Pterostilbene, a Natural Methoxylated Analog of Resveratrol, Exhibits Antifungal Activity Induced by Reactive Oxygen Species Production and Plasma Membrane Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Strain and Culture Conditions
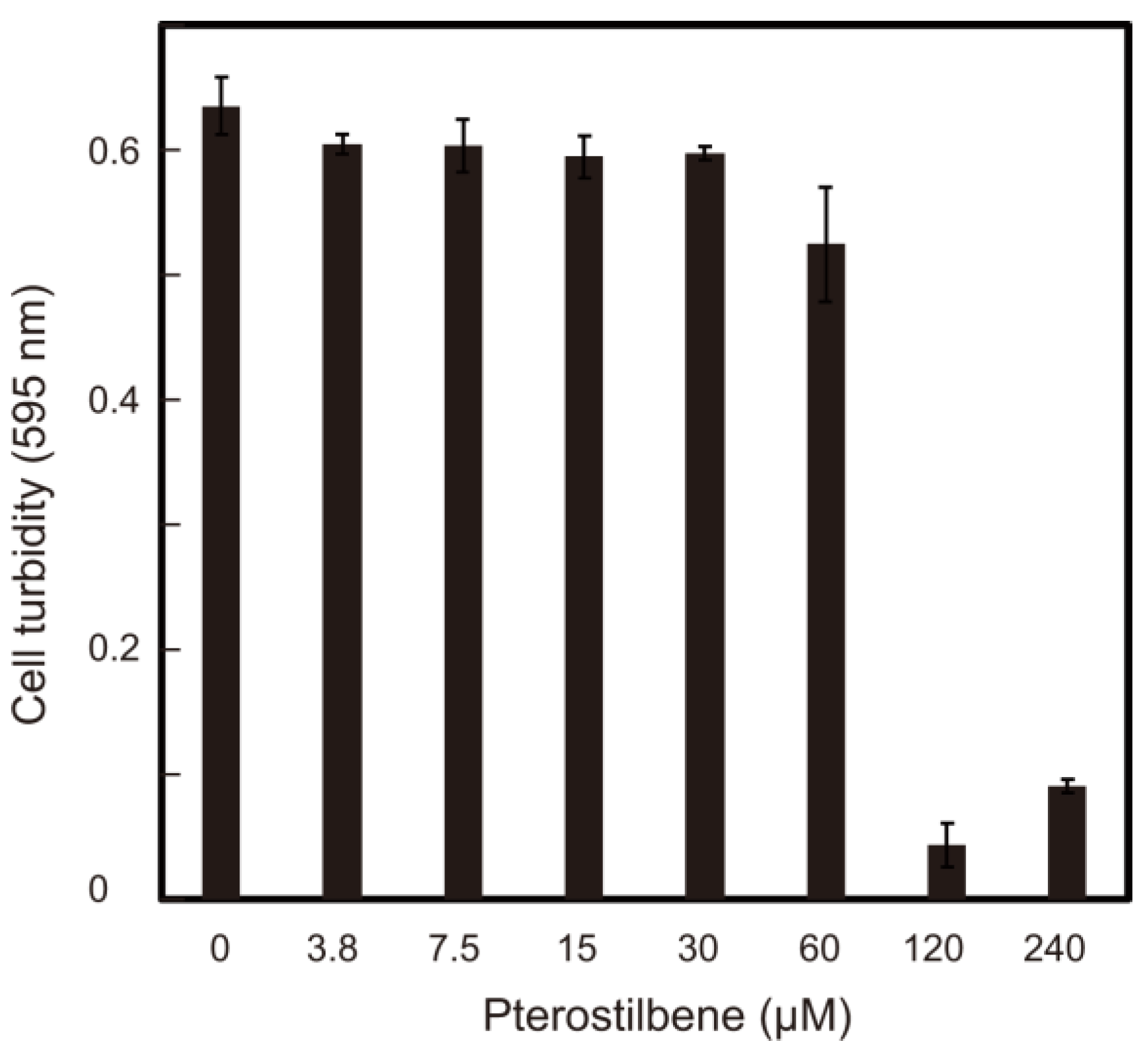
2.3. Measurement of Cell Growth and Viability
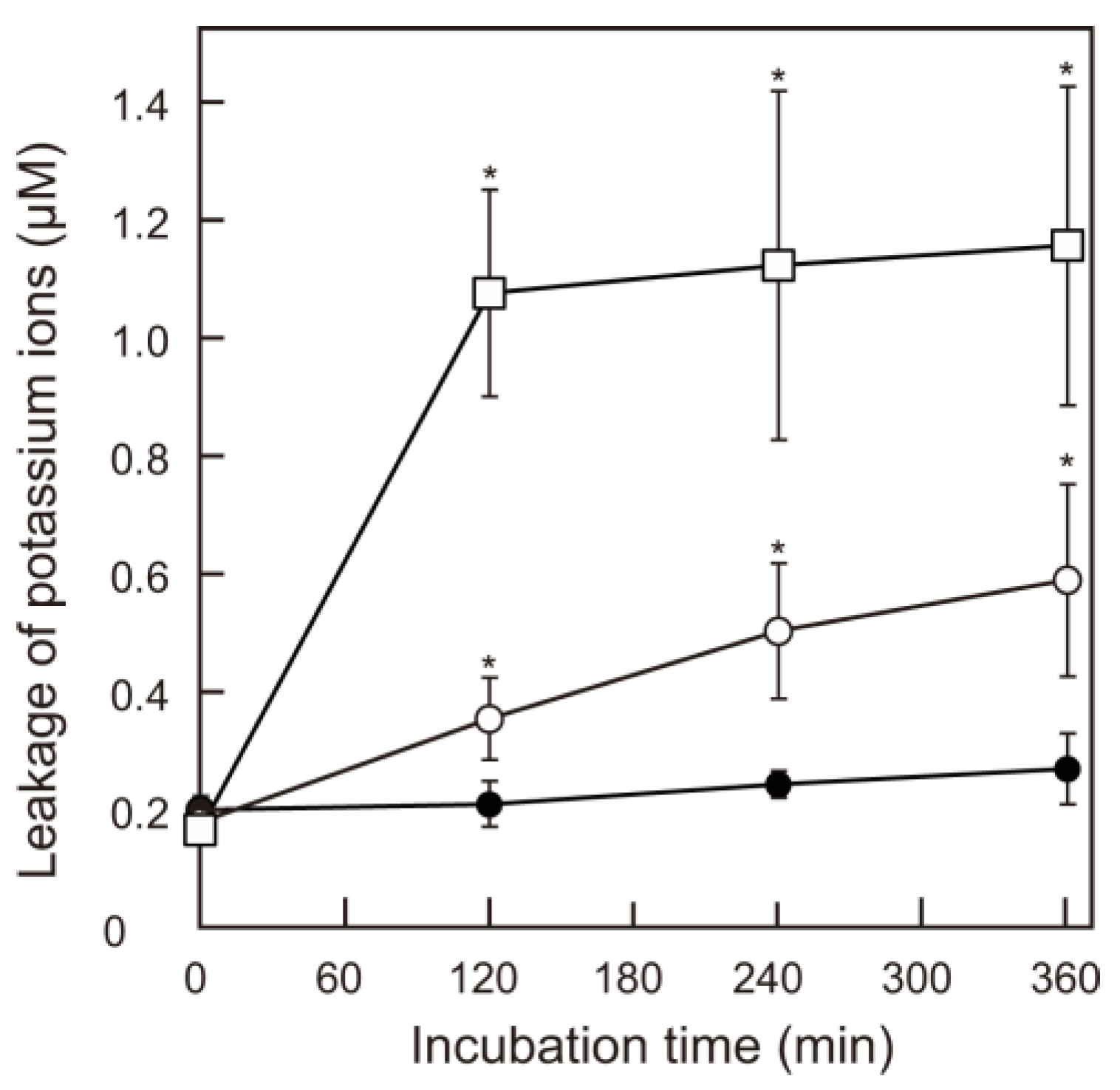
2.4. Leakage of K+ Ions
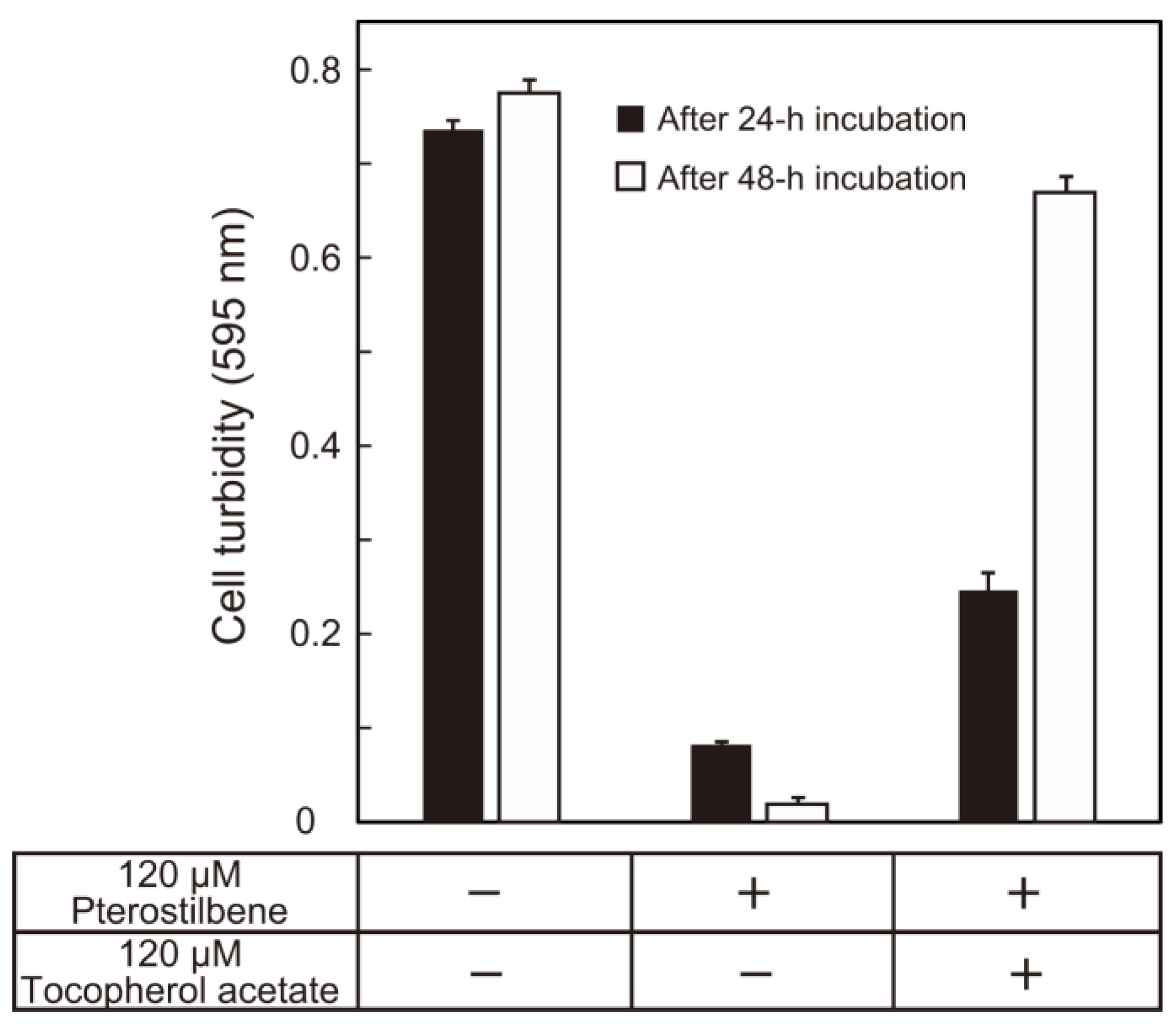
2.5. Determination of Cellular Reactive Oxygen Species (ROS) Levels
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loureiro, V.; Querol, A. The prevalence and control of spoilage yeasts in foods and beverages. Trends Food Sci. Technol. 1999, 10, 356–365. [Google Scholar] [CrossRef]
- Stratford, M.; Vallières, C.; Geoghegan, I.A.; Archer, D.B.; Avery, S.V. The preservative sorbic acid targets respiration, explaining the resistance of fermentative spoilage yeast species. mSphere 2020, 5, e00273-20. [Google Scholar] [CrossRef] [PubMed]
- Sundh, I.; Melin, P. Safety and regulation of yeasts used for biocontrol or biopreservation in the food or feed chain. Antonie Van Leeuwenhoek 2011, 99, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.; Verstrepen, K.; Derdelinckx, G. Brewing yeasts. In Yeasts in Food–Beneficial and Detrimental Aspects; Boekhout, T., Robert, V., Eds.; Behr’s Verlag: Hamburg, Germany, 2003; pp. 347–388. [Google Scholar]
- Timke, M.; Wang-Lieu, N.Q.; Altendorf, K.; Lipski, A. Identity, beer spoiling and biofilm forming potential of yeasts from beer bottling plant associated biofilms. Antonie Van Leeuwenhoek 2008, 93, 151–161. [Google Scholar] [CrossRef]
- Bonjean, B.; Guillaume, L.D. Yeasts in bread and baking products. In Yeasts in Food–Beneficial and Detrimental Aspects; Boekhout, T., Robert, V., Eds.; Behr’s Verlag: Hamburg, Germany, 2003; pp. 289–307. [Google Scholar]
- Lanciotti, R.; Sinigaglia, M.; Gardini, F.; Guerzoni, M.E. Hansenula anomala as spoilage agent of cream-filled cakes. Microbiol. Res. 1998, 153, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Ullah, A.; Orij, R.; Brul, S.; Smits, G.J. Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8377–8387. [Google Scholar] [CrossRef]
- Bulle, S.; Reddyvari, H.; Nallanchakravarthula, V.; Vaddi, D.R. Therapeutic potential of Pterocarpus santalinus L.: An update. Pharmacogn. Rev. 2016, 10, 43–49. [Google Scholar] [PubMed]
- Majeed, M.; Majeed, S.; Jain, R.; Mundkur, L.; Rajalakshmi, H.R.; Lad, P.S.; Neupane, P. An open-sabel single-arm, monocentric study assessing the efficacy and safety of natural pterostilbene (Pterocarpus marsupium) for skin brightening and antiaging effects. Clin. Cosmet. Investig. Dermatol. 2020, 13, 105–116. [Google Scholar] [CrossRef]
- Nagarajan, S.; Mohandas, S.; Ganesan, K.; Xu, B.; Ramkumar, K.M. New Insights into dietary pterostilbene: Sources, metabolism, and health promotion effects. Molecules 2022, 27, 6316. [Google Scholar] [CrossRef]
- Vaňková, E.; Paldrychová, M.; Kašparová, P.; Lokočová, K.; Kodeš, Z.; Maťátková, O.; Kolouchová, I.; Masák, J. Natural antioxidant pterostilbene as an effective antibiofilm agent, particularly for gram-positive cocci. World J. Microbiol. Biotechnol. 2020, 36, 101. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elmawla, M.A.; Abdelalim, E.; Ahmed, K.A.; Rizk, S.M. The neuroprotective effect of pterostilbene on oxaliplatin-induced peripheral neuropathy via its anti-inflammatory, anti-oxidative and anti-apoptotic effects: Comparative study with celecoxib. Life Sci. 2023, 315, 121364. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Akhtar, M.F.; Saleem, A.; Sharif, A.; Shah, S.; Khan, M.I.; Anwar, F.; Abbas, G.; Zubair, H.M.; Sohail, M.F. Pterostilbene improves CFA-induced arthritis and peripheral neuropathy through modulation of oxidative stress, inflammatory cytokines and neurotransmitters in Wistar rats. Inflammopharmacology 2022, 30, 2285–2300. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Pezzuto, J.M.; Farnsworth, N.R.; Santisuk, T.; Reutrakul, V. Revision of the NMR assignments of pterostilbene and of dihydrodehydrodiconieferyl alcohol: Cytotoxic constituents from Anogeissus acuminata. Nat. Prod.Lett. 1994, 4, 267–272. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Zhang, Y.; Huang, H.; Chen, H.; Chen, J.; Han, L.; Chen, X.; Chen, X.; Zhang, Y. Subacute oral toxicology and toxicokinetics of pterostilbene, a novel Top1/Tdp1 inhibiting anti-tumor reagent. Drug Chem. Toxicol. 2023, 46, 392–399. [Google Scholar] [CrossRef]
- Li, D.D.; Zhao, L.X.; Mylonakis, E.; Hu, G.H.; Zou, Y.; Huang, T.K.; Yan, L.; Wang, Y.; Jiang, Y.Y. In vitro and in vivo activities of pterostilbene against Candida albicans biofilms. Antimicrob. Agents Chemother. 2014, 58, 2344–2355. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Pont, V.; Pezet, R. Relation between the chemical structure and the biological activity of hydroxystilbenes against Botrytis cinerea. J. Phytopathol. 1990, 130, 1–8. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef]
- Yamada, N.; Murata, W.; Yamaguchi, Y.; Fujita, K.I.; Ogita, A.; Tanaka, T. Enhancing the fungicidal activity of amphotericin B via vacuole disruption by benzyl isothiocyanate, a cruciferous plant constituent. Lett. Appl. Microbiol. 2021, 72, 390–398. [Google Scholar] [CrossRef]
- Fujita, K.; Tatsumi, M.; Ogita, A.; Kubo, I.; Tanaka, T. Anethole induces apoptotic cell death accompanied by reactive oxygen species production and DNA fragmentation in Aspergillus fumigatus and Saccharomyces cerevisiae. FEBS J. 2014, 281, 1304–1313. [Google Scholar] [CrossRef]
- Tsukuda, Y.; Mizuhara, N.; Usuki, Y.; Yamaguchi, Y.; Ogita, A.; Tanaka, T.; Fujita, K. Structure-activity relationships of antifungal phenylpropanoid derivatives and their synergy with n-dodecanol and fluconazole. Lett. Appl. Microbiol. 2022, 74, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.S.; Ma, S.M.; Kamaya, H.; Ueda, I. Anesthesia cutoff phenomenon: Interfacial hydrogen bonding. Science 1990, 248, 583–585. [Google Scholar] [CrossRef]
- Fujita, K.; Kubo, I. Antifungal activity of octyl gallate. Int. J. Food Microbiol. 2002, 79, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubo, I. Plasma membrane injury induced by nonyl gallate in Saccharomyces cerevisiae. J. Appl. Microbiol. 2002, 92, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, T.; Kubo, A.; Fujita, K.I. Modes of antifungal action of alkanols against Saccharomyces cerevisiae. Bioorg. Med. Chem. 2003, 11, 1117–1122. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and other polyenes: Discovery, clinical use, mode of action and drug resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Fujita, K.I.; Fujita, T.; Kubo, I. Anethole, a potential antimicrobial synergist, converts a fungistatic dodecanol to a fungicidal agent. Phytother. Res. 2007, 21, 47–51. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Innokentev, A.; Kanki, T. Mitophagy in yeast: Molecular mechanism and regulation. Cells 2021, 10, 3569. [Google Scholar] [CrossRef]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilicstress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K.; Machida, K.; Nakamura, H.; Nakayama, K.; Fujita, K.; Tanaka, T.; Otani, S.; Taniguchi, M. Protective effect of antioxidants against para-nonylphenol-induced inhibition of cell growth in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2000, 185, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.G.; Tan, S.X.; Dawes, I.W. Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta. 2008, 1783, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Tani, K.; Usuki, Y.; Tanaka, T.; Taniguchi, M. Growth inhibition dependent on reactive oxygen species generated by C9-UK-2A, a derivative of the antifungal antibiotic UK-2A, in Saccharomyces cerevisiae. J. Antibiot. 2004, 57, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.F.; Li, L.; Zhu, X.M.; Liu, X.H.; Klionsky, D.J.; Lin, F.C. Current opinions on mitophagy in fungi. Autophagy. 2022, 19, 747–757. [Google Scholar] [CrossRef]
- Belenky, P.; Camacho, D.; Collins, J.J. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013, 3, 350–358. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Ricardo, E.; Guerreiro, S.G.; Costa-de-Oliveira, S.; Rodrigues, A.G.; Pina-Vaz, C. New insights regarding yeast survival following exposure to liposomal amphotericin B. Antimicrob. Agents Chemother. 2015, 59, 6181–6187. [Google Scholar] [CrossRef]
- Taniguchi, M.; Yano, Y.; Tada, E.; Ikenishi, K.; Oi, S.; Haraguchi, H.; Hashimoto, K.; Kubo, I. Mode of action of polygodial, an antifungal sesquiterpene dialdehyde. Appl. Biol. Chem. 1988, 52, 1409–1414. [Google Scholar]
- Machida, K.; Tanaka, T.; Taniguchi, M. Depletion of glutathione as a cause of the promotive effects of polygodial, a sesquiterpene on the production of reactive oxygen species in Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 88, 526–530. [Google Scholar] [CrossRef]
- Machida, K.; Tanaka, T.; Fujita, K.I.; Taniguchi, M. Farnesol-induced generation of reactive oxygen species via indirect inhibition of the mitochondrial electron transport chain in the yeast Saccharomyces cerevisiae. J. Bacteriol. 1998, 180, 4460–4465. [Google Scholar] [CrossRef] [PubMed]
- Kalamkar, S.D.; Bose, G.S.; Ghaskadbi, S.; Mittal, S. Andrographolide and pterostilbene inhibit adipocyte differentiation by downregulating PPARγ through different regulators. Nat. Prod. Res. 2022, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S. Anti-microbial activity of essential oils involving reactive oxygen species. Aroma Res. 2010, 11, 307–311. [Google Scholar]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuhara, N.; Inoue, M.; Kurotaki, H.; Matsumoto, K.; Ogita, A.; Fujita, K.-I. Pterostilbene, a Natural Methoxylated Analog of Resveratrol, Exhibits Antifungal Activity Induced by Reactive Oxygen Species Production and Plasma Membrane Injury. Appl. Microbiol. 2023, 3, 666-674. https://doi.org/10.3390/applmicrobiol3030045
Mizuhara N, Inoue M, Kurotaki H, Matsumoto K, Ogita A, Fujita K-I. Pterostilbene, a Natural Methoxylated Analog of Resveratrol, Exhibits Antifungal Activity Induced by Reactive Oxygen Species Production and Plasma Membrane Injury. Applied Microbiology. 2023; 3(3):666-674. https://doi.org/10.3390/applmicrobiol3030045
Chicago/Turabian StyleMizuhara, Naoko, Moe Inoue, Hideki Kurotaki, Kazuyori Matsumoto, Akira Ogita, and Ken-Ichi Fujita. 2023. "Pterostilbene, a Natural Methoxylated Analog of Resveratrol, Exhibits Antifungal Activity Induced by Reactive Oxygen Species Production and Plasma Membrane Injury" Applied Microbiology 3, no. 3: 666-674. https://doi.org/10.3390/applmicrobiol3030045
APA StyleMizuhara, N., Inoue, M., Kurotaki, H., Matsumoto, K., Ogita, A., & Fujita, K.-I. (2023). Pterostilbene, a Natural Methoxylated Analog of Resveratrol, Exhibits Antifungal Activity Induced by Reactive Oxygen Species Production and Plasma Membrane Injury. Applied Microbiology, 3(3), 666-674. https://doi.org/10.3390/applmicrobiol3030045





