Photocatalytic Inactivation of Viruses and Prions: Multilevel Approach with Other Disinfectants
Abstract
:1. Introduction
2. Decontamination of Influenza Viruses
3. Decontamination of SARS-CoV-2
3.1. Decontamination of SARS-CoV-2 by Photocatalysts
3.2. Decontamination of SARS-CoV-2 by 222-nm UV Light
4. Decontamination of Scrapie Prions
5. Future Prospects for Decontamination of Prionoids
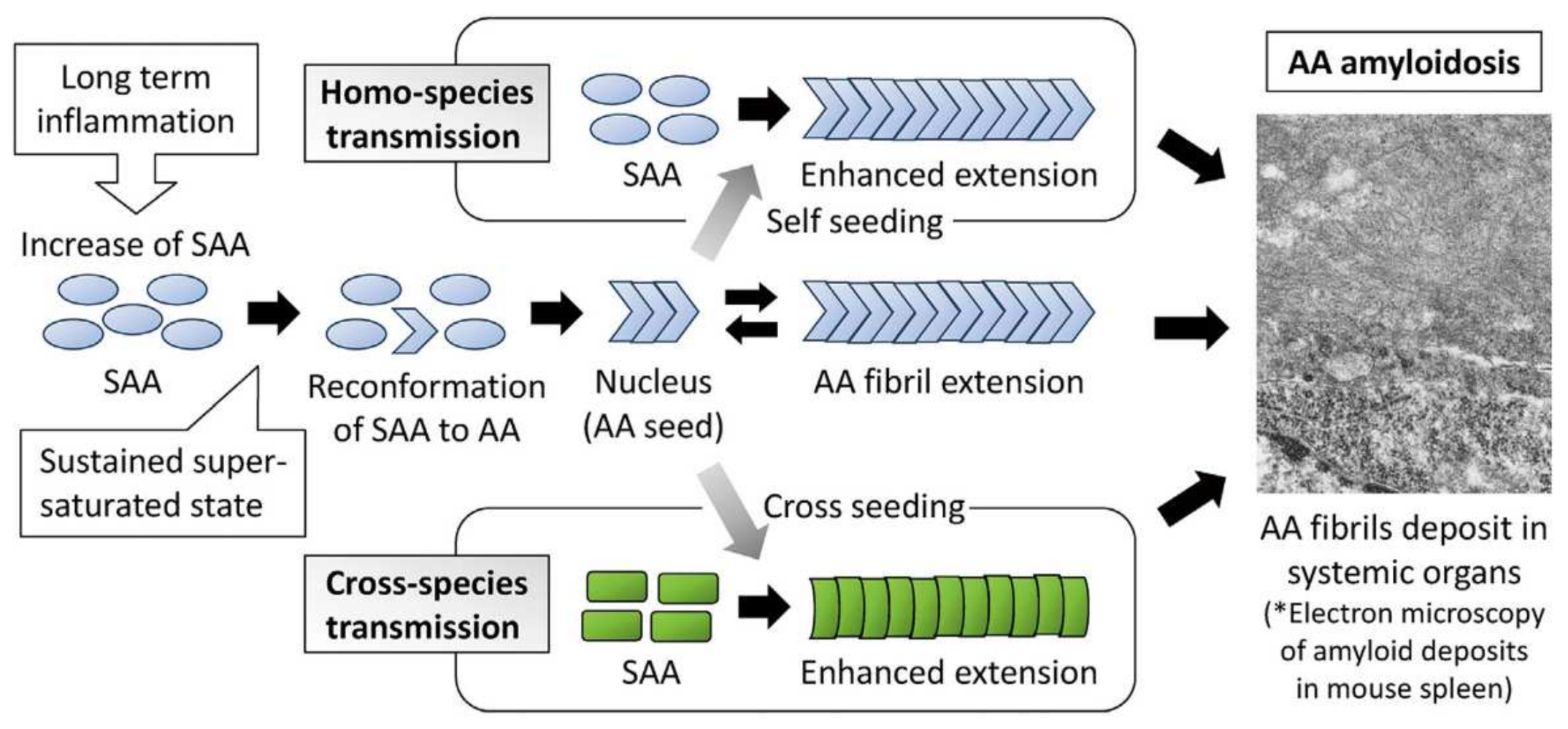
5.1. Transmission of Amyloid A Amyloidosis and Bovine Aβ
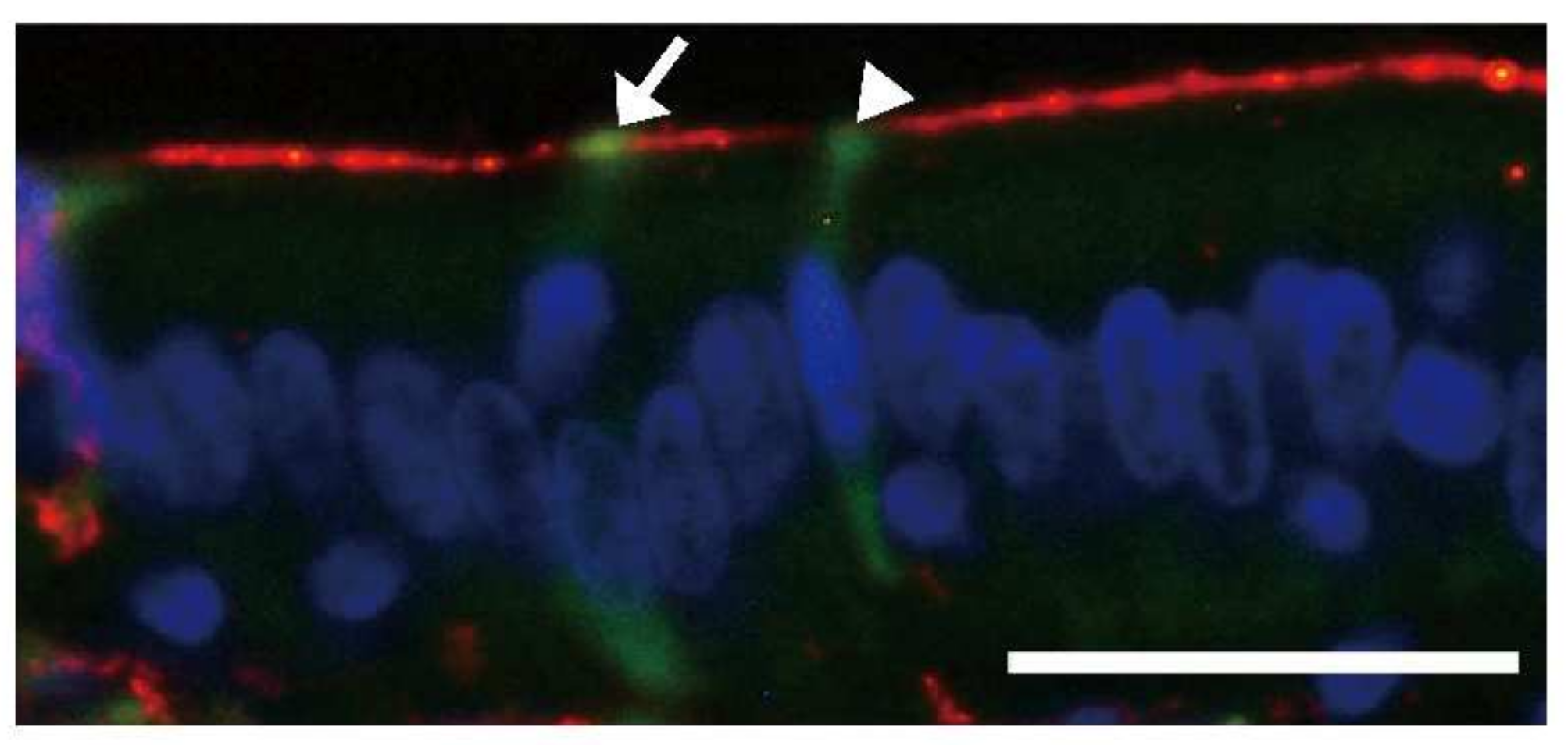
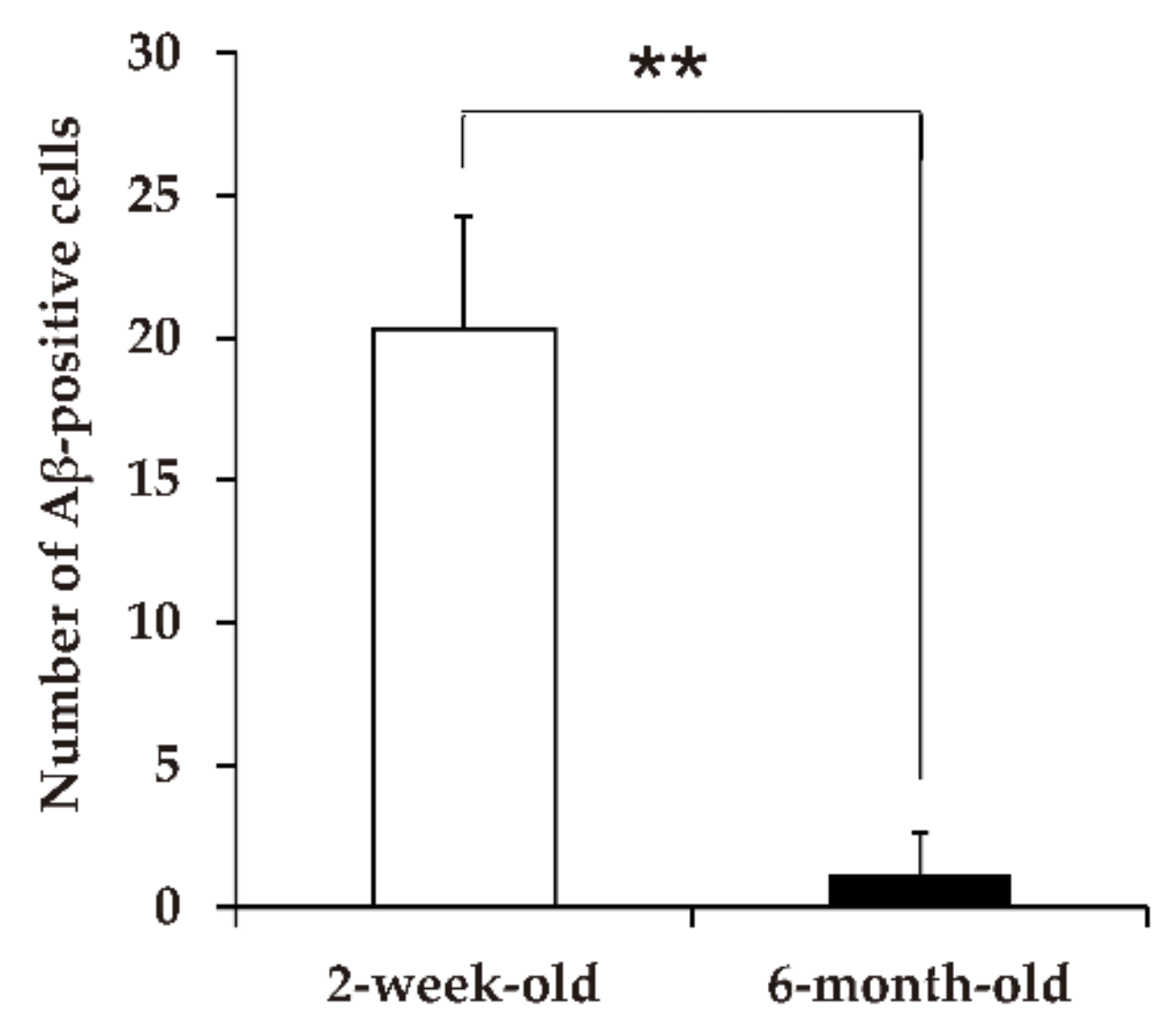
5.2. Induction of Aβ Deposition, Islet Amyloid Polypeptide Deposition, and Cerebral Aβ-Amyloid Angiopathy
5.3. Aggregates of α-Synuclein
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advance in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Sun, D.S.; Wu, W.S.; Chan, H.; Syue, M.S.; Ho, H.C.; Chang, H.H. Antibacterial performance of nanoscaled visible-light responsive platinum-containing titania photocatalyst in vitro and in vivo. Biochim. Biophys. Acta 2013, 1830, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Shen, Z.; Huang, G.; Wang, W.; Yu, J.C.; Wong, P.K. Red phosphorus: An earth-abundant elemental photocatalyst for “green” bacterial inactivation under visible light. Environ. Sci. Technol. 2015, 49, 6264–6273. [Google Scholar] [CrossRef] [PubMed]
- Leyland, N.S.; Podporska-Carroll, J.; Browne, J.; Hinder, S.J.; Quilty, B.; Pillai, S.C. Highly efficient F, Cu doped TiO2 anti-bacterial visible light active photocatalytic coatings to combat hospital-acquired infections. Sci. Rep. 2016, 6, 24770. [Google Scholar] [CrossRef]
- Wajima, T.; Nakaminami, H.; Aoki, S.; Seyama, S.; Noguchi, N. Evaluation of the antimicrobial effects of a novel visible light-driven photocatalyst in vitro and in the environment. Yakugaku Zasshi 2021, 141, 135–142. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, H.A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on heterogenous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interface Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Li, Y.; Shuai, D. Visible-light-driven photocatalytic disinfection of human adenovirus by a novel heterostructure of oxygen-doped graphitic carbon nitride and hydrothermal carbonation carbon. Appl. Catal. B Environ. 2019, 248, 11–21. [Google Scholar] [CrossRef]
- Hiragond, C.B.; Kshirsagar, A.S.; Dhapte, V.V.; Khanna, T.; Joshi, P.; More, P.V. Enhanced anti-microbial response of commercial face mask using colloidal silver nanoparticles. Vacuum 2018, 156, 475–482. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R.; Röhl, C.; Shukla, D.; Spors, F.; Tiwari, V. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral Res. 2011, 92, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Nino, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine 2010, 6, 681–688. [Google Scholar] [CrossRef]
- Perni, S.; Piccirillo, C.; Pratten, J.; Prokopovich, P.; Chrzanowski, W.; Parkin, I.P.; Wilson, M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials 2009, 30, 89–93. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. The latest strategies in the fight against the COVID-19 pandemic: The role of metal and metal oxide nanoparticles. New J. Chem. 2021, 45, 6167–6179. [Google Scholar] [CrossRef]
- Matsuura, R.; Lo, C.W.; Wada, S.; Somei, J.; Ochiai, H.; Murakami, T.; Saito, N.; Ogawa, T.; Shinjo, A.; Benno, Y.; et al. SARS-CoV-2 disinfection of air and surface contamination by TiO2 photocatalyst-mediated damage to viral morphology, RNA, and protein. Viruses 2021, 13, 942. [Google Scholar] [CrossRef]
- Patial, S.; Kumar, A.; Raizada, P.; Van Le, Q.V.; Nguyen, V.H.; Selvasembian, R.; Singh, P.; Thakur, S.; Hussain, C.M. Potential of graphene based photocatalyst for antiviral activity with emphasis on COVID-19: A review. J. Environ. Chem. Eng. 2022, 10, 107527. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Ishihara, R.; Omiya, D.; Ishitsuka, M.; Hirano, S.; Suzuki, T. Application of a photocatalyst as an inactivator of bovine coronavirus. Viruses 2020, 12, 1372. [Google Scholar] [CrossRef]
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic Inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012, 11, 1293–1298. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, K.; Wang, J.; Cui, D.; Zhao, M. Fabrication and characterization of CdS nanowires templated in tobacco mosaic virus with improved photocatalytic ability. Appl. Microbiol. Biotechnol. 2021, 105, 8255–8264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Kang, M.; Shen, Z.P.; Shi, L.; Zheng, X. Visible-light-driven photocatalytic inactivation of bacteriophage f2 by Cu-TiO2 nanofibers in the presence of humic acid. J. Environ. Sci. 2019, 77, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Fa, W.; Peng, T.; Gong, Z.K. Photocatalysis effect of nanometer TiO2 and TiO2-coated ceramic plate on hepatitis B virus. J. Photochem. Photobiol. B 2007, 86, 165–169. [Google Scholar] [CrossRef]
- Paspaltsis, I.; Kotta, K.; LagoudSakudo, A.; Lagoudaki, R.; Grigoriadis, N.; Poulios, I.; Sklaviadis, T. Titanium dioxide photocatalytic inactivation of prions. J. Gen. Virol. 2006, 87, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Berberidou, C.; Xanthopoulos, K.; Paspaltsis, I.; Lourbopoulos, A.; Polyzoidou, E.; Sklaviadis, T.; Poulios, I. Homologous photocatalytic decontamination of prion infected stainless steel and titanium surfaces. Prion 2014, 7, 488–495. [Google Scholar] [CrossRef]
- Ashikaga, T.; Wada, W.; Kobayashi, H.; Mori, M.; Katsumura, Y.; Fukui, H.; Kato, S.; Yamanuichi, M.; Takamatsu, T. Effect of the photocatalytic activity of TiO2 on plasmid DNA. Mutat. Res. Genet. Toxicol. Environ. Mutag. 2000, 466, 1–7. [Google Scholar] [CrossRef]
- CDC. HPAI a H5 Virus Background and Clinical Illness. Available online: https://www.cdc.gov/flu/avianflu/hapi-background-clinical-illness.htm (accessed on 8 May 2022).
- Takehara, K.; Yamazaki, K.; Miyazaki, M.; Yamada, Y.; Ruenphet, S.; Jahangir, A.; Shoham, D.; Okamura, M.; Nakamura, M. Inactivation of avian influenza virus H1N1 by photocatalyst under visible light irradiation. Virus Res. 2010, 151, 102–103. [Google Scholar] [CrossRef]
- Uema, M.; Yonemitsu, K.; Momose, Y.; Ishii, Y.; Takeda, K.; Inoue, T.; Asakura, H. Effect of photocatalyst under visible light irradiation in SARS-CoV-2 stability on an abiotic surface. Biocontrol Sci. 2021, 26, 119–125. [Google Scholar] [CrossRef]
- Performance Standard (Photocatalysis Industry Association of Japan). Available online: https://www.piaj.gr.jp/roller/entry/20090121 (accessed on 20 October 2021).
- Holmes, E.C. COVID-19—Lessons for zoonotic disease. Science 2022, 375, 1114–1115. [Google Scholar] [CrossRef]
- Lam, T.T.-Y.; Ja, N.; Zhang, Y.-W.; Shum, M.H.-H.; Jiang, J.-F.; Zhu, H.-C.; Tong, Y.-G.; Shi, Y.-X.; Ni, X.-B.; Liao, Y.-S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Chen, W.; Chen, J.-P. Viral metagenomics revealed Sendai virus and coronavirus infection of Malayan pangolins (Manis javanica). Viruses 2019, 11, 979. [Google Scholar] [CrossRef]
- Carbis Bay G7 Summit Communique. Our Shared Agenda for Global Action to Build Back Better. Available online: https://www.g7/uk.org/wp-content/uploads/2021/06/Carbis-Bay-G7-Summit-Comunique-PDP-430KB-25-pages-3.pdf82021 (accessed on 20 July 2022).
- Xu, G.J.; Kula, T.; Xu, Q.; Li, M.Z.; Vernon, S.D.; Ndung’u, T.; Ruxrungtham, K.; Sanchez, J.; Brander, C.; Chung, R.T.; et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science 2015, 348, aaa0698. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; An, S.; Sohn, Y.; Cho, Y.; Hyun, J.H.; Baek, Y.J.; Kim, M.H.; Jeong, S.J.; Kim, J.H.; Ku, N.S.; et al. Environmental contamination in the isolation of COVID-19 patients with severe pneumonia requiring mechanical ventilation of high-flow oxygen therapy. J. Hosp. Infect. 2020, 106, 570–576. [Google Scholar] [CrossRef]
- Hu, T.; Ji, Y.; Fei, F.; Zhu, M.; Jin, T.; Xue, P.; Zhang, N. Optimization of COVID-19 prevention and control with low building energy consumption. Build. Environ. 2022, 219, 109233. [Google Scholar] [CrossRef] [PubMed]
- Leslie, R.A.; Zhou, S.S.; Macinga, D.R. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Am. J. Infect. Control 2021, 49, 401–402. [Google Scholar] [CrossRef]
- Akhavan, O.; Choobtashani, M.; Ghaderi, E. Protein degradation and RNA efflux of viruses photocatalyzed by graphene-tungstene oxide composite under visible light irradiation. J. Phys. Chem. C 2012, 116, 9653–9659. [Google Scholar] [CrossRef]
- Donskyi, I.S.; Nie, C.; Ludwig, K.; Trimpert, J.; Ahmed, R.; Quaas, E.; Achzi, K.; Radnik, J.; Adeli, M.; Haag, R. Graphene sheets with defined dual functionalies for the strong SARS-CoV-2 interactions. Small 2021, 17, 2007091. [Google Scholar] [CrossRef]
- Xu, R.; Liu, X.; Zhang, P.; Ma, H.; Liu, G.; Xia, Z. The photodestruction of virus in nano-TiO2 suspension. J. Wuhan Univ. Technol. 2007, 22, 422–425. [Google Scholar] [CrossRef]
- Liga, M.V.; Bryant, E.L.; Colvin, V.L.; Li, Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011, 45, 535–544. [Google Scholar] [CrossRef]
- Liga, M.V.; Maguire-Boyle, S.J.; Jafry, H.R.; Barron, A.R.; Li, Q. Silica decorated TiO2 for virus inactivation in drinking water-simple synthesis method and mechanisms of enhanced inactivation kinetics. Environ. Sci. Technol. 2013, 47, 6463–6470. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, I.; Yao, Y.; Ochiai, I.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad spectrum microbial activity of photocatalysis by TiO2. Catalysis 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Zhang, L.; Rao, L.; Wang, P.; Guo, X.; Wang, Y. Fabrication and photocatalytic performance evaluation of hydrodynamic erosion-resistant nano-TiO2-silicone resin composite films. Environ. Sci. Pollut. Res. 2019, 26, 4997–5007. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Feng, D.; Song, A.; Gong, H.; Zhu, S. Effects of organic-inorganic hybrid coating on the color stability of denture base resins. J. Prosthet. Dent. 2016, 115, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Ge, J.; Cheng, S.; Han, H.; Cui, S. Synthesis and characterization of titania/mq silicon resin hybrid nanocomposite via sol-gel process. J. Sol-Gel Sci. Technol. 2011, 59, 635–639. [Google Scholar] [CrossRef]
- Kitagawa, H.; Nomura, T.; Nazmul, T.; Omori, K.; Shigemoto, N.; Sakaguchi, T.; Ohge, H. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control 2021, 49, 299–301. [Google Scholar] [CrossRef]
- Mariita, R.M.; Davis, J.H.; Randive, R.V. Illuminating human noroviruses: A perspective on disinfection of water and surfaces using uvc, norovirus model organisms, and radiation safety considerations. Pathogens 2022, 11, 226. [Google Scholar] [CrossRef]
- Kaiki, Y.; Kitagawa, H.; Hara, T.; Nomura, T.; Omori, K.; Shigemoto, N.; Takahashi, S.; Ohge, H. Methicillin-resistant Staphylococcus aureus Contamination of hospital-use-only mobile phones and efficacy of 222-nm ultraviolet disinfection. Am. J. Infect. Control 2021, 49, 800–803. [Google Scholar] [CrossRef]
- Yamano, N.; Kunisada, M.; Kaidzu, S.; Sugihara, K.; Nishiaki-Sawada, A.; Ohashi, H.; Yoshioka, A.; Igarashi, T.; Ohira, A.; Tanito, M.; et al. Long-term effects of 222-nm ultraviolet radiation C sterilizing lamps on mice susceptible to ultraviolet radiation. Photochem. Photobiol. 2020, 96, 853–862. [Google Scholar] [CrossRef]
- Aguzzi, A.; Calella, A.M. Prions: Protein aggregation and infectious diseases. Physiol. Rev. 2009, 89, 1105–1152. [Google Scholar] [CrossRef]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef] [Green Version]
- Sakudo, A.; Xue, G.; Kawashita, N.; Ano, Y.; Takagi, T.; Shintani, H.; Tanaka, Y.; Onodera, T.; Ikuta, K. Structure of the prion protein and its gene: An analysis using bioinformatics and computer simulation. Curr. Protein Pept. Sci. 2010, 11, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Stahl, N.; Brochelt, D.R.; Hsiao, K.; Prusiner, S.B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 1987, 51, 229–240. [Google Scholar] [CrossRef]
- Matsuura, Y.; Ishikawa, Y.; Murayama, Y.; Yokoyama, T.; Somerville, R.A.; Kitamoto, T.; Mohri, S. Eliminating transmissibility of bovine spongiform encephalopathy by dry-heat treatment. J. Gen. Virol. 2020, 101, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Paspaltis, I.; Berberidou, C.; Poulios, I.; Sklaviadis, T. Photocatalytic degradation of prions using the photo-fenton reagent. J. Hosp. Infect. 2009, 71, 149–156. [Google Scholar] [CrossRef]
- Bodgan, J.; Zarzynnka, J.; Plawrinska-Czarnak, J. Comparison of infectious agents susceptibility to photocatalytic effects of nanosized titanium and zinc oxides: A practical approach. Nanoscale Res. Lett. 2015, 10, 1–5. [Google Scholar] [CrossRef]
- Scheckel, C.; Aguzzi, A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018, 19, 405–418. [Google Scholar] [CrossRef]
- Murakami, T.; Inoshima, Y.; Ishiguro, N. Systemic AA Amyloidosis as a prion-like disorder. Virus Res. 2015, 207, 76–81. [Google Scholar] [CrossRef]
- Soto, C.; Estrada, L.; Castilla, J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem. Sci. 2006, 31, 150–155. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kamatari, Y.O.; Fukuoka, M.; Miyaji, R.; Kuwata, K. Nearly reversible conformational change of amyloid fibrils as revealed by pH-jump experiments. Biochemistry 2013, 52, 6797–6806. [Google Scholar] [CrossRef]
- Miyagawa, I.; Nakayamada, S.; Saito, K.; Hanami, K.; Nawata, M.; Sawamukai, N.; Nakano, K.; Yamaoka, K.; Tanaka, Y. Study on the safety and efficacy of tocilizumab, an Anti-IL-6 receptor antibody, in patients with rheumatoid arthritis complicated with AA amyloidosis. Mod. Rheumatol. 2014, 24, 405–409. [Google Scholar] [CrossRef]
- Tojo, K.; Tokuda, T.; Hoshii, Y.; Fu, X.; Higuchi, K.; Matsui, T.; Kametani, F.; Ikeda, S. Unexpectedly high incidence of visceral AA-amyloidosis in slaughtered cattle in japan. Amyloid 2005, 12, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Richey, T.; Murchy, C.L.; Weiss, D.T.; Wall, J.S.; Westermark, G.T.; Westermark, P. Amyloidogenic potential of foie gras. Proc. Natl. Acad. Sci. USA 2007, 104, 10998–11001. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Kawano, H.; Hoshii, Y.; Liu, Y.; Ishihara, T. Acceleration of murine AA amyloid deposition by bovine amyloid fibril and tissue homogenates. Amyloid 2008, 15, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, D.; Hoshii, Y.; Kwano, H.; Une, Y.; Gondo, T.; Ishihara, T. Induction murine AA Amyloidosis by various homogenous amyloid fibrils and amyloid-like synthetic peptides. Scand. J. Immunol. 2007, 66, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Murakami, T.; Inoshima, Y.; Ishiguro, N. Effect of heating on the stability of amyloid a (AA) fibrils and the intra- and cross-species transmission of aa amyloidosis. Amyloid 2015, 22, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Iwaide, S.; Ujike, N.; Kobayashi, K.; Sassa, Y.; Murakami, T. Species-barrier on the cross-species oral transmission of bovine aa amyloidosis in mice. J. Vet. Med. Sci. 2012, 83, 962–967. [Google Scholar] [CrossRef]
- Ano, Y.; Nakayama, H.; Sakai, Y.; Sakudo, A.; Endo, M.; Ebisu, S.; Li, J.-Y.; Uetsuka, K.; Manabe, N.; Onodera, T. Incorporation of β-amyloid protein through the bovine ileal epithelium before and after weaning: Model for orally transmitted amyloidosis. Microbiol. Immunol. 2008, 52, 429–434. [Google Scholar] [CrossRef]
- Beery, M.A.; Jacobsen, L.M.; Atkinson, M.A.; Butler, A.E.; Campbell-Thompson, M. Islet amyloidosis in a child with type 1 diabetes. Islet 2019, 11, 44–49. [Google Scholar] [CrossRef]
- Costes, S.; Langen, R.; Gurlo, T.; Matveyenko, A.V.; Butler, P.C. Beta-cell failure in type 2 diabetes: A case of asking too much of too few? Diabetes 2013, 62, 327–335. [Google Scholar] [CrossRef]
- Gary, C.; Lam, S.; Herard, A.-S.; Koch, J.E.; Petit, F.; Gipchtein, P.; Sawiak, S.J.; Caillrerez, R.; Eddarkaoui, S.; Colin, M.; et al. Encephalopathy induced by Alzheimer brain inoculation in a non-human primate. Acta Neuropathol. Commun. 2019, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Stohr, J.; Watts, J.C.; Mensinger, Z.L.; Oehler, A.; Grillo, S.K.; DeArmond, S.J.; Prusiner, S.B.; Giles, K. Purified and synthetic Alzheimer’s amyloid beta [Aβ] proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 11025–11130. [Google Scholar] [CrossRef]
- Frontzek, K.; Lutz, M.I.; Aguzzi, A.; Kovacs, G.G.; Budka, H. Amyloid-β pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt-Jakob disease after dural grafting. Swiss Med. Wkly. 2016, 146, w14287. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Sazdovitch, V.; Ando, K.; Seihean, D.; Privat, N.; Yilmaz, Z.; Peckeu, L.; Amar, E.; Comoy, E.; Maceski, A.; et al. Neuropathology of iatrogenic creutzfeldt-jakob disease and immunoassay of French Cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of aβ pathology. Acta Neuropathol. 2018, 135, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Purro, S.A.; Farrow, M.A.; Linehan, J.; Nazari, T.; Thomas, D.X.; Chen, Z.; Mengel, D.; Saito, T.; Saido, T.; Rudge, P.; et al. Transmission of amyloid-β protein pathology from cadaveric pituitary growth hormone. Nature 2018, 564, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Taniguchi, Y.; Sakai, K.; Kitamoto, T.; Takao, M.; Murayama, S.; Iwasaki, Y.; Yoshida, M.; Shimizu, H.; Kakita, A.; et al. Significant association cadaveric dura mater grafting with subpial aβ deposition and meningeal amyloid angiopathy. Acta Neuropathol. 2016, 132, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Daviglus, M.L.; Plassman, B.; Pirzada, A.; Bell, C.C.; Bowen, P.E.; Burke, J.R.; Connolly, E.S.; Dunbar-Jakob, J.M.; Granieri, E.C.; McGarry, K.; et al. Risk factors and preventive interventions for Alzheimer disease: State of the science. Arch. Neurol. 2011, 68, 1185–1190. [Google Scholar] [CrossRef]
- O’Meara, E.S.; Kukull, W.A.; Schellenberg, G.D.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Pfanschmidt, M.; Thompson, J.D.; Larson, E.B. Alzheimer’s disease and history of blood transfusion by apolipoprotein-e genotype. Neuroepidemiology 1997, 16, 86–93. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Quaegebeur, A.; Taipa, R.; Viana-Baptista, M.; Barbosa, R.; Koriath, C.; Sciot, R.; Mead, S.; Brandner, S. Evidence of amyloid-β cerebral amyloid angiopathy transmission through neurosurgery. Acta Neuropathol. 2018, 135, 671–679. [Google Scholar] [CrossRef]
- Watts, J.C.; Giles, K.; Oehler, A.; Middleton, L.; Dexter, D.T.; Gentleman, S.M.; DeArmond, S.J.; Prusiner, S.B. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. USA 2013, 110, 1955–19560. [Google Scholar] [CrossRef]
- Prusiner, S.B.; Woeman, A.; Mordes, D.A.; Watts, J.C.; Rampersaud, R.; Berry, D.B.; Patel, S.; Oehler, A.; Lowe, J.K.; Kravitz, S.; et al. Evidence for α-synuclein prions causing multiple system atrophy in human with Parkinsonism. Proc. Natl. Acad. Sci. USA 2015, 112, E5308–E5317. [Google Scholar] [CrossRef] [Green Version]
- Sigurdson, C.J.; Nilsson, K.P.; Hornemann, S.; Manco, G.; Fernandez-Borges, N.; Schwarz, P.; Castilla, J.; Wuthrich, K.; Aguzzi, A. A molecular switch controls interspecies prion disease transmission in mice. J. Clin. Investig. 2010, 120, 2590–2599. [Google Scholar] [CrossRef]
- Sacino, A.N.; Ayers, J.; Brooks, M.M.; Chakrabarty, P.; Hudson, V.J., 3rd; Howard, J.K.; Golde, T.; Giasson, B.I.; Borchelt, D.R. Non-prion type transmission in a53t α-synuclein transgenic mice: A normal component of spinal homogenates from naïve non-transgenic mice induces robust α-synuclein pathology. Acta Neuropathol. 2016, 131, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, Z.; Englund, E.; Winder, H.; Lindvall, O.; Li, J.-Y.; Brundin, P. Signs of degeneration in 12-22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson’s disease. J. Parkinson’s Dis. 2011, 1, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Chu, Y.; Hauser, R.A.; Freeman, T.B.; Olanow, C.W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008, 14, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Englund, E.; Widner, H.; Mattson, B.; van Westen, D.; Latt, J.; Rehncrona, S.; Brundin, P.; Bjorklund, A.; Lindvall, O.; et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating Parkinsonian brain. Proc. Natl. Acad. Sci. USA 2016, 113, 6544–6549. [Google Scholar] [CrossRef]
- Kahn, K.; Marita, R.M. Quantifying the Impact of Ultraviolet Subtype C in Reducing Airborne Pathogen Transmission and Improving Energy Efficiency in Healthy Buildings: A Kahn-Marita equivalent ventilation model. Front. Built Environ. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Cadet, J. Harmless effects of sterilizing 222-nm far-UV radiation on mouse skin and eye tissues. Photochem. Photobiol. 2020, 96, 949–950. [Google Scholar] [CrossRef]
- Capellari, S.; Zaidi, S.I.; Urig, C.B.; Perry, G.; Smith, M.A.; Petersen, R.B. Prion protein glycosylation is sensitive to redox change. J. Biol. Chem. 1999, 274, 34846–34850. [Google Scholar] [CrossRef]
- Nakashima, R.; Kawamoto, M.; Miyazaki, S.; Onishi, R.; Furusaki, K.; Osaki, M.; Kirisawa, R.; Sakudo, A.; Onodera, T. Evaluation of calcium hydrogen carbonate mesoscopic crystals as a disinfectant for influenza a viruses. J. Vet. Med. Sci. 2017, 79, 939–942. [Google Scholar] [CrossRef]
- Shimakura, H.; Gen-Nagata, F.; Haritani, M.; Furusaki, K.; Kato, Y.; Yamashita-Kawanishi, N.; Le, D.T.; Tsuzuki, M.; Tohya, Y.; Kyuwa, S.; et al. Inactivation of human norovirus and its surrogate by the disinfectant consisting of calcium hydrogen carbonate mesoscopic crystals. FEMS Microbiol. Lett. 2019, 366, fnz235. [Google Scholar] [CrossRef]
- Yokoyama, T.; Nishimura, T.; Uwamino, Y.; Kosaki, K.; Furusaki, K.; Onishi, R.; Onodera, T.; Haritani, M.; Sugiura, K.; Kirisawa, R.; et al. Virucidal effect of the mesoscopic structure of CAC-717 against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Microorganisms 2021, 9, 2096. [Google Scholar] [CrossRef] [PubMed]
- Kirisawa, R.; Kato, R.; Furusaki, K.; Onodera, T. Universal virucidal activity of calcium bicarbonate mesoscopic crystals that provides an effective and biosafe disinfectant. Microorganisms 2022, 10, 262. [Google Scholar] [CrossRef]
- Sakudo, A.; Iwamaru, Y.; Furusaki, K.; Haritani, M.; Onishi, R.; Imamura, M.; Yokoyama, T.; Yoshikawa, Y.; Onodera, T. Inactivation of scrapie prions by the electrically charged disinfectant CAC-717. Pathogens 2020, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.L. Photocatalysis and virus. From theory to applications. J. Photocatal. 2021, 2, 25–34. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization of microorganisms using gas plasma: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
- Sakudo, A.; Yamashiro, R.; Onodera, T. Recent advances in prion inactivation by plasma sterilizer. Int. J. Mol. Sci. 2022, 23, 10241. [Google Scholar] [CrossRef]
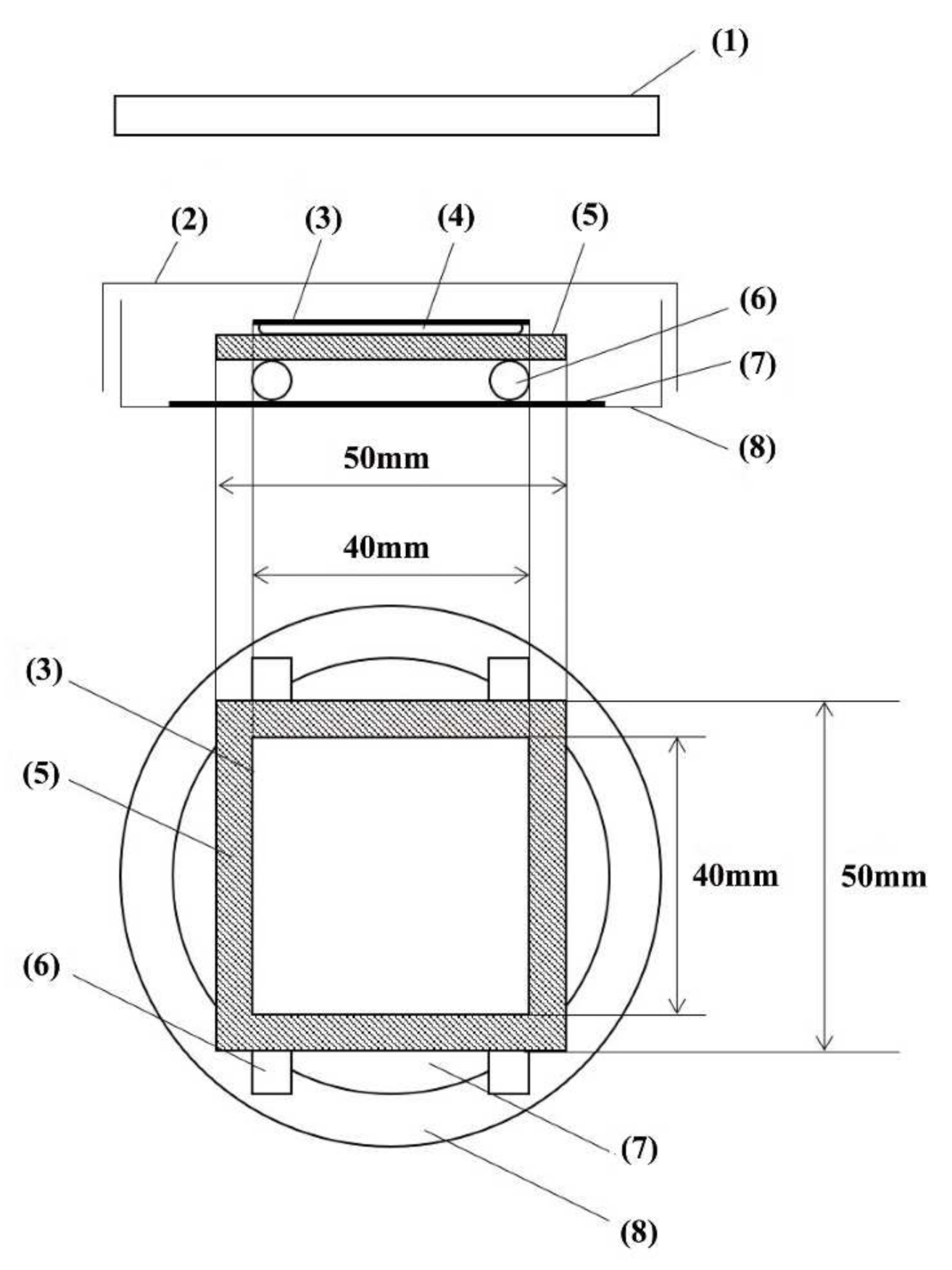

| Photocatalyst | Agent | Pathogen Load | Duration of Treatments | Reference | |
|---|---|---|---|---|---|
| Photocatalysis-Untreated | Photocatalysis-Treated | ||||
| Influenza | |||||
| TiO2/Black Light (352 nm) | H1N1 | >1.0 × 108.0 (TCID50/mL) | undetectable | 8 h | [20] |
| Pt-WO3 | H1N1 | 1.0 × 107.0 (TCID50/mL) | <1.0 × 101.5 (TCID50/mL) | 6 h | [28] |
| SARS-CoV-2 | |||||
| WO3 | JPN/TY/WK-521 | 5.98 ± 0.38 log10 (TCID50/mL) | 3.05 ± 0.25 log10 (TCID50/mL) | 6 h | [29] |
| TiO2/LED | JPN/TY/WK-521 | 1.0 × 105 (TCID50/mL) | undetectable | 2 h | [17] |
| Bovine coronavirus | |||||
| Peroxo titanium acid (70%) + peroxo-modified anatase | Hokkaido/9/03 | 4.4 ± 0.3 log10 (TCID50/0.1 mL) | 1.6 ± 0.1 log10 (TCID50/0.1 mL) | 4 h | [19] |
| Photocatalysts | Agent | Reactor Volume | pH during Reaction | Temperature | Photocatalyst Retained Material | Light | Reference |
|---|---|---|---|---|---|---|---|
| Influenza | |||||||
| TiO2/Black Light | H1N1 | 100 µL | PBS | 25 °C | sprayed to glass with 1 g TiO2 to 300 m2 | UV (352 nm) | [20] |
| Pt-WO3 | H1N1 | 100 µL | PBS | 25 °C | coated glass * | visible light (410–470 nm) | [28] |
| SARS-CoV-2 | |||||||
| WO3 | JPN/TY/WK-521 | 30 µL | 6.8 (MEM) + 2% FBS | 20 °C | 4 g/m2 mixed with silica binder | visible light (>380 nm) | [29] |
| TiO2/LED | JPN/TY/WK-521 | 1 mL | 6.8 (MEM) + 5% FBS | 20 °C | sprayed to glass fiber sheet ** | UV (405 nm LED) | [17] |
| Bovine coronavirus | |||||||
| Peroxo titanium acid (70%) + peroxo-modified anatase | Hokkaido/9/03 | 150 µL | PBS | 25 °C | 0.2 mg/m2 sprayed to projector film | visible light (>410 nm) | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onodera, T.; Sugiura, K.; Haritani, M.; Suzuki, T.; Imamura, M.; Iwamaru, Y.; Ano, Y.; Nakayama, H.; Sakudo, A. Photocatalytic Inactivation of Viruses and Prions: Multilevel Approach with Other Disinfectants. Appl. Microbiol. 2022, 2, 701-715. https://doi.org/10.3390/applmicrobiol2040054
Onodera T, Sugiura K, Haritani M, Suzuki T, Imamura M, Iwamaru Y, Ano Y, Nakayama H, Sakudo A. Photocatalytic Inactivation of Viruses and Prions: Multilevel Approach with Other Disinfectants. Applied Microbiology. 2022; 2(4):701-715. https://doi.org/10.3390/applmicrobiol2040054
Chicago/Turabian StyleOnodera, Takashi, Katsuaki Sugiura, Makoto Haritani, Tohru Suzuki, Morikazu Imamura, Yoshifumi Iwamaru, Yasuhisa Ano, Hiroyuki Nakayama, and Akikazu Sakudo. 2022. "Photocatalytic Inactivation of Viruses and Prions: Multilevel Approach with Other Disinfectants" Applied Microbiology 2, no. 4: 701-715. https://doi.org/10.3390/applmicrobiol2040054
APA StyleOnodera, T., Sugiura, K., Haritani, M., Suzuki, T., Imamura, M., Iwamaru, Y., Ano, Y., Nakayama, H., & Sakudo, A. (2022). Photocatalytic Inactivation of Viruses and Prions: Multilevel Approach with Other Disinfectants. Applied Microbiology, 2(4), 701-715. https://doi.org/10.3390/applmicrobiol2040054









