Trichoderma: Population Structure and Genetic Diversity of Species with High Potential for Biocontrol and Biofertilizer Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Trichoderma in Soil and Endophytes
2.2. Harzianum Complex Clade Species
2.3. Phylogenetic Analysis
3. Results
3.1. Trichoderma Soil and Endophyte Survey Compilation
3.2. Population Structure and Genetic Diversity of T. atroviride
3.3. Population Structure and Genetic Diversity of the T. asperellum/asperelloides Species Group
3.4. Population Structure and Genetic Diversity of T. hamatum
3.5. Genetic Diversity of Harzianum Complex Clade Species
3.6. Population Structure and Genetic Diversity of T. virens
3.7. Nearest-Relative Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harman, G.E.; Obregón, M.A.; Samuel, G.J.; Lorito, M. Changing models for commercialization and implementation of biocontrol in the developing and the developed world. Plant Dis. 2010, 8, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Rush, T.A.; Shrestha, H.K.; Gopalakrishnan, M.M.; Spangler, M.K.; Ellis, J.C.; Labbé, J.L.; Abraham, P.E. Bioprospecting Trichoderma: A systematic roadmap to screen genomes and natural products for biocontrol applications. Front. Fungal Biol. 2021, 2, 716511. [Google Scholar] [CrossRef]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genet. Biol. 2009, 46, 615–631. [Google Scholar] [CrossRef]
- Samuels, G.J.; Ismaiel, A.; Bon, M.C.; De Respinis, S.; Petrini, O. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 2010, 102, 944–966. [Google Scholar] [CrossRef]
- Smith, A.; Beltrán, C.A.; Kusunoki, M.; Cotes, A.M.; Motohashi, K.; Kondo, T.; Deguchi, M. Diversity of soil-dwelling Trichoderma in Colombia and their potential as biocontrol agents against the phytopathogenic fungus Sclerotinia sclerotiorum (Lib.) de Bary. J. Gen. Plant Pathol. 2013, 79, 74–85. [Google Scholar] [CrossRef]
- Inglis, P.W.; Mello, S.C.M.; Martins, I.; Silva, J.B.T.; Macêdo, K.; Sifuentes, D.N.; Inglis, M.C.V. Trichoderma from Brazilian garlic and onion crop soils and description of two new species: Trichoderma azevedoi and Trichoderma peberdyi. PLoS ONE 2020, 15, e0228485. [Google Scholar] [CrossRef]
- Druzhinina, I.; Kopchinski, A.; Komoñ-Zelazowska, M.; Kubicek, C.P. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet. Biol. 2005, 42, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, L.; Popiel, D.; Chełkowski, J.; Koczyk, G.; Samuels, G.J.; Sobieralski, K.; Siwulski, M. Species diversity of Trichoderma in Poland. J. Appl. Genet. 2011, 52, 233–243. [Google Scholar] [CrossRef]
- Migheli, Q.; Balmas, V.; Komoñ-Zelazowska, M.; Scherm, B.; Fiori, S.; Kopchinskiy, A.G.; Kubicek, C.P.; Druzhinina, I.S. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environ. Microbiol. 2009, 11, 35–46. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Tomaselli, E.; Pollard-Flamand, J.; Boule, J.; Gerin, D.; Pollastro, S. Characterization of Trichoderma isolates from southern Italy, and their potential biocontrol activity against grapevine trunk disease fungi. Phytopathol. Mediterr. 2020, 59, 425–439. [Google Scholar] [CrossRef]
- Mulatu, A.; Megersa, N.; Abena, T.; Kanagarajan, S.; Liu, Q.; Tenkegna, T.A.; Vetukuri, R.R. Biodiversity of the genus Trichoderma in the rhizosphere of coffee (Coffea arabica) plants in Ethiopia and their potential use in biocontrol of coffee wilt Disease. Crops 2022, 2, 120–141. [Google Scholar] [CrossRef]
- Sadfi-Zouaoui, N.; Hannachi, I.; Rouaissi, M.; Hajlaoui, M.R.; Rubio, M.B.; Monte, E.; Boudabous, A.; Hermosa, M.R. Biodiversity of Trichoderma strains in Tunisia. Can. J. Microbiol. 2009, 55, 154–162. [Google Scholar] [CrossRef]
- Haouhach, S.; Karkachi, N.; Oguiba, B.; Sidaoui, A.; Chamorro, I.; Kihal, M.; Monte, E. Three new reports of Trichoderma in Algeria: T. atrobrunneum, (South) T. longibrachiatum (South), and T. afroharzianum (Northwest). Microorganisms 2020, 8, 1455. [Google Scholar] [CrossRef]
- Gherbawy, Y.; Druzhinina, I.; Shaban, G.M.; Wuczkowsky, M.; Yasar, M.; El-Naghy, M.A.; Prillinger, H.J.; Kubicek, C.P. Trichoderma populations from alkaline agricultural soil in the Nile valley, Egypt, consist of only two species. Mycol. Prog. 2004, 3, 211–218. [Google Scholar] [CrossRef]
- Kullnig, C.; Szakacs, G.; Kubicek, C.P. Molecular identification of Trichoderma species from Russia, Siberia and the Himalaya. Mycol. Res. 2000, 104, 1117–1125. [Google Scholar] [CrossRef]
- Mirkhani, F.; Alaei, H. Species diversity of indigenous Trichoderma from alkaline pistachio soils in Iran. Mycol. Iran 2015, 2, 22–37. [Google Scholar] [CrossRef]
- Khadka, R.B.; Miller, S.A. Synergy of anaerobic soil disinfestation and Trichoderma spp. in Rhizoctonia root rot suppression. Front. Sustain. Food Syst. 2021, 5, 76. [Google Scholar] [CrossRef]
- Rahman, S.S.M.S.A.; Zainudin, N.A.I.M.; Aziz, N.A.A. Evaluation of Trichoderma asperellum B1902 in controlling Fusarium wilt of Cavendish banana cultivar. Sains Malays. 2021, 50, 2549–2561. [Google Scholar] [CrossRef]
- Jang, S.; Jang, Y.; Kim, C.W.; Lee, H.; Hong, J.H.; Heo, Y.M.; Lee, Y.M.; Lee, D.W.; Lee, H.B.; Kim, J.J. Five new records of soil-derived Trichoderma in Korea: T. albolutescens, T. asperelloides, T. orientale, T. spirale, and T. tomentosum. Mycobiology 2017, 45, 1–8. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.; Wang, J.L.; Chen, J.; Mao, L.J.; Feng, X.X.; Zhang, C.L.; Lin, F.C. Trichoderma biodiversity of agricultural fields in east China reveals a gradient distribution of species. PLoS ONE 2016, 11, e0160613. [Google Scholar] [CrossRef]
- Ma, J.; Tsegaye, E.; Li, M.; Wu, B.; Jiang, X. Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 2020, 10, 362. [Google Scholar] [CrossRef]
- Zhang, C.L.; Druzhinina, I.S.; Kubicek, C.P.; Xu, T. Trichoderma biodiversity in China: Evidence for a North to South distribution of species in East Asia. FEMS Microbiol. Lett. 2005, 251, 251–257. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Yu, C.; Dou, K.; Wang, M.; Li, Y.; Chen, J. Biodiversity of Trichoderma community in the tidal flats and wetland of southeastern China. PLoS ONE 2016, 11, e0168020. [Google Scholar] [CrossRef]
- Xue, M.; Wang, R.; Zhang, C.; Wang, W.; Zhang, F.; Chen, D.; Ren, S.; Manman, Z.; Hou, J.; Liu, T. Screening and identification of Trichoderma strains isolated from natural habitats in China with potential agricultural applications. Biomed. Res. Int. 2021, 2021, 7913950. [Google Scholar] [CrossRef]
- Tang, G.T.; Li, Y.; Zhou, Y.; Zhou, Y.; Zhu, Y.H.; Zheng, X.J.; Chang, X.L.; Zhang, S.R.; Gong, G.S. Diversity of Trichoderma species associated with soil in the Zoige alpine wetland of Southwest China. Sci. Rep. 2022, 12, 21709. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Bissett, J.; Druzhinina, I.; Kullnig-Gradinger, C.; Szakacs, G. Genetic and metabolic diversity of Trichoderma: A case study on South-East Asian isolates. Fungal Genet. Biol. 2003, 38, 310–319. [Google Scholar] [CrossRef]
- Pollard-Flamand, J.; Boulé, J.; Hart, M.; Úrbez-Torres, J.R. Biocontrol activity of Trichoderma species isolated from grapevines in British Columbia against Botryosphaeria dieback fungal pathogens. J. Fungi. 2022, 8, 409. [Google Scholar] [CrossRef]
- Nascimento Brito, V.; Lana Alves, J.; Sírio Araújo, K.; de Souza Leite, T.; Borges de Queiroz, C.; Liparini Pereira, O.; de Queiroz, M.V. Endophytic Trichoderma species from rubber trees native to the Brazilian Amazon, including four new species. Front. Microbiol. 2023, 14, 1095199. [Google Scholar] [CrossRef]
- Morais, E.M.; Silva, A.A.R.; Sousa, F.W.A.; Azevedo, I.M.B.; Silva, H.F. Endophytic Trichoderma strains isolated from forest species of the Cerrado-Caatingaecotone are potential biocontrol agents against crop pathogenic fungi. PLoS ONE 2022, 17, e0265824. [Google Scholar] [CrossRef]
- Gazis, R.; Chaverri, P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010, 3, 240–254. [Google Scholar] [CrossRef]
- Rees, H.J.; Bashir, N.; Drakulic, J.; Cromey, M.G.; Bailey, A.M.; Foster, G.D. Identification of native endophytic Trichoderma spp. for investigation of in vitro antagonism towards Armillaria mellea using synthetic- and plant-based substrates. J. Appl. Microbiol. 2021, 131, 392–403. [Google Scholar] [CrossRef]
- Kovács, C.; Csótó, A.; Pál, K.; Nagy, A.; Fekete, E.; Karaffa, L.; Kubicek, C.P.; Sándor, E. The biocontrol potential of endophytic Trichoderma fungi isolated from Hungarian grapevines. Part I. Isolation, identification and in vitro studies. Pathogens 2021, 10, 1612. [Google Scholar] [CrossRef]
- Rodriguez, M.C.H.; Evans, H.C.; de Abreu, L.M.; Macedo, D.M.; Ndacnou, M.K.; Bekele, B.K.; Barreto, R.W. New species and records of Trichoderma isolated as mycoparasites and endophytes from cultivated and wild coffee in Africa. Sci. Rep. 2021, 11, 5671. [Google Scholar] [CrossRef]
- Mulaw, T.B.; Druzhinina, I.S.; Kubicek, C.P.; Atanasova, L. Novel endophytic Trichoderma spp. isolated from healthy Coffea arabica roots are capable of controlling coffee tracheomycosis. Diversity 2013, 5, 750–766. [Google Scholar] [CrossRef]
- Cummings, N.J.; Ambrose, A.; Braithwaite, M.; Bissett, J.; Roslan, H.A.; Abdullah, J.; Stewart, A.; Agbayani, F.V.; Steyaert, J.; Hill, R.A. Diversity of root-endophytic Trichoderma from Malaysian Borneo. Mycol. Prog. 2016, 15, 50. [Google Scholar] [CrossRef]
- Rosmana, A.; Samuels, G.J.; Ismaiel, A.; Ibrahim, E.S.; Chaverri, P.; Herawati, Y.; Asman, A. Trichoderma asperellum: A dominant endophyte species in cacao grown in Sulawesi with potential for controlling vascular streak dieback disease. Trop. Plant Pathol. 2015, 40, 19–25. [Google Scholar] [CrossRef]
- Sirikamonsathien, T.; Kenji, M.; Dethop, T. Potential of endophytic Trichoderma in controlling Phytophthora leaf fall disease in rubber (Hevea brasiliensis). Biol. Control 2023, 179, 105175. [Google Scholar] [CrossRef]
- Leylaie, S.; Zafari, D. Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Front. Microbiol. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Hosseyni-Moghaddam, M.S.; Soltani, J. Bioactivity of endophytic Trichoderma fungal species from the plant family Cupressaceae. Ann. Microbiol. 2014, 64, 753–761. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.R.; Tan, P.; Jiang, Y.L.; Hyde, K.D.; Mckenzie, E.H.C.; Bahkali, A.H.; Kang, J.C.; Wang, Y. A novel Trichoderma species isolated from soil in Guizhou, T. guizhouense. Mycol. Prog. 2013, 12, 167–172. [Google Scholar] [CrossRef]
- Rifai, M.A. A revision of the genus Trichoderma. Mycol. Pap. 1969, 116, 1–56. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Qiao, M.; Lv, Y.; Du, X.; Zhang, K.Q.; Yu, Z. New Species of Trichoderma isolated as endophytes and saprobes from southwest China. J. Fungi 2021, 64, 67. [Google Scholar] [CrossRef] [PubMed]
- Samuels, G.J.; Ismaiel, A. Trichoderma evansii and T. lieckfeldtiae: Two new T. hamatum-like species. Mycologia 2009, 1, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Samuels, G.J.; Hebbar, P.K. Trichoderma: Identification and Agricultural Application, 1st ed.; The American Phytopathological Society: St. Paul, MI, USA, 2015; p. 204. [Google Scholar]
- Bissett, J. A revision of the genus Trichoderma. I. Sect. Longibrachiatum sect. nov. Can. J. Bot. 1984, 62, 924–931. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. II. Infrageneric classification. Can. J. Bot. 1991, 69, 2357–2372. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. III. Sect. Pachybasium. Can. J. Bot. 1991, 69, 2373–2417. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. IV. Additional notes on section Longibrachiatum. Can. J. Bot. 1991, 69, 2418–2420. [Google Scholar] [CrossRef]
- Kindermann, J.; El-Ayouti, Y.; Samuels, G.J.; Kubicek, C.P. Phylogeny of the genus Trichoderma based on sequence analysis of the internal transcribed spacer region 1 of the rDNA clade. Fungal Genet. Biol. 1998, 24, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.M.; DiLeo, M.V. From the lab to the farm: An industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 2016, 7, 1100. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Agric. Sci. 2020, 65, 68–178. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harmen, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Skoneczny, D.; Oskiera, M.; Szczech, M.; Bartoszewski, G. Genetic diversity of Trichoderma atroviride strains collected in Poland and identification of loci useful in detection of within-species diversity. Folia Microbiol. 2015, 60, 297–307. [Google Scholar] [CrossRef]
- Dodd, S.L.; Lieckfeldt, E.; Samuels, G.J. Hypocrea atroviridis sp. Nov., the teleomorph of Trichoderma atroviride. Mycologia 2003, 95, 27–40. [Google Scholar] [CrossRef]
- Schalamun, M.; Schmoll, M. Trichoderma – genomes and genomics as treasure troves for research towards biology, biotechnology and agriculture. Front. Fungal Biol. 2022, 3, 1002161. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of versatile biocontrol agent, its secrets and insights into mechanism of biocontrol potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Scherm, B.; Schmoll, M.; Balmas, V.; Kubicek, C.P.; Migheli, Q. Identification of potential marker genes for Trichoderma harzianum strains with high antagonistic potential against Rhizoctonia solani by a rapid subtraction hybridization approach. Curr. Genet. 2009, 55, 81–91. [Google Scholar] [CrossRef]
- Nagy, V.; Seidl, V.; Szakacs, G.; Komon-Zelazowska, M.; Kubicek, C.P.; Druzhinina, I.S. Application of DNA bar codes for screening of industrially important fungi: The haplotype of Trichoderma harzianum sensu stricto indicates superior chitinase formation. Appl. Environ. Microbiol. 2007, 73, 7048–7058. [Google Scholar] [CrossRef]
- Marco, S.; Larendana, M.; Riccardo, V.; Raffaella, B.; Walter, C.; Luca, N. Microbe-assisted crop improvement: A sustainable weapon to restore holobiont functionality and resilience. Hort. Res. 2022, 9, uhac160. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H.; Gashaw, T. Biocontrol potential of Trichoderma and yeast against post harvest fruit fungal diseases: A review. World News Nat. Sci. 2019, 27, 153–173. [Google Scholar]
- González-Estrada, R.; Blancas-Benítez, F.; Montaño-Leyva, B.; Moreno-Hernández, C.; Romero-Islas, L.D.C.; Romero-Islas, J.; Avila-Pena, R.; Ramos-Guerrero, A.; Fonseca-Cantabrana, A.; Gutierrez-Martinez, P. A review study on the postharvest decay control of fruit by Trichoderma. Trichoderma—The Most Widely Used Fungicide. IntechOpen 2019. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma volatile organic compounds as a biofumigation tool against late blight pathogen Phytophthora infestans in postharvest potato tubers. J. Agric. Food Chem. 2020, 68, 8163–8171. [Google Scholar] [CrossRef]
- Intana, W.; Kheawleng, S.; Sunpapao, A. Trichoderma asperellum T76-14 released volatile organic compounds against postharvest fruit rot in muskmelons (Cucumis melo) caused by Fusarium incarnatum. J. Fungi 2021, 7, 46. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Li, Y.; Yu, C.; Wang, Q.Q.; Wang, M.; Sun, J.; Gao, J.X.; Chen, J. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium stalk rot. Sci. Rep. 2017, 7, 1771. [Google Scholar] [CrossRef]
- Xie, P.; Yang, S.; Liu, X.; Zhang, T.; Zhao, X.; Wen, T.; Zhang, J.; Xue, C.; Shen, Q.; Yuan, J. Learning from seed microbes: Trichoderma coating intervenes in rhizosphere microbiome assembly. Microbiol. Spectr. 2023, 11, e0309722. [Google Scholar] [CrossRef]
- Gao, P.; Qi, K.; Han, Y.; Ma, L.; Zhang, B.; Zhang, Y.; Guan, X.; Qi, J. Effect of Trichoderma viride on rhizosphere microbial communities and biocontrol of soybean root rot. Front Microbiol. 2023, 14, 1204688. [Google Scholar] [CrossRef]
- Di Lelio, I.; Forni, G.; Magoga, G.; Brunetti, M.; Bruno, D.; Becchimanzi, A.; De Luca, M.G.; Sinno, M.; Barra, E.; Bonelli, M.; et al. A soil fungus confers plant resistance against a phytophagous insect by disrupting the symbiotic role of its gut microbiota. Proc. Natl. Acad. Sci. USA 2023, 120, e2216922120. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tan, Y.; Yan, Z.; Liao, Y.; Chen, J.; De Boevre, M.; De Saeger, S.; Wu, A. Antagonistic and detoxification potentials of Trichoderma isolates for control of zearalenone (ZEN) producing Fusarium graminearum. Front. Microbiol. 2018, 8, 2017. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Song, Y.; Dianzhen, Y.; Na, L.; Yan, T.; Yingying, F.; Cheng, W.; Aibo, W. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control 2019, 106, 106748. [Google Scholar] [CrossRef]
- Dini, I.; Alborino, V.; Lanzuise, S.; Lombardi, N.; Marra, R.; Balestrieri, A.; Ritieni, A.; Woo, S.L.; Vinale, F. Trichoderma enzymes for degradation of aflatoxin B1 and ochratoxin A. Molecules 2022, 27, 3959. [Google Scholar] [CrossRef] [PubMed]
- Modrzewska, M.; Błaszczyk, L.; Stępień, Ł.; Urbaniak, M.; Waśkiewicz, A.; Yoshinari, T.; Bryła, M. Trichoderma versus Fusarium—Inhibition of pathogen growth and mycotoxin biosynthesis. Molecules 2022, 27, 8146. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Li, P.; Zhao, S.; Altomare, C. Potential of Trichoderma spp. for biocontrol of aflatoxin-producing Aspergillus flavus. Toxins 2022, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Yazid, S.N.E.; Tajudin, N.I.; Razman, N.A.A.; Selamat, J.; Ismail, S.I.; Sanny, M.; Samsudin, N.I.P. Mycotoxigenic fungal growth inhibition and multi-mycotoxin reduction of potential biological control agents indigenous to grain maize. Mycotoxin Res. 2023, 39, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Kubheka, B.P.; Ziena, L.W. Trichoderma: A biofertilizer and a bio-fungicide for sustainable crop production. IntechOpen. 2022. [Google Scholar] [CrossRef]
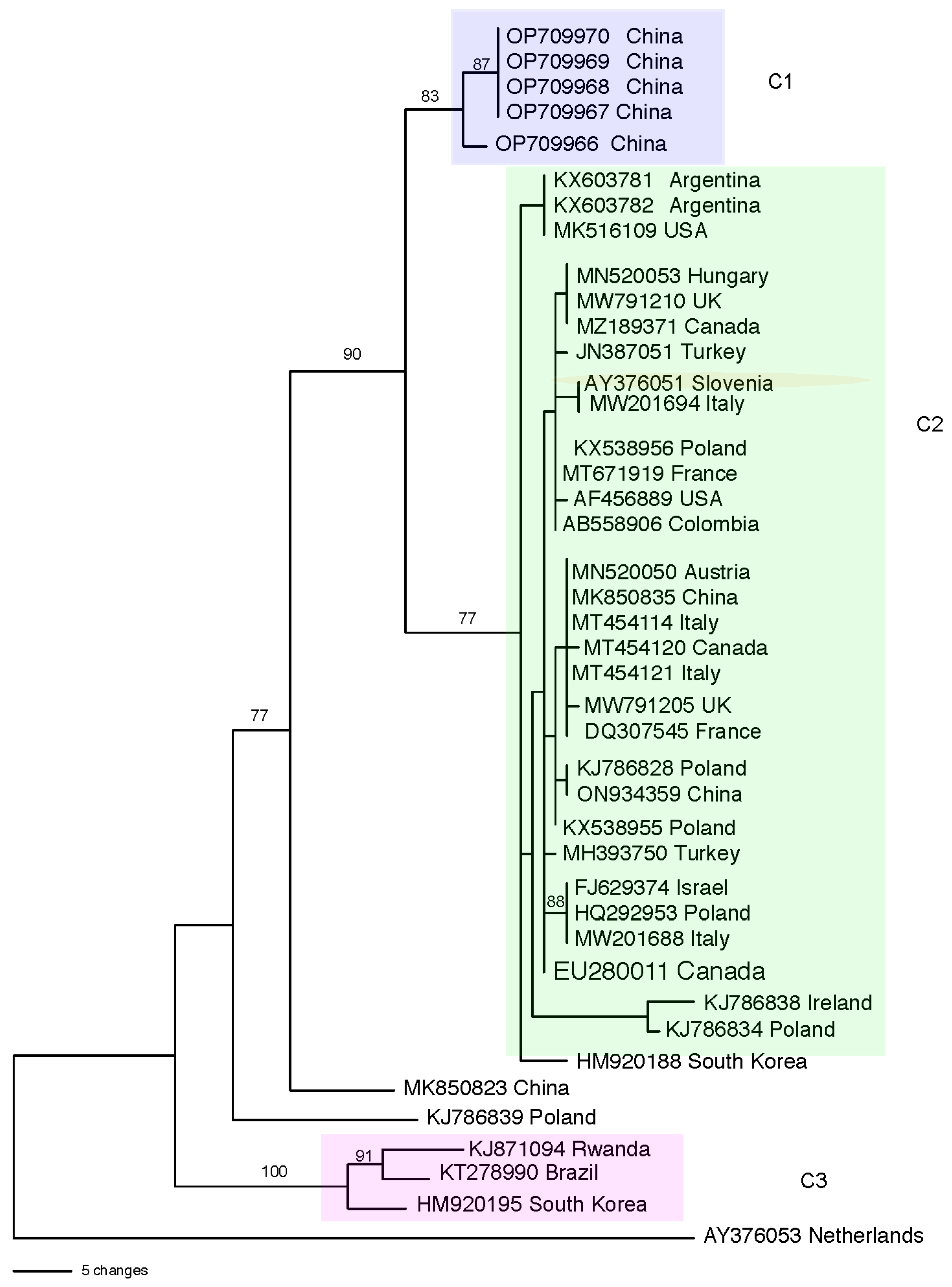
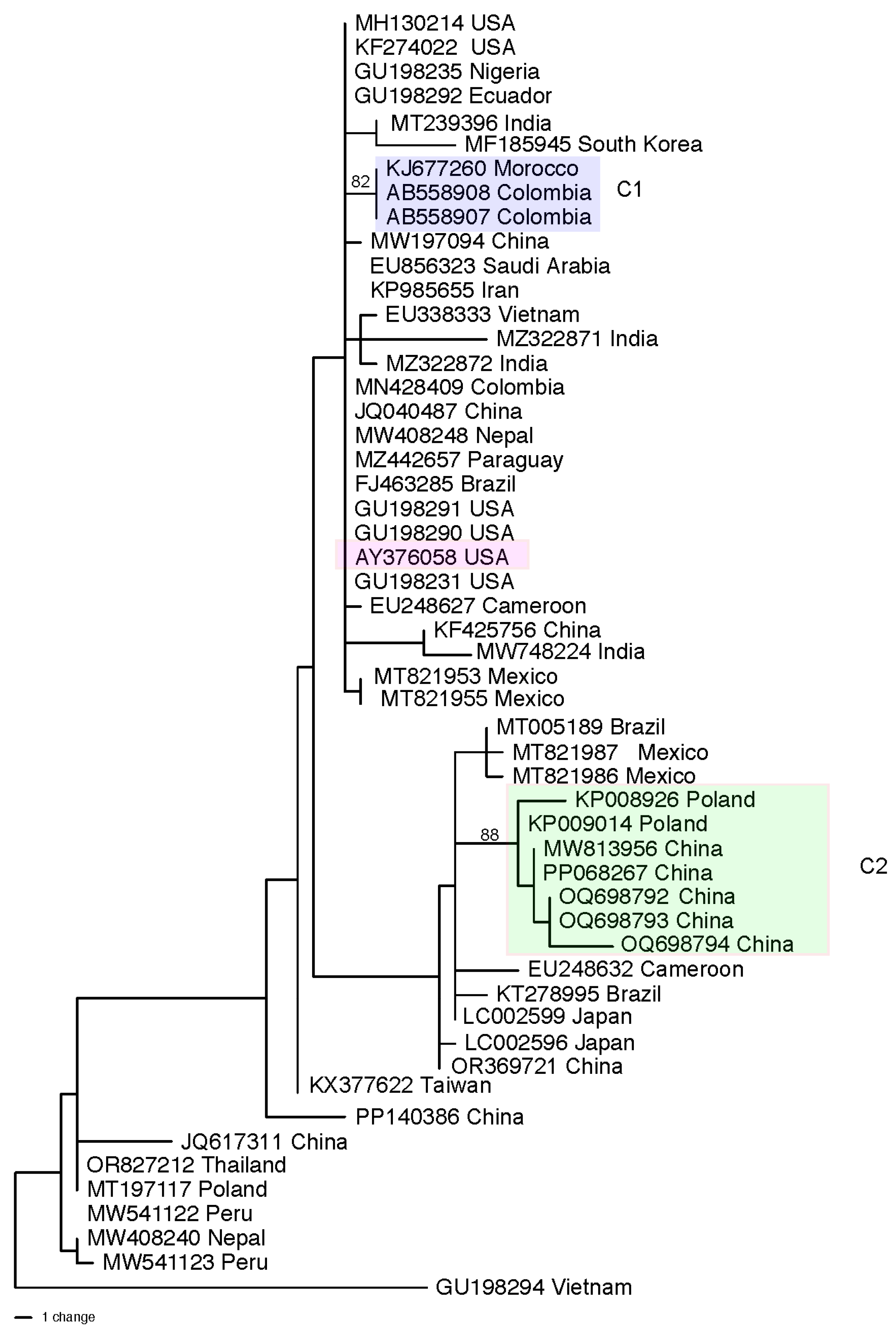
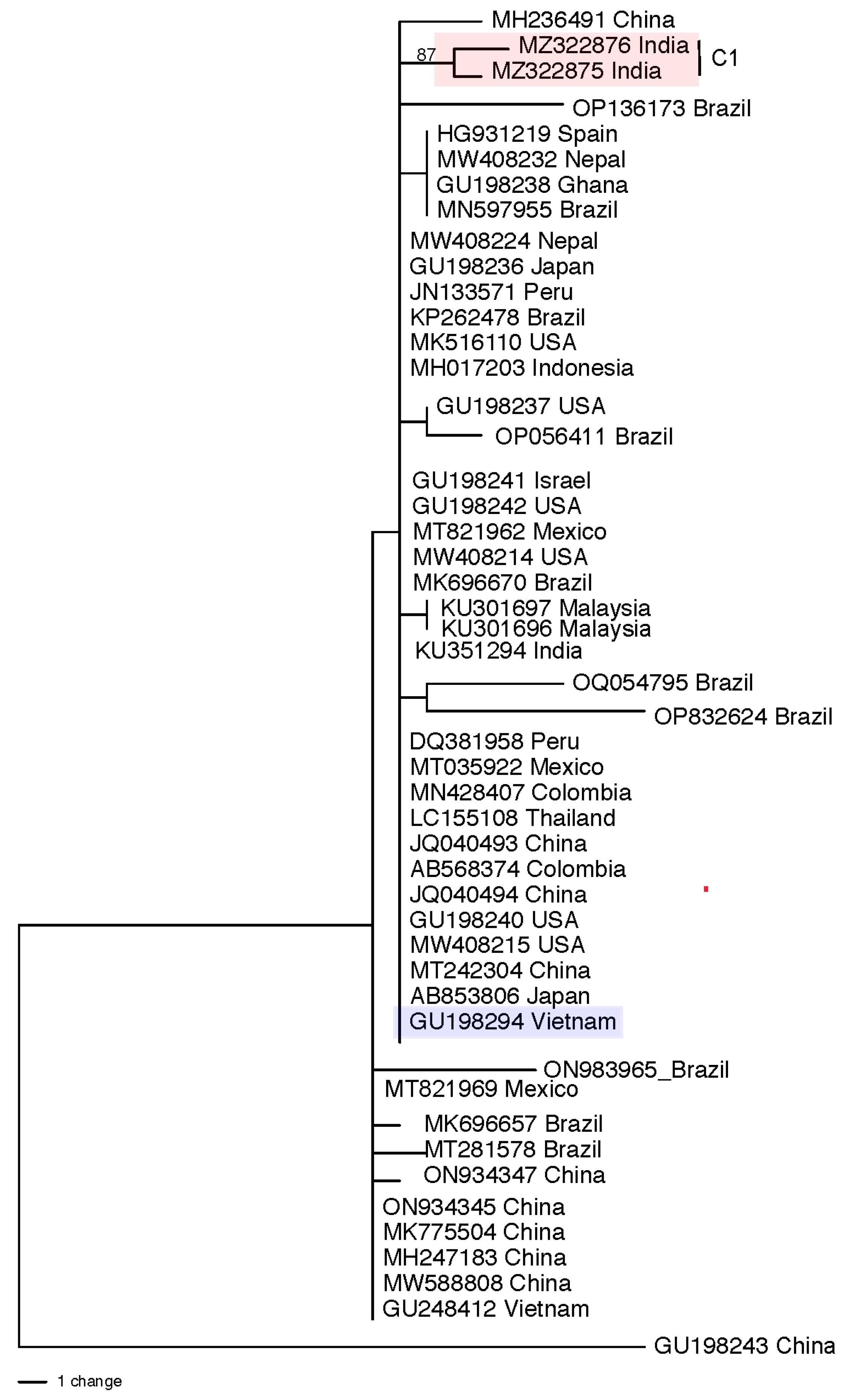


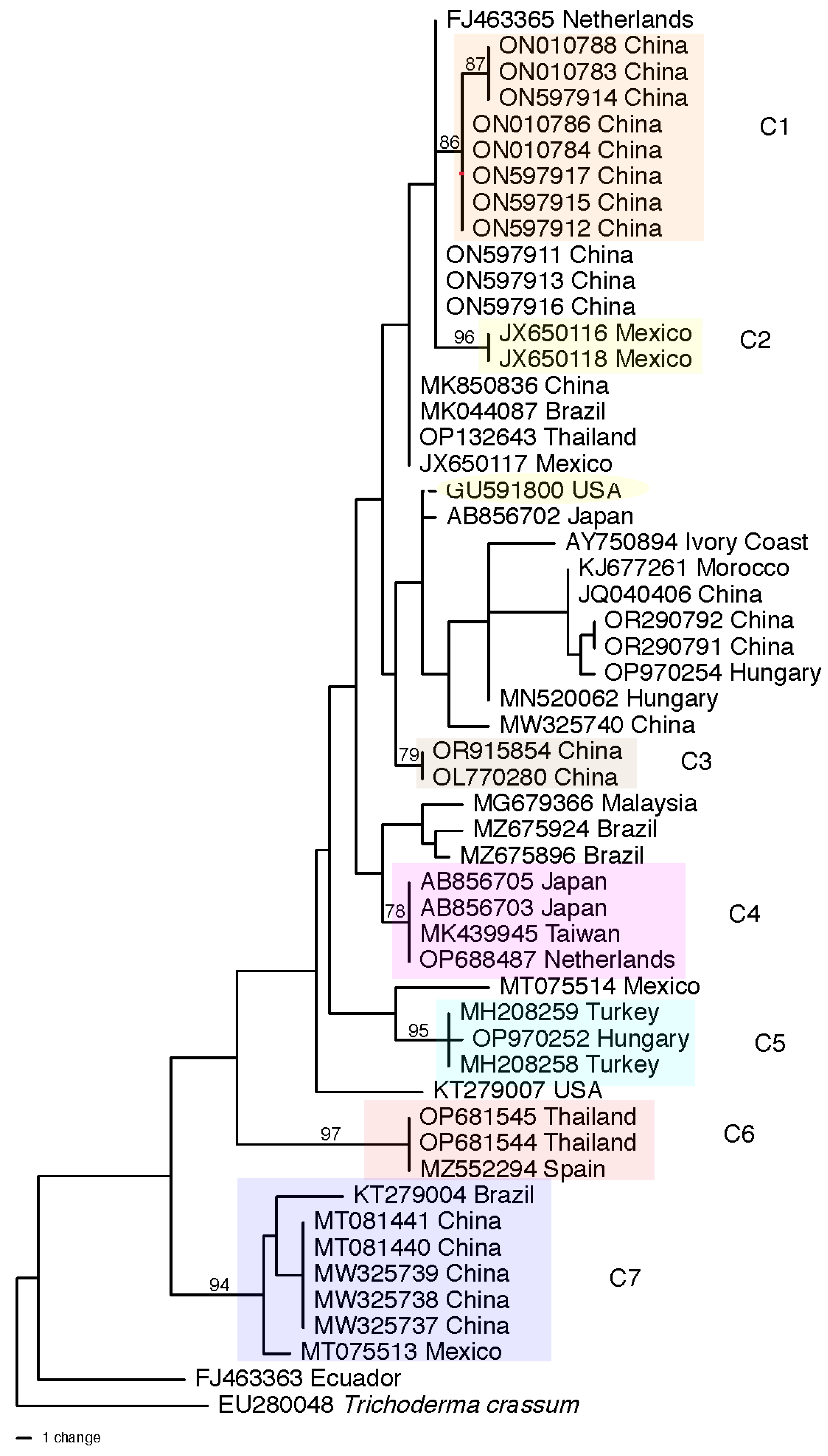

| Region or Country | Total Isolates Identified b | asperellum/asperelloides c | harzianum d | virens | atroviride | hamatum | Total (% of All) e Isolates | Reference f |
|---|---|---|---|---|---|---|---|---|
| Central and South America | ||||||||
| South America | 183 | 60/0 | 49 | 8 | 3 | 2 | 122 (67%) | [5] |
| Colombia | 21 | 10/0 | 3 | 0 | 4 | 0 | 17 (81%) | [7] |
| Brazil | 54 | 2/13 | 23 | 0 | 0 | 2 | 40 (74%) | [8] |
| Central and South America | 54 | 4/0 | 20 | 2 | 4 | 0 | 30 (56%) | [9] |
| Europe | ||||||||
| Poland | 170 | 0 | 43 | 6 | 20 | 9 | 78 (46%) | [10] |
| Island of Sardinia | 482 | 3/0 | 277 | 19 | 0 | 22 | 321 (67%) | [11] |
| Southern Italy | 16 | 0 | 6 | 0 | 4 | 0 | 10 (63%) | [12] |
| Africa | ||||||||
| Ethiopia | 164 | 64/32 | 8 | 0 | 0 | 6 | 110 (67%) | [13] |
| Tunisia | 53 | 0 | 15 | 0 | 7 | 14 | 36 (68%) | [14] |
| Algeria | 9 | 0 | 4 | 0 | 0 | 0 | 4 (44%) | [15] |
| Egypt | 20 | 0 | 14 | 0 | 0 | 0 | 14 (70%) | [16] |
| Asia | ||||||||
| Russia, Siberia, Himalaya | 75 | 2/0 | 31 | 5 | 14 | 15 | 67 (89%) | [17] |
| Iran | 159 | 0 | 87 | 19 | 0 | 0 | 106 (67%) | [18] |
| Nepal | 41 | 18/19 | 4 | 0 | 0 | 0 | 41 (100%) | [19] |
| Malaysia | 326 | 86/0 | 156 | 9 | 0 | 20 | 271 (83%) | [20] |
| South Korea | 26 | 2/4 | 3 | 6 | 1 | 1 | 17 (65%) | [21] |
| China (East agri. fields) | 2078 | 425/0 | 429 | 340 | 73 | 397 | 1664 (80%) | [22] |
| China (northwest) | 312 | 20/0 | 108 | 0 | 3 | 1 | 132 (42%) | [23] |
| China (four regions) | 64 | 4/0 | 26 | 2 | 13 | 0 | 45 (70%) | [24] |
| China (southeast sediments) | 254 | 32/0 | 63 | 1 | 134 | 1 | 231 (91%) | [25] |
| China | 13 | 12/0 | 1 | 0 | 0 | 0 | 13 (100%) | [26] |
| China (southwest) | 57 | 0 | 49 | 0 | 0 | 0 | 49 (86%) | [27] |
| Southeast Asia | 78 | 4/0 | 37 | 16 | 3 | 1 | 61 (78%) | [28] |
| Total g | 4709 | 816 | 1456 | 417 | 283 | 491 | 3479 (74%) | |
| detection frequency among studies h | N/A i | 70%/17% | 100% | 52% | 57% | 57% | N/A |
| Country | Host | Total Isolates b | asp/aspo c | Harzianum Complex d | virens | atroviride | hamatum | Total and (%) | Reference e |
|---|---|---|---|---|---|---|---|---|---|
| North America | |||||||||
| Canada | Grapevines | 29 | 0/4 | 8 | 0 | 7 | 0 | 19 (66%) | [29] |
| South America | |||||||||
| Brazil | Rubber trees | 30 | 0 | 0 | 0 | 0 | 0 | 0 | [30] |
| Brazil | Cerrado-Caatinga ecotone | 19 | 0 | 0 | 0 | 0 | 0 | 0 | [31] |
| Peru | Wild rubber tree | 39 | 0 | 31 | 0 | 0 | 0 | 31 (79%) | [32] |
| Europe | |||||||||
| United Kingdom | Various garden trees | 40 | 0 | 15 | 1 | 0 | 4 | 20 (50%) | [33] |
| Hungary | Grapevines | 10 | 0 | 8 | 0 | 0 | 0 | 8 (80%) | [34] |
| Africa | |||||||||
| Ethiopia, Cameroon, Kenya | Coffee (cultivated and wild) | 76 | 0 | 46 | 1 | 1 | 3 | 51 (67%) | [35] |
| Ethiopia | Coffee | 48 | 0 | 14 | 0 | 0 | 20 | 14 (48%) | [36] |
| Asia | |||||||||
| Malaysia | 35 plant families | 93 | 13/22 | 27 | 22 | 0 | 0 | 84 (90%) | [37] |
| Indonesia | Theobroma cacao | 21 | 19 | 0 | 2 | 0 | 0 | 21 (100%) | [38] |
| Thailand | Rubber trees | 12 | 3/0 | 3 | 2 | 0 | 3 | 11 (92%) | [39] |
| Iran | Vinca sp. | 7 | 1/0 | 0 | 0 | 0 | 0 | 1 (14%) | [40] |
| Iran | Cuppressaceae family plants | 5 | 0 | 0 | 0 | 4 | 0 | 4 (80%) | [41] |
| Total g | N/A i | 429 | 62 | 152 | 28 | 12 | 27 | 281 (66%) | |
| Detection frequency among studies h | N/A | N/A | 38% | 61% | 38% | 23% | 30% |
| Species b | GenBank Hits c | Habitat | Geographic Region | Reference d |
|---|---|---|---|---|
| T. lentiforme | 481 | Endophytes; few from soil | South America | [42] |
| T. inhamatum | 117 | soil | South America | MycoBank# 104673 |
| T. guizhouense | 204 | Commonly in soil; endophytes in Africa | Worldwide | [43] |
| T. afroharzianum | 644 | Commonly in soil; few endophytic | Worldwide | [42] |
| T. pyramidale | 26 | Soil and decaying wood | Europe | [42] |
| T. atrobrunneum | 234 | commonly in soil | Europe | [42] |
| T. simmonsii | 176 | Commonly in soil or decaying wood | North America, Europe | [42] |
| T. harzianum | N/A e | Soil, endophytic | North America, Europe | [29,44] |
| T. camerunense | 38 | Commonly in soil | Africa | [42] |
| T. botryosum | 44 | Endophytic in coffee | Africa | [35] |
| T. pseudopyramidale | 82 | Endophytic in coffee and mycoparasite | Africa | [35] |
| T. afarasin | 27 | Mostly endophytic | Africa | [42] |
| T. neotropicale | 37 | Endophytes of tropical trees | South America | [42] |
| T. endophyticum | 72 | Endophytes of tropical trees | South America | [42] |
| T. rifaii | 40 | Endophytes of tropical trees | South America | [42] |
| T. lixii | 113 | Soil or decaying wood | Southeast Asia | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismaiel, A.; Lakshman, D.K.; Jambhulkar, P.P.; Roberts, D.P. Trichoderma: Population Structure and Genetic Diversity of Species with High Potential for Biocontrol and Biofertilizer Applications. Appl. Microbiol. 2024, 4, 875-893. https://doi.org/10.3390/applmicrobiol4020060
Ismaiel A, Lakshman DK, Jambhulkar PP, Roberts DP. Trichoderma: Population Structure and Genetic Diversity of Species with High Potential for Biocontrol and Biofertilizer Applications. Applied Microbiology. 2024; 4(2):875-893. https://doi.org/10.3390/applmicrobiol4020060
Chicago/Turabian StyleIsmaiel, Adnan, Dilip K. Lakshman, Prashant P. Jambhulkar, and Daniel P. Roberts. 2024. "Trichoderma: Population Structure and Genetic Diversity of Species with High Potential for Biocontrol and Biofertilizer Applications" Applied Microbiology 4, no. 2: 875-893. https://doi.org/10.3390/applmicrobiol4020060
APA StyleIsmaiel, A., Lakshman, D. K., Jambhulkar, P. P., & Roberts, D. P. (2024). Trichoderma: Population Structure and Genetic Diversity of Species with High Potential for Biocontrol and Biofertilizer Applications. Applied Microbiology, 4(2), 875-893. https://doi.org/10.3390/applmicrobiol4020060






