rhizo platform to study root traits such as root depth, lateral root growth, and root angle in response to drought. The platform utilized a combination of non-invasive imaging and automated data collection to phenotype root architecture with high precision. The study showed that deeper root systems were associated with better drought tolerance, and phenomics tools like GROWSCREEN-Rhizo have since been integrated into breeding programs to select root traits that confer resilience to water scarcity [32]. Similarly, Atkinson et al. (2019) employed X-ray computed tomography (CT) to phenotype root systems in controlled environments, allowing the researchers to visualize root growth in three dimensions [35]. CT scanning provided detailed insights into how different root traits, such as root diameter and volume, contribute to drought resistance [91]. These advancements in root phenomics are significant because they provide a complete picture of plant responses to abiotic stress, combining shoot and root data to improve breeding outcomes.
One of the most promising areas of phenomics is the integration of multiple imaging techniques into a single platform, allowing for the simultaneous capture of various physiological and morphological traits [62]. A study by Jahnke et al. (2009) combined PET and MRI to investigate carbon allocation and water transport in sugar beet taproots under drought conditions [34]. This study demonstrated how the integration of PET and MRI provided real-time data on metabolic and structural changes, offering a holistic view of how plants cope with water scarcity. Another breakthrough in integrated imaging systems is multispectral imaging combined with three imaging technologies. Rahaman et al. (2015) [27] showed that by combining these two methods, researchers could capture spatial and spectral data on plant architecture and stress responses. This integrated approach allowed for more precise measurements of traits such as leaf area, biomass accumulation, and pigment content, which are critical for understanding how plants adapt to stress over time. These integrated systems benefit the field by enabling researchers to phenotype more complex, previously difficult-to-quantify traits [62]. The ability to capture detailed physiological data alongside structural changes provides a more comprehensive understanding of how plants respond to abiotic stress, ultimately leading to more targeted breeding strategies [22].
6. Challenges and Limitations
HTP has transformed plant science, yet significant challenges hinder its broader application in research and commercial agriculture. Addressing limitations in cost, scalability, standardization, and data processing is crucial for aligning phenomics with sustainable agriculture [84].
A major barrier is the high cost of advanced phenotyping platforms like LiDAR, hyperspectral imaging, and robotic-assisted systems, which limits accessibility, particularly in low-resource settings [63,85]. While low-cost alternatives such as smartphone-based imaging and UAVs exist, they often lack automation and precision, reducing their utility in large-scale breeding programs [3]. Additionally, AI-driven phenotyping remains constrained by proprietary datasets and biased training models, restricting its effectiveness across diverse crop species and environments [88].
Lack of standardization and reproducibility further complicates phenomics research. Studies using UAV-based imaging and hyperspectral sensors often yield inconsistent results due to variations in sensor configurations, imaging conditions, and environmental factors [90]. Without universal data collection protocols, cross-study comparisons and large-scale implementation remain difficult [86]. Data processing bottlenecks also pose challenges, as HTP generates massive datasets requiring advanced computational tools [63]. While cloud computing solutions such as Google Cloud and AWS facilitate large-scale data analysis and collaboration, adoption remains limited in agricultural research [84]. Emerging technologies like blockchain offer secure data tracking, but their application in phenomics is still in its infancy [64].
Field implementation remains problematic, as many HTP platforms optimized for controlled environments struggle in real-world conditions due to environmental variability [89]. For example, thermal imaging, effective in greenhouses, loses accuracy in open fields due to canopy shading and background interference. Similarly, UAV-based imaging is affected by motion artifacts and wind variability, reducing data reliability [88]. Developing sensor fusion techniques that integrate multiple imaging modalities, such as LiDAR with hyperspectral sensors, could improve field phenotyping accuracy [23].
Additionally, multi-omics integration remains a challenge, as bioinformatics pipelines struggle to process complex datasets linking phenotypic traits with genomic, transcriptomic, and metabolomic data [85]. Current genomic selection models often fail to account for genotype × environment interactions, limiting their predictive power [84]. Strengthening AI-driven predictive models and computational frameworks can improve breeding efficiency by making better use of high-dimensional phenotypic data [91].
To address these challenges, research must prioritize standardized phenotyping protocols, improved AI models, expanded cloud computing adoption, and adaptive field-based sensing [17]. By overcoming these limitations, HTP can become a more scalable, accessible, and effective tool for breeding climate-resilient crops, contributing to sustainable agriculture and food security [18].
7. Future Directions in Phenomics for Sustainable Agriculture
To overcome the limitations of HTP and align phenomics with sustainable agriculture, advancements in cost reduction, AI-driven analysis, and interdisciplinary collaboration are essential. Integrating affordable, scalable phenotyping solutions, AI-enhanced predictive analytics, and collaborative multi-disciplinary research will be key to expanding the impact of phenomics in both research and commercial agricultural settings.
7.1. Cost and Scalability
The high cost of phenotyping platforms remains a major limitation, particularly for resource-limited institutions. Technologies such as hyperspectral imaging, root phenotyping platforms, and robotic-assisted systems require substantial investments in infrastructure, specialized hardware, and proprietary software [5,10]. Addressing these cost barriers requires scalable, field-deployable solutions that balance affordability with accuracy.
A promising approach involves low-cost handheld and smartphone-based sensors using RGB, infrared, and multispectral imaging to assess plant traits such as chlorophyll content, water status, and biomass accumulation [18,21]. These tools provide real-time phenotypic assessments, reducing dependence on expensive lab-based imaging systems. Similarly, UAV based platforms equipped with multispectral and thermal sensors offer efficient large-scale field phenotyping, significantly reducing labor costs and time while improving data collection efficiency across diverse agroecological regions [20,38,62].
Additionally, open-source software and AI-powered cloud computing platforms are making phenotypic data analysis more accessible. Instead of relying on costly proprietary software, researchers can use AI-driven open-source tools such as PlantCV, DeepPlantPheno, and OpenPheno, which provide automated solutions for large-scale phenotypic data processing and real-time trait analysis [66,70]. Expanding the adoption of cloud-based infrastructure will further reduce computational costs and improve global data-sharing in phenomics.
7.2. Integration of AI and Machine Learning
The integration of AI and ML is revolutionizing phenomics by enabling automated analysis of large-scale, high-dimensional datasets generated from imaging systems, sensors, and environmental monitoring tools [91]. These technologies enhance the speed and accuracy of data interpretation, eliminating labor-intensive manual assessments. AI models such as CNNs and random forests can predict stress responses, yield potential, and nutrient-use efficiency across different environmental conditions [88,93]. For instance, ML-based hyperspectral data analysis has successfully predicted drought stress tolerance with high accuracy [77]. Moreover, AI-powered real-time stress detection models can identify early physiological changes before visible symptoms appear, allowing for timely interventions to prevent crop losses and optimize resource use [5,91].
Further advancements in deep learning frameworks, e.g., TensorFlow, PyTorch will enable AI-driven models to autonomously analyze multimodal datasets, integrating data from hyperspectral, thermal, and 3D imaging to generate comprehensive stress-response profiles. These real-time, scalable AI-powered systems will be crucial for commercial phenotyping applications and large-scale breeding programs.
7.3. Interdisciplinary Collaboration
The future of phenomics depends on interdisciplinary collaboration between plant scientists, engineers, data scientists, and computer scientists. Effective phenotyping requires not just advanced sensor and AI technologies but also domain-specific biological expertise to ensure the collected data are accurately interpreted and applied to breeding programs [94,95]. Collaborations between engineers and plant scientists have led to the development of multi-sensor phenotyping platforms, which simultaneously measure root and shoot traits, water use efficiency, and stress responses under different environmental conditions [96]. For instance, combining multispectral imaging with 3D reconstruction provides deeper insights into plant architecture and biomass accumulation, while thermal sensors improve real-time stress monitoring [32,39].
Additionally, partnerships with agricultural economists and policymakers will help translate phenomics insights into actionable strategies for sustainable agriculture. AI-driven phenomics models can support decision-making on water use, crop management, and climate resilience, ensuring that research findings inform real-world agricultural policies [31,32]. By integrating affordable phenotyping technologies, AI-driven predictive analytics, and interdisciplinary collaborations, phenomics can become a scalable and effective tool for breeding climate-resilient crops, contributing to global food security and sustainable agricultural practices (Figure 4).
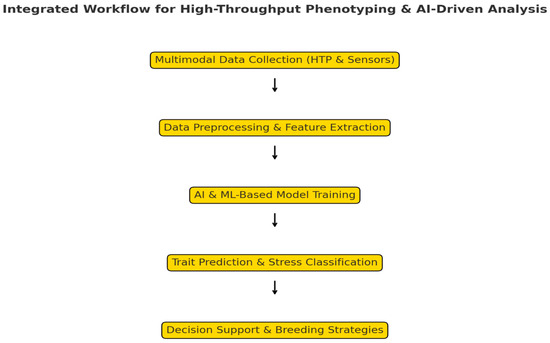
Figure 4.
Integration of HTP and AI-driven analysis.
8. Conclusions
The evolution of HTP and phenomics has revolutionized plant science, particularly in addressing the challenges of abiotic stresses such as drought, salinity, and heat. By enabling precise, large-scale, and non-destructive trait measurement, phenomics has transformed crop improvement strategies, accelerating the selection of stress-resilient genotypes. This review has underscored how advanced imaging techniques, sensor-based platforms, and AI-driven data analytics contribute to a deeper understanding of plant responses to environmental stresses.
Beyond its technical advancements, phenomics plays a pivotal role in ensuring agricultural sustainability and food security in the face of climate change and increasing global demand for resilient crops. However, several challenges must be addressed to fully harness its potential. The high costs and scalability limitations of sophisticated phenotyping platforms remain significant barriers, particularly in resource-constrained regions. Additionally, the vast amounts of data generated require robust management systems, standardized protocols, and efficient computational tools to extract meaningful insights. Artificial intelligence and machine learning have begun to address these challenges, streamlining data processing, improving predictive accuracy, and enhancing real-time decision-making in breeding programs.
Moving forward, the integration of low-cost, field-deployable phenotyping solutions, AI-driven analytical frameworks, and interdisciplinary collaborations will be critical in making phenomics more accessible and impactful. The convergence of phenomics with multi-omics approaches, cloud computing, and automated data pipelines will further bridge the gap between genotype and phenotype, enhancing breeding efficiency and precision agriculture applications.
In conclusion, phenomics is not just a technological advancement, it is an essential driver of next-generation crop improvement strategies. Its ability to rapidly and accurately identify stress-resilient genotypes makes it a cornerstone of sustainable agriculture. As phenotyping technologies continue to evolve, their integration into breeding programs will be fundamental in ensuring that global agriculture remains adaptable, productive, and resilient to future environmental challenges.
Author Contributions
S.A. and K.M. prepared the conception and design. K.M., S.A., A.B. and M.M.T. wrote the first draft of the manuscript, and all authors commented on previous versions. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose. The authors declare no conflict of interest.
References
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Ashraful Alam, M.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy 2020, 11, 241. [Google Scholar] [CrossRef]
- Bakala, H.S.; Singh, G.; Srivastava, P. Smart breeding for climate resilient agriculture. In Plant Breeding-Current and Future Views; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Song, P.; Wang, J.; Guo, X.; Yang, W.; Zhao, C. High-throughput phenotyping: Breaking through the bottleneck in future crop breeding. Crop J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Al-Tamimi, N.; Langan, P.; Bernád, V.; Walsh, J.; Mangina, E.; Negrão, S. Capturing crop adaptation to abiotic stress using image-based technologies. Open Biol. 2022, 12, 210–353. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.; Gill, S.K.; Saini, D.K.; Chopra, Y.; de Koff, J.P.; Sandhu, K.S. A comprehensive review of high-throughput phenotyping and machine learning for plant stress phenotyping. Phenomics 2022, 2, 156–183. [Google Scholar] [CrossRef]
- Arya, S.; Sandhu, K.S.; Singh, J.; Kumar, S. Deep learning: As the new frontier in high-throughput plant phenotyping. Euphytica 2022, 218, 47. [Google Scholar] [CrossRef]
- Khan, A.; Vibhute, A.D.; Mali, S.; Patil, C.H. A systematic review on hyperspectral imaging technology with a machine and deep learning methodology for agricultural applications. Ecol. Inform. 2022, 69, 101–678. [Google Scholar] [CrossRef]
- Su, W.H. Advanced machine learning in point spectroscopy, RGB-and hyperspectral-imaging for automatic discriminations of crops and weeds: A review. Smart Cities 2020, 3, 767–792. [Google Scholar] [CrossRef]
- Atefi, A.; Ge, Y.; Pitla, S.; Schnable, J. Robotic technologies for high-throughput plant phenotyping: Contemporary reviews and future perspectives. Front. Plant Sci. 2021, 12, 611–940. [Google Scholar] [CrossRef]
- Li, D.; Quan, C.; Song, Z.; Li, X.; Yu, G.; Li, C.; Muhammad, A. High-throughput plant phenotyping platform (HT3P) as a novel tool for estimating agronomic traits from the lab to the field. Front. Bioeng. Biotechnol. 2021, 8, 623–705. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Z.; Huang, C.; Duan, L.; Chen, G.; Jiang, N.; Fang, W.; Feng, H.; Xie, W.; Lian, X.; et al. Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat. Commun. 2014, 5, 50–87. [Google Scholar] [CrossRef]
- Smith, D.T.; Potgieter, A.B.; Chapman, S.C. Scaling up high-throughput phenotyping for abiotic stress selection in the field. Theor. Appl. Genet. 2021, 134, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.I.; Hu, H.; Gill, M.; Batley, J.; Edwards, D. Crop breeding for a changing climate: Integrating phenomics and genomics with bioinformatics. Theor. Appl. Genet. 2021, 134, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.S.M.; Neik, T.X.; Thomas, W.J.; Amas, J.C.; Cantila, A.Y.; Craig, R.J.; Edwards, D.; Batley, J. Advancing designer crops for climate resilience through an integrated genomics approach. Curr. Opin. Plant Biol. 2022, 67, 102–220. [Google Scholar] [CrossRef]
- Zavafer, A.; Bates, H.; Mancilla, C.; Ralph, P.J. Phenomics: Conceptualization and importance for plant physiology. Trends Plant Sci. 2023, 28, 1004–1013. [Google Scholar] [CrossRef]
- Sen, P.; Orešič, M. Integrating Omics Data in Genome-Scale Metabolic Modeling: A Methodological Perspective for Precision Medicine. Metabolites 2023, 13, 855. [Google Scholar] [CrossRef]
- Roitsch, T.; Himanen, K.; Chawade, A.; Jaakola, L.; Nehe, A.; Alexandersson, E. Functional phenomics for improved climate resilience in Nordic agriculture. J. Exp. Bot. 2022, 73, 5111–5127. [Google Scholar] [CrossRef]
- Houle, D.; Govindaraju, D.R.; Omholt, S. Phenomics: The next challenge. Nat. Rev. Genet. 2010, 11, 855–866. [Google Scholar] [CrossRef]
- Cobb, J.N.; DeClerck, G.; Greenberg, A.; Clark, R.; McCouch, S. Next-generation phenotyping: Requirements and strategies for enhancing our understanding of genotype–phenotype relationships and its relevance to crop improvement. Theor. Appl. Genet. 2013, 126, 867–887. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Yan, J. Crop phenomics and high-throughput phenotyping: Past decades, current challenges, and future perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, X.; Lu, X.; Wu, S. Spectral–spatial attention network for hyperspectral image classification. IEEE Trans. Geosci. Remote Sens. 2019, 58, 3232–3245. [Google Scholar] [CrossRef]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A Physio-Morphological Trait-Based Approach for Breeding Drought Tolerant Wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Golzarian, M.R.; Frick, R.A.; Rajendran, K.; Berger, B.; Roy, S.; Tester, M.; Lun, D.S. Accurate inference of shoot biomass from high-throughput images of cereal plants. Plant Methods 2011, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Gilmer, F.; Biskup, B.; Nagel, K.A.; Rascher, U.; Fischbach, A.; Briem, S.; Dreissen, G.; Tittmann, S.; Braun, S.; et al. Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants. Funct. Plant Biol. 2009, 36, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Awlia, M.; Nigro, A.; Fajkus, J.; Schmoeckel, S.M.; Negrão, S.; Santelia, D.; Trtílek, K.; Tester, M.; Julkowska, M.M.; Panzarová, K. High-throughput non-destructive phenotyping of traits that contribute to salinity tolerance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1414. [Google Scholar] [CrossRef]
- Czedik-Eysenberg, A.; Seitner, S.; Güldener, U.; Koemeda, S.; Jez, J.; Colombini, M.; Djamei, A. The ‘PhenoBox’, a flexible, automated, open-source plant phenotyping solution. New Phytol. 2018, 219, 808–823. [Google Scholar] [CrossRef]
- Rahaman, M.; Chen, D.; Gillani, Z.; Klukas, C.; Chen, M. Advanced phenotyping and phenotype data analysis for the study of plant growth and development. Front. Plant Sci. 2015, 6, 619. [Google Scholar] [CrossRef]
- Reuzeau, C.; Frankard, V.; Hatzfeld, Y.; Sanz, A.; Van Camp, W.; Lejeune, P.; De Wilde, C.; Lievens, K.; de Wolf, J.; Vranken, E. TraitmillTM: A functional genomics platform for the phenotypic analysis of cereals. Plant Gen. Res. 2006, 4, 20–24. [Google Scholar] [CrossRef]
- Kumar, M.; Mahato, A.; Kumar, S.; Mishra, V.K. Phenomics-assisted breeding: An emerging way for stress management. In New Frontiers in Stress Management for Durable Agriculture; Springer: Singapore, 2020; pp. 295–310. [Google Scholar] [CrossRef]
- Yazdanbakhsh, N.; Fisahn, J. High throughput phenotyping of root growth dynamics, lateral root formation, root architecture and root hair development enabled by PlaRoM. Funct. Plant Biol. 2009, 36, 938–946. [Google Scholar] [CrossRef]
- Galkovskyi, T.; Mileyko, Y.; Bucksch, A.; Moore, B.; Symonova, O.; Price, C.A.; Topp, C.N.; Iyer-Pascuzzi, A.S.; Zurek, P.R.; Fang, S. GiA Roots: Software for the high throughput analysis of plant root system architecture. BMC Plant Biol. 2012, 12, 116. [Google Scholar] [CrossRef]
- Nagel, K.A.; Putz, A.; Gilmer, F.; Heinz, K.; Fischbach, A.; Pfeifer, J.; Faget, M.; Blossfeld, S.; Ernst, M.; Dimaki, C.; et al. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Funct. Plant Biol. 2012, 39, 891–904. [Google Scholar] [CrossRef]
- Garbout, A.; Munkholm, L.J.; Hansen, S.B.; Petersen, B.M.; Munk, O.L.; Pajor, R. The use of PET/CT scanning technique for 3D visualization and quantification of real-time soil/plant interactions. Plant Soil 2012, 352, 113–127. [Google Scholar] [CrossRef]
- Jahnke, S.; Menzel, M.I.; van Dusschoten, D.; Roeb, G.W.; Buhler, J.; Minwuyelet, S.; Blumler, P.; Temperton, V.M.; Hombach, T.; Streun, M.; et al. Combined MRI-PET dissects dynamic changes in plant structures and functions. Plant J. 2009, 59, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.A.; Pound, M.P.; Bennett, M.J.; Wells, D.M. Uncovering the hidden half of plants using new advances in root phenotyping. Curr. Opin. Biotechnol. 2019, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Tehran, P.; Sabermanesh, K.; Virlet, N.; Hawkesford, M.J. Automated method to determine two critical growth stages of wheat: Heading and flowering. Front. Plant Sci. 2017, 8, 252. [Google Scholar] [CrossRef]
- Weight, C.; Parnham, D.; Waites, R. TECHNICAL ADVANCE: Leaf Analyser: A computational method for rapid and large-scale analyses of leaf shape variation. Plant J. 2007, 53, 578–586. [Google Scholar] [CrossRef]
- Busemeyer, L.; Mentrup, D.; Möller, K.; Wunder, E.; Alheit, K.; Hahn, V.; Maurer, H.P.; Reif, J.C.; Würschum, T.; Müller, J.; et al. BreedVision—A multi-sensor platform for non-destructive field-based phenotyping in plant breeding. Sensors 2013, 13, 2830–2847. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Perspectives for remote sensing with unmanned aerial vehicles in precision agriculture. Trends Plant Sci. 2019, 24, 152–164. [Google Scholar] [CrossRef]
- Zhang, X.; Ibrahim, Z.; Khaskheli, M.B.; Raza, H.; Zhou, F.; Shamsi, I.H. Integrative Approaches to Abiotic Stress Management in Crops: Combining Bioinformatics Educational Tools and Artificial Intelligence Applications. Sustainability 2024, 16, 7651. [Google Scholar] [CrossRef]
- Confalonieri, R.; Paleari, L.; Foi, M.; Movedi, E.; Vesely, F.M.; Thoelke, W.; Agape, C.; Borlini, G.; Ferri, I.; Massara, F.; et al. PocketPlant3D: Analysing canopy structure using a smartphone. Biosyst. Eng. 2017, 164, 1–12. [Google Scholar] [CrossRef]
- Duan, L.; Yang, W.; Huang, C.; Liu, Q. A novel machine-vision-based facility for the automatic evaluation of yield-related traits in rice. Plant Methods 2011, 7, 44. [Google Scholar] [CrossRef]
- Crowell, S.; Falcão, A.X.; Shah, A.; Wilson, Z.; Greenberg, A.J.; McCouch, S.R. High-resolution inflorescence phenotyping using a novel image-analysis pipeline, PANorama. Plant Physiol. 2014, 165, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Al-Tam, F.; Adam, H.; Anjos, A.D.; Lorieux, M.; Larmande, P.; Ghesquière, A.; Jouannic, S.; Shahbazkia, H.R. P-TRAP: A panicle trait phenotyping tool. BMC Plant Biol. 2013, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Hughes, N.; Oliveira, H.R.; Fradgley, N.; Corke, F.M.; Cockram, J.; Doonan, J.H.; Nibau, C. Trait analysis reveals morphometric differences between domesticated temperate small grain cereals and their wild relatives. Plant J. 2019, 99, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Askew, K.; Scotson, C.P.; Williams, K.; Sauze, C.; Corke, F.; Doonan, J.H.; Nibau, C. Non-destructive, high-content analysis of wheat grain traits using X-ray micro computed tomography. Plant Methods 2017, 13, 76. [Google Scholar] [CrossRef]
- Harris, Z.N.; Awale, M.; Bhakta, N.; Chitwood, D.H.; Fennell, A.; Frawley, E.; Klein, L.L.; Kovacs, L.G.; Kwasniewski, M.; Londo, J.P.; et al. Multi-dimensional leaf phenotypes reflect root system genotype in grafted grapevine over the growing season. Giga Sci. 2021, 10, giab087. [Google Scholar] [CrossRef]
- Clark, R.T.; MacCurdy, R.B.; Jung, J.K.; Shaff, J.E.; McCouch, S.R.; Aneshansley, D.J.; Kochian, L.V. Three-dimensional root phenotyping with a novel imaging and software platform. Plant Physiol. 2011, 156, 455–465. [Google Scholar] [CrossRef]
- Armengaud, P.; Zambaux, K.; Hills, A.; Sulpice, R.; Pattison, R.J.; Blatt, M.R.; Amtmann, A. EZ-Rhizo: Integrated software for the fast and accurate measurement of root system architecture. Plant J. 2009, 57, 945–956. [Google Scholar] [CrossRef]
- Naeem, A.; French, A.P.; Wells, D.M.; Pridmore, T.P. High throughput feature counting and measurement of roots. Bioinformatics 2011, 27, 1337–1338. [Google Scholar] [CrossRef]
- French, A.; Ubeda-Tomás, S.; Holman, T.J.; Bennett, M.J.; Pridmore, T. High-throughput quantification of root growth using a novel image-analysis tool. Plant Physiol. 2009, 150, 1784–1795. [Google Scholar] [CrossRef]
- Le Bot, J.; Serra, V.; Fabre, J.; Draye, X.; Adamowicz, S.; Pagés, L. DART: A software to analyse root system architecture and development from captured images. Plant Soil. 2010, 326, 261–273. [Google Scholar] [CrossRef]
- Lobet, G.; Pagés, L.; Draye, X. A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiol. 2011, 157, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; Aguirrezabal, L.; Chenu, K.; Cookson, S.J.; Dauzat, M.; Hamard, P.; Thioux, J.J.; Rolland, G.; Bouchier-Combaud, S.; Lebaudy, A. PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytol. 2006, 169, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Czauderna, T.; Hoffmann, R.; Stein, N.; Schreiber, F. HTPheno: An image analysis pipeline for highthroughput plant phenotyping. BMC Bioinform. 2011, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Napier, J.D.; Heckman, R.W.; Juenger, T.E. Gene-by-environment interactions in plants: Molecular mechanisms, environmental drivers, and adaptive plasticity. Plant Cell 2023, 35, 109–124. [Google Scholar] [CrossRef]
- Iwata, H.; Ukai, Y. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 2002, 93, 384–385. [Google Scholar] [CrossRef]
- Iwata, H.; Ebana, K.; Uga, Y.; Hayashi, T.; Jannink, J.L. Genome-wide association study of grain shape variation among Oryza sativa L. germplasms based on elliptic Fourier analysis. Mol. Breed. 2010, 25, 203–215. [Google Scholar] [CrossRef]
- Tanabata, T.; Shibaya, T.; Hori, K.; Ebana, K.; Yano, M. SmartGrain: High-throughput phenotyping software for measuring seed shape. Plant Physiol. 2012, 160, 1871–1880. [Google Scholar] [CrossRef]
- Boer, M.M.; Macfarlane, C.; Norris, J.; Sadler, R.J.; Wallace, J.; Grierson, P.F. Mapping Burned Areas and Burn Severity Patterns in SW Australian Eucalypt Forest Using Remotely-Sensed Changes in Leaf Area Index. Remote Sens. Environ. 2008, 112, 4358–4369. [Google Scholar] [CrossRef]
- Yao, Y.; Yue, J.; Liu, Y.; Yang, H.; Feng, H.; Shen, J.; Hu, J.; Liu, Q. Classification of Maize Growth Stages Based on Phenotypic Traits and UAV Remote Sensing. Agriculture 2024, 14, 11–75. [Google Scholar] [CrossRef]
- Williamson, H.F.; Leonelli, S. Accelerating agriculture: Data-intensive plant breeding and the use of genetic gain as an indicator for agricultural research and development. Stud. Hist. Philos. Sci. 2002, 95, 167–176. [Google Scholar] [CrossRef]
- Mansoor, S.; Karunathilake, E.M.B.M.; Tuan, T.T.; Chung, Y.S. Genomics, Phenomics, and Machine Learning in Transforming Plant Research: Advancements and Challenges. Hortic. Plant J. 2024. [Google Scholar] [CrossRef]
- Yan, J.; Wang, X. Machine learning bridges omics sciences and plant breeding. Trends Plant Sci. 2023, 28, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Puneet, M.; Lohumi, S.; Khan, H.A.; Nordon, A. Close-range hyperspectral imaging of whole plants for digital phenotyping: Recent applications and illumination correction approaches. Comput. Electron. Agric. 2020, 178, 105780. [Google Scholar]
- Fiorani, F.; Schurr, U. Future scenarios for plant phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef]
- Khasanova, A.; Lovell, J.T.; Bonnette, J.; Weng, X.; Jenkins, J.; Yoshinaga, Y.; Schmutz, J.; Juenger, T.E. The Genetic Architecture of Shoot and Root Trait Divergence Between Mesic and Xeric Ecotypes of a Perennial Grass. Front. Plant Sci. 2019, 10, 366. [Google Scholar] [CrossRef]
- Fahlgren, N.; Gehan, M.A.; Baxter, I. Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Curr. Opin. Plant Biol. 2015, 24, 93–99. [Google Scholar] [CrossRef]
- Neilson, E.H.; Edwards, A.M.; Blomstedt, C.K.; Berger, B.; Møller, B.L.; Gleadow, R.M. Utilization of a high-throughput shoot imaging system to examine the dynamic phenotypic responses of a C4 cereal crop plant to nitrogen and water deficiency over time. J. Exp. Bot. 2015, 66, 1817–1832. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Tahir, M.U.; Al-Suhaibani, N.; Elsayed, S.; Elsherbiny, O.; Elsharawy, H. Potential of thermal and RGB imaging combined with artificial neural networks for assessing salt tolerance of wheat genotypes grown in real-field conditions. Agronomy 2024, 14, 13–90. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Sirault, X.R.; Furbank, R.T.; Jones, H.G. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J. Exp. Bot. 2010, 61, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Ramanathan, V.; Jagadeeshselvam, N.; Ramasamy, S.; Rajendran, S.; Ramachandran, M.; Sudheer, P.D.; Chauhan, S.; Natesan, S.; Muthurajan, R. Phenomics: Technologies and Applications in Plant and Agriculture. In PlantOmics: The Omics of Plant Science; Barh, D., Khan, M., Davies, E., Eds.; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Hairmansis, A.; Berger, B.; Tester, M.; Roy, S.J. Image-based phenotyping for non-destructive screening of different salinity tolerance traits in rice. Rice 2014, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Rascher, U.; Hütt, M.T.; Siebke, K.; Osmond, B.; Beck, F.; Lüttge, U. Spatiotemporal variation of metabolism in a plant circadian rhythm: The biological clock as an assembly of coupled individual oscillators. Proc. Natl. Acad. Sci. USA 2001, 98, 11801–11805. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Role of Phenomics in Screening Abiotic Stress Tolerance in Plants. In Plant Abiotic Stress Physiology; Apple Academic Press: Point Pleasant, NJ, USA, 2022; pp. 47–59. [Google Scholar]
- Yang, L.; Yang, S.; Jin, P.; Zhang, R. Semi-supervised hyperspectral image classification using spatio-spectral Laplacian support vector machine. IEEE Geosci. Remote Sens. Lett. 2013, 11, 651–655. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Borisjuk, L.; Rolletschek, H.; Neuberger, T. Surveying the plant’s world by magnetic resonance imaging. Plant J. 2012, 70, 129–146. [Google Scholar] [CrossRef]
- Jones, A.M.; Danielson, J.A.; Kumar, M.S.N.; Lanquar, V.; Grossmann, G.; Frommer, W.B. Abscisic acid dynamics in roots detected with genetically encoded FRET sensors. eLife 2014, 3, 17–41. [Google Scholar] [CrossRef]
- Okumoto, S.; Jones, A.; Frommer, W.B. Quantitative imaging with fluorescent biosensors. Annu. Rev. Plant Biol. 2012, 63, 663–706. [Google Scholar] [CrossRef]
- Coppens, F.; Wuyts, N.; Inzé, D.; Dhondt, S. Unlocking the potential of plant phenotyping data through integration and data-driven approaches. Curr. Opin. Syst. Biol. 2017, 4, 58–63. [Google Scholar] [CrossRef]
- Honecker, A.; Schumann, H.; Becirevic, D.; Klingbeil, L.; Volland, K.; Forberig, S.; Jansen, M.; Paulsen, H.; Kuhlmann, H.; Léon, J. Plant, space and time-linked together in an integrative and scalable data management system for phenomic approaches in agronomic field trials. Plant Methods 2020, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Gilliham, M.; Able, J.A.; Roy, S.J. Translating knowledge about abiotic stress tolerance to breeding programmes. Plant J. 2017, 90, 898–917. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Goldman, I.; Ceccarelli, S.; Ortiz, R. Advanced analytics, phenomics and biotechnology approaches to enhance genetic gains in plant breeding. Adv. Agron. 2020, 162, 89–142. [Google Scholar] [CrossRef]
- Walsh, J.; Mangina, E.E.; Negrão, S. Advancements in Imaging Sensors and AI for Plant Stress Detection: A Systematic Literature Review. Plant Phenomics 2024, 6, 0153. [Google Scholar] [CrossRef]
- Machwitz, M.; Pieruschka, R.; Berger, K.; Schlerf, M.; Aasen, H.; Fahrner, S.; Jiménez-Berni, J.; Baret, F.; Rascher, U. Bridging the gap between remote sensing and plant phenotyping—Challenges and opportunities for the next generation of sustainable agriculture. Front. Plant Sci. 2021, 12, 749374. [Google Scholar] [CrossRef]
- Xu, L.; Cruz, J.A.; Savage, L.J.; Kramer, D.M.; Chen, J. Plant photosynthesis phenomics data quality control. Bioinformatics 2015, 31, 1796–1804. [Google Scholar] [CrossRef]
- Nabwire, S.; Suh, H.K.; Kim, M.S.; Baek, I.; Cho, B.K. Application of artificial intelligence in phenomics. Sensors 2021, 21, 43–63. [Google Scholar] [CrossRef]
- Römer, C.; Wahabzada, M.; Ballvora, A.; Pinto, F.; Rossini, M.; Panigada, C.; Behmann, J.; Léon, J.; Thurau, C.; Bauckhage, C.; et al. Early drought stress detection in cereals: Simplex volume maximisation for hyperspectral image analysis. Funct. Plant Biol. 2012, 39, 878–890. [Google Scholar] [CrossRef]
- Van Dyck, L.E.; Kwitt, R.; Denzler, S.J.; Gruber, W.R. Comparing object recognition in humans and deep convolutional neural networks—An eye tracking study. Front. Neurosci. 2021, 15, 750639. [Google Scholar] [CrossRef]
- Cembrowska-Lech, D.; Krzemińska, A.; Miller, T.; Nowakowska, A.; Adamski, C.; Radaczyńska, M.; Mikiciuk, G.; Mikiciuk, M. An integrated multi-omics and artificial intelligence framework for advance plant phenotyping in horticulture. Biology 2023, 12, 1298. [Google Scholar] [CrossRef]
- Mostafa, S.; Mondal, D.; Panjvani, K.; Kochian, L.; Stavness, I. Explainable deep learning in plant phenotyping. Front. Artif. Intell. 2023, 6, 1203546. [Google Scholar] [CrossRef]
- Jurado, J.M.; López, A.; Pádua, L.; Sousa, J.J. Remote sensing image fusion on 3D scenarios: A review of applications for agriculture and forestry. Int. J. Appl. Earth Obs. Geoinf. 2022, 112, 102856. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).