Microbial Biopesticides against Bacterial, Fungal and Oomycete Pathogens of Tomato, Cabbage and Chickpea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anti-Fungal and Antioomycete Assays
2.2. Biopesticide and Plant Bacterial Disease Suppression Assays
2.3. Biopesticide and Plant Fungal Disease Suppression Assays
2.4. Biopesticide and Plant Oomycete Disease Suppression Assays
2.5. Statistical Analyses
3. Results
3.1. Anti-Fungal and Antioomycete Assays
3.2. Use of Biocontrol Bacteria as Biopesticides to Control Bacterial Plant Pathogens in Tomato
3.3. Use of Biocontrol Bacteria as Biopesticides to Control Fungal Plant Pathogens in Cabbage
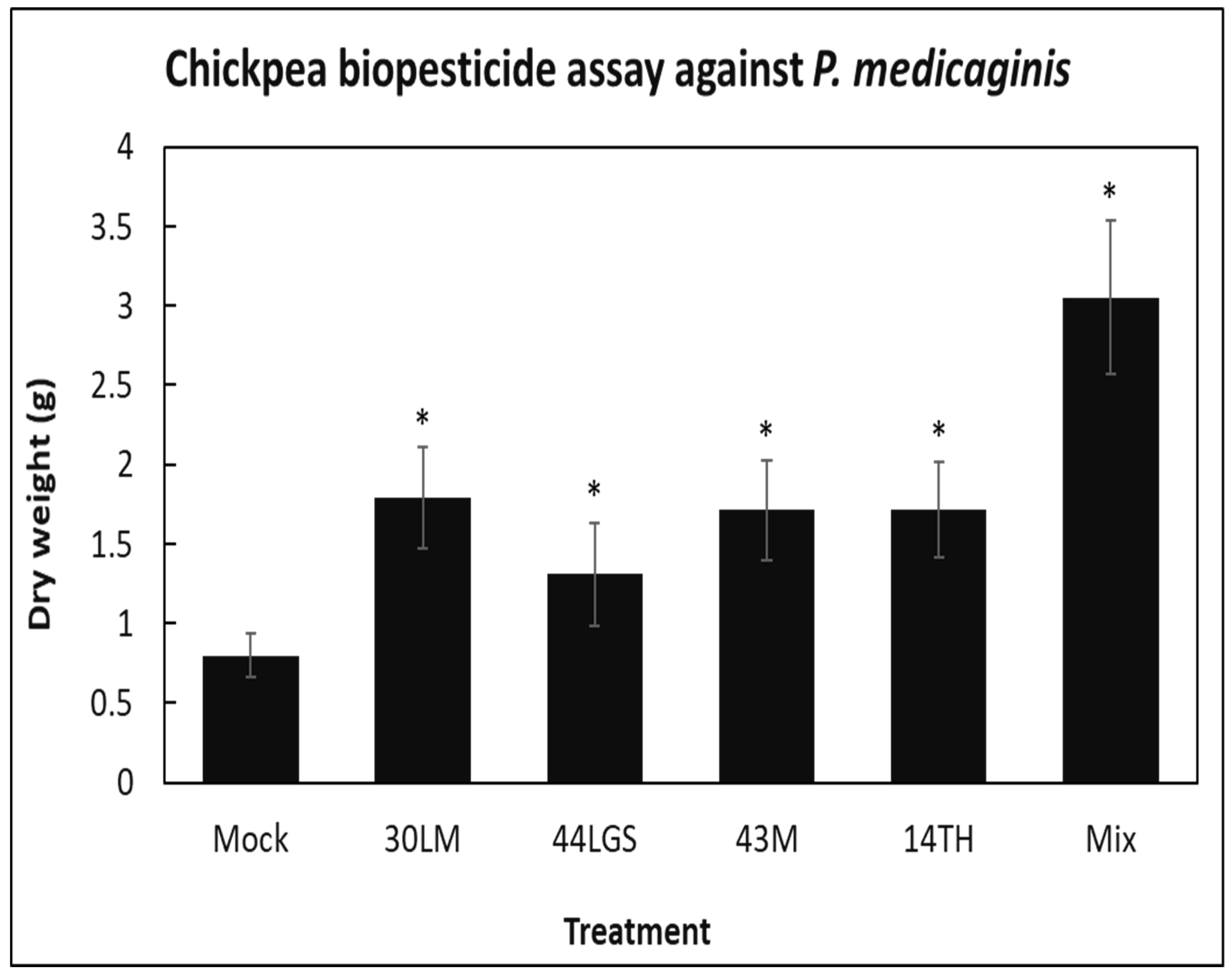
3.4. Use of Biocontrol Bacteria as Biopesticides to Control Oomycete Plant Pathogens in Chickpea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wirthmueller, L.; Maqbool, A.; Banfield, M.J. On the front line: Structural insights into plant-pathogen interactions. Nat. Rev. Micro. 2013, 11, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Abizgil’dina, R.R.; Pusenkova, L.I. Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (review). Appl. Biochem. Microbiol. 2011, 47, 333–345. [Google Scholar] [CrossRef]
- Søgaard Jørgensen, P.; Aktipis, A.; Brown, Z.; Carriere, Y.; Downes, S.; Dunn, R.R.; Epstein, G.; Frisvold, G.B.; Hawthorne, D.; Grohn, Y.T. Antibiotic and pesticide susceptibility and the Anthropocene operating space. Nat. Sustain. 2018, 1, 632–641. [Google Scholar]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101. [Google Scholar]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Ruiu, L. Microbial biopesticides in agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Marrone, P.G. Pesticidal natural products–status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Thakur, N.; Kaur, S.; Tomar, P.; Thakur, S.; Yadav, A.N. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–282. [Google Scholar]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Oberhardt, M.A.; Zarecki, R.; Gronow, S.; Lang, E.; Klenk, H.-P.; Gophna, U.; Ruppin, E. Harnessing the landscape of microbial culture media to predict new organism–media pairings. Nat. Commun. 2015, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Wintzingerode, F.V.; Göbel, U.B.; Stackebrandt, E. Determination of microbial diversity in environmental samples: Pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 1997, 21, 213–229. [Google Scholar] [CrossRef]
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998, 5, R245–R249. [Google Scholar] [CrossRef] [Green Version]
- Besset-Manzoni, Y.; Joly, P.; Brutel, A.; Gerin, F.; Soudière, O.; Langin, T.; Prigent-Combaret, C. Does in vitro selection of biocontrol agents guarantee success in planta? A study case of wheat protection against Fusarium seedling blight by soil bacteria. PLoS ONE 2019, 14, e0225655. [Google Scholar] [CrossRef] [Green Version]
- Khalaf, E.M.; Raizada, M.N. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol. 2018, 9, 42. [Google Scholar] [CrossRef]
- Rajesh Kannan, V.; Bastas, K.; Rajendran, S. Scientific and economic impact of plant pathogenic bacteria. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Mirzaee, H.; Ariens, E.; Blaskovich, M.A.; Clark, R.J.; Schenk, P.M. Biostimulation of Bacteria in Liquid Culture for Identification of New Antimicrobial Compounds. Pharmaceuticals 2021, 14, 1232. [Google Scholar] [CrossRef] [PubMed]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.; Xiao, Y.; Wass, T.J.; Schenk, P.M. Identification of soil bacterial isolates suppressing different Phytophthora spp. and promoting plant growth. Front. Plant Sci. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, S.R.; Ishiga, Y.; Wangdi, T.; Kunkel, B.N.; Anand, A.; Mysore, K.S.; Bender, C.L. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 2007, 20, 955–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, G.C.; Mayers, P.; Henry, R. A simplified method for the preparation of fungal genomic DNA for PCR and RAPD analysis. Biotechniques 1994, 16, 48. [Google Scholar] [PubMed]
- Prada-Ramírez, H.A.; Pérez-Mendoza, D.; Felipe, A.; Martínez-Granero, F.; Rivilla, R.; Sanjuán, J.; Gallegos, M.T. AmrZ regulates cellulose production in Pseudomonas syringae pv. tomato DC 3000. Mol. Microbiol. 2016, 99, 960–977. [Google Scholar]
- Mascia, T.; Santovito, E.; Gallitelli, D.; Cillo, F. Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol. Plant Pathol. 2010, 11, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant. Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Su’udi, M.; Park, J.-M.; Park, S.-R.; Hwang, D.-J.; Bae, S.-C.; Kim, S.; Ahn, I.-P. Quantification of Alternaria brassicicola infection in the Arabidopsis thaliana and Brassica rapa subsp. pekinensis. Microbiology 2013, 159, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Berg, T.; Tesoriero, L.; Hailstones, D. A multiplex real-time PCR assay for detection of Xanthomonas campestris from brassicas. Lett. Appl. Microbiol. 2006, 42, 624–630. [Google Scholar] [CrossRef]
- Ozgonen, H.; Erkilic, A. Growth enhancement and Phytophthora blight (Phytophthora capsici Leonian) control by arbuscular mycorrhizal fungal inoculation in pepper. J. Crop Prot. 2007, 26, 1682–1688. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.-C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell. Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Panda, A.K.; Bisht, S.S.; DeMondal, S.; Kumar, N.S.; Gurusubramanian, G.; Panigrahi, A.K. Brevibacillus as a biological tool: A short review. Anton. Leeuw. 2014, 105, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Messens, W.; De Vuyst, L. Inhibitory substances produced by Lactobacilli isolated from sourdoughs—A review. Int. J. Food Microbiol. 2002, 72, 31–43. [Google Scholar] [CrossRef]
- Zhao, L.; Teng, S.; Liu, Y. Characterization of a versatile rhizospheric organism from cucumber identified as Ochrobactrum haematophilum. J. Basic. Microbiol. 2012, 52, 232–244. [Google Scholar] [CrossRef]
- Nogueira, M.F.; Pereira, L.; Jenull, S.; Kuchler, K.; Lion, T. Klebsiella pneumoniae prevents spore germination and hyphal development of Aspergillus species. Sci. Rep. 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Al-Rubaye, A.F.; Kadhim, M.J.; Hameed, I.H. Characterization of antifungal secondary metabolites produced by Klebsiella pneumoniae and screening of its chemical compounds using GC-MS. J. Curr. Pharm. Res. 2017, 8, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Gao, H.; Li, H.; Qin, Y.; Tang, W.; Lu, J.; Li, M.; Shao, L.; Liu, H. Change of growth promotion and disease resistant of wheat seedling by application of biocontrol bacterium Pseudochrobactrum kiredjianiae A4 under simulated microgravity. Acta Astronaut. 2017, 139, 222–227. [Google Scholar] [CrossRef]
- Cai, R.; Lewis, J.; Yan, S.; Liu, H.; Clarke, C.R.; Campanile, F.; Almeida, N.F.; Studholme, D.J.; Lindeberg, M.; Schneider, D. The plant pathogen Pseudomonas syringae pv. tomato is genetically monomorphic and under strong selection to evade tomato immunity. PLoS Pathog. 2011, 7, e1002130. [Google Scholar]
- Nikolić, I.; Berić, T.; Dimkić, I.; Popović, T.; Lozo, J.; Fira, D.; Stanković, S. Biological control of Pseudomonas syringae pv. aptata on sugar beet with Bacillus pumilus SS-10.7 and Bacillus amyloliquefaciens (SS-12.6 and SS-38.4) strains. J. Appl. Microbiol. 2019, 126, 165–176. [Google Scholar]
- Al-Lami, H.; You, M.; Barbetti, M. Role of foliage component and host age on severity of Alternaria leaf spot (caused by Alternaria japonica and A. brassicae) in canola (Brassica napus) and mustard (B. juncea) and yield loss in canola. Crop. Pasture Sci. 2019, 70, 969–980. [Google Scholar] [CrossRef]
- Pane, C.; Zaccardelli, M. Evaluation of Bacillus strains isolated from solanaceous phylloplane for biocontrol of Alternaria early blight of tomato. Biol. Control 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Khan, N.; Mishra, A.; Nautiyal, C.S. Paenibacillus lentimorbus B-30488r controls early blight disease in tomato by inducing host resistance associated gene expression and inhibiting Alternaria solani. Biol. Control 2012, 62, 65–74. [Google Scholar] [CrossRef]
- Barbieri, G.; Ferrari, C.; Mamberti, S.; Gabrieli, P.; Castelli, M.; Sassera, D.; Ursino, E.; Scoffone, V.C.; Radaelli, G.; Clementi, E. Identification of a Novel Brevibacillus laterosporus Strain With Insecticidal Activity Against Aedes albopictus Larvae. Front. Microbiol. 2021, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koornneef, A.; Pieterse, C.M. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Amalraj, A.; Taylor, J.; Bithell, S.; Li, Y.; Moore, K.; Hobson, K.; Sutton, T. Mapping resistance to Phytophthora root rot identifies independent loci from cultivated (Cicer arietinum L.) and wild (Cicer echinospermum PH Davis) chickpea. Theor. Appl. Genet. 2019, 132, 1017–1033. [Google Scholar] [CrossRef]
- Jahan, M.; Shazad, U.; Naqvi, S.; Tahir, I.; Abbas, T.; Iqbal, M. Effects of Mesorhizobium ciceri and Biochar on the Growth, Nodulation and Antifungal Activity Against Root Pathogenic Fungi in Chickpea (Cicer arietinum L.). J. Plant. Pathol. Microbiol. 2020, 11, 520. [Google Scholar]
- Kannan, V.; Sureendar, R. Synergistic effect of beneficial rhizosphere microflora in biocontrol and plant growth promotion. J. Basic Microbiol. 2009, 49, 158–164. [Google Scholar] [CrossRef]
- Ruiu, L. Brevibacillus laterosporus, a pathogen of invertebrates and a broad-spectrum antimicrobial species. Insects 2013, 4, 476–492. [Google Scholar] [CrossRef] [Green Version]
- Desjardine, K.; Pereira, A.; Wright, H.; Matainaho, T.; Kelly, M.; Andersen, R.J. Tauramamide, a lipopeptide antibiotic produced in culture by Brevibacillus laterosporus isolated from a marine habitat: Structure elucidation and synthesis. J. Nat. Prod. 2007, 70, 1850–1853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, L.; Zeng, H.; Yang, X.; Yuan, J.; Shi, H.; Xiong, Y.; Chen, M.; Han, L.; Qiu, D. Purification and characterization of a novel antimicrobial peptide from Brevibacillus laterosporus strain A60. Peptides 2012, 33, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.E.; An, F.Y.; Dholpe, V.; Ramamoorthy, A.; Lopatin, D.E.; Lantz, M.S. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J. Antimicrob. Chemother. 2007, 59, 297–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, R.; Vater, J.; Budiharjo, A.; Wang, Z.; He, Y.; Dietel, K.; Schwecke, T.; Herfort, S.; Lasch, P.; Borriss, R. Amylocyclicin, a Novel Circular Bacteriocin Produced by Bacillus amyloliquefaciens FZB42. J. Bacteriol. 2014, 196, 1842–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Hiradate, S.; Tsukamoto, T.; Hatakeda, K.; Shirata, A. Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology 2001, 91, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Von der Weid, I.; Alviano, D.S.; Santos, A.L.S.; Soares, R.M.A.; Alviano, C.S.; Seldin, L. Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J. Appl. Microbiol. 2003, 95, 1143–1151. [Google Scholar] [CrossRef]
- Frikha-Gargouri, O.; Ben Abdallah, D.; Ghorbel, I.; Charfeddine, I.; Jlaiel, L.; Triki, M.A.; Tounsi, S. Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress Agrobacterium crown gall tumours on tomato plants. Pest Manag. Sci. 2017, 73, 568–574. [Google Scholar] [CrossRef]
- He, C.-N.; Ye, W.-Q.; Zhu, Y.-Y.; Zhou, W.-W. Antifungal activity of volatile organic compounds produced by Bacillus methylotrophicus and Bacillus thuringiensis against five common spoilage fungi on loquats. Molecules 2020, 25, 3360. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; Hu, J.; Wang, S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012, 65, 317. [Google Scholar] [CrossRef] [Green Version]
- Jasim, B.; Sreelakshmi, S.; Mathew, J.; Radhakrishnan, E.K. Identification of endophytic Bacillus mojavensis with highly specialized broad spectrum antibacterial activity. 3 Biotech 2016, 6, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apetroaie-Constantin, C.; Mikkola, R.; Andersson, M.A.; Teplova, V.; Suominen, I.; Johansson, T.; Salkinoja-Salonen, M. Bacillus subtilis and B. mojavensis strains connected to food poisoning produce the heat stable toxin amylosin. J. Appl. Microbiol. 2009, 106, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-N.; Zhang, J.; Chen, Q.; He, J.; Li, Q.-F.; Li, S.-P. Comamonas jiangduensis sp. nov., a biosurfactant-producing bacterium isolated from agricultural soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 2168–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoud, W.; Jakobsen, M. Surface ripened cheeses: The effects of Debaryomyces hansenii, NaCl and pH on the intensity of pigmentation produced by Brevibacterium linens and Corynebacterium flavescens. Int. Dairy J. 2003, 13, 231–237. [Google Scholar] [CrossRef]
- Pattnaik, P.; Kaushik, J.; Grover, S.; Batish, V. Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J. Appl. Microbiol. 2001, 91, 636–645. [Google Scholar] [CrossRef]
- Kayalvizhi, N.; Gunasekaran, P. Production and characterization of a low-molecular-weight bacteriocin from Bacillus licheniformis MKU3. Lett. Appl. Microbiol. 2008, 47, 600–607. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, Q.; Ji, Y.; Xie, A.; Yang, L.; Mu, S.; Li, Z.; He, T.; Xiao, Y.; Zhao, J. Isolation and identification of antibacterial bioactive compounds from Bacillus megaterium L2. Front. Microbiol. 2021, 12, 645484. [Google Scholar] [CrossRef]
- Al-Thubiani, A.S.; Maher, Y.A.; Fathi, A.; Abourehab, M.A.; Alarjah, M.; Khan, M.S.; Al-Ghamdi, S.B. Identification and characterization of a novel antimicrobial peptide compound produced by Bacillus megaterium strain isolated from oral microflora. Saudi Pharm. J. 2018, 26, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Kim, S.G.; Khan, Z.; Jeon, Y.H.; Kim, Y.H. Inhibitory effect of Paenibacillus polymyxa GBR-462 on Phytophthora capsici causing phytophthora blight in chili pepper. J. Phytopathol. 2009, 157, 329–337. [Google Scholar] [CrossRef]
- Timmusk, S.; Van West, P.; Gow, N.; Paul Huffstutler, R. Paenibacillus polymyxa antagonizes oomycete plant pathogens Phytophthora palmivora and Pythium aphanidermatum. J. Appl. Microbiol. 2009, 106, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Almeida, F.C.R.; Magalhães, D.M.; Favaris, A.P.; Rodríguez, J.; Azevedo, K.E.X.; Bento, J.M.S.; Alves, D.A. Side effects of a fungus-based biopesticide on stingless bee guarding behaviour. Chemosphere 2022, 287, 132147. [Google Scholar] [CrossRef]
- Trivedi, P.; Schenk, P.M.; Wallenstein, M.D.; Singh, B.K. Tiny microbes, big yields: Enhancing food crop production with biological solutions. Microb. Biotechnol. 2017, 10, 999–1003. [Google Scholar] [CrossRef] [Green Version]
- Sudakin, D.L. Biopesticides. Toxicol. Rev. 2003, 22, 83–90. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Booth, J.; Schenk, P.M.; Mirzaee, H. Microbial Biopesticides against Bacterial, Fungal and Oomycete Pathogens of Tomato, Cabbage and Chickpea. Appl. Microbiol. 2022, 2, 288-301. https://doi.org/10.3390/applmicrobiol2010021
Booth J, Schenk PM, Mirzaee H. Microbial Biopesticides against Bacterial, Fungal and Oomycete Pathogens of Tomato, Cabbage and Chickpea. Applied Microbiology. 2022; 2(1):288-301. https://doi.org/10.3390/applmicrobiol2010021
Chicago/Turabian StyleBooth, James, Peer M. Schenk, and Hooman Mirzaee. 2022. "Microbial Biopesticides against Bacterial, Fungal and Oomycete Pathogens of Tomato, Cabbage and Chickpea" Applied Microbiology 2, no. 1: 288-301. https://doi.org/10.3390/applmicrobiol2010021





